Genetic Modifiers Associated with Vaso-Occlusive Crises and Acute Pain Phenomena in Sickle Cell Disease: A Scoping Review
Abstract
1. Introduction
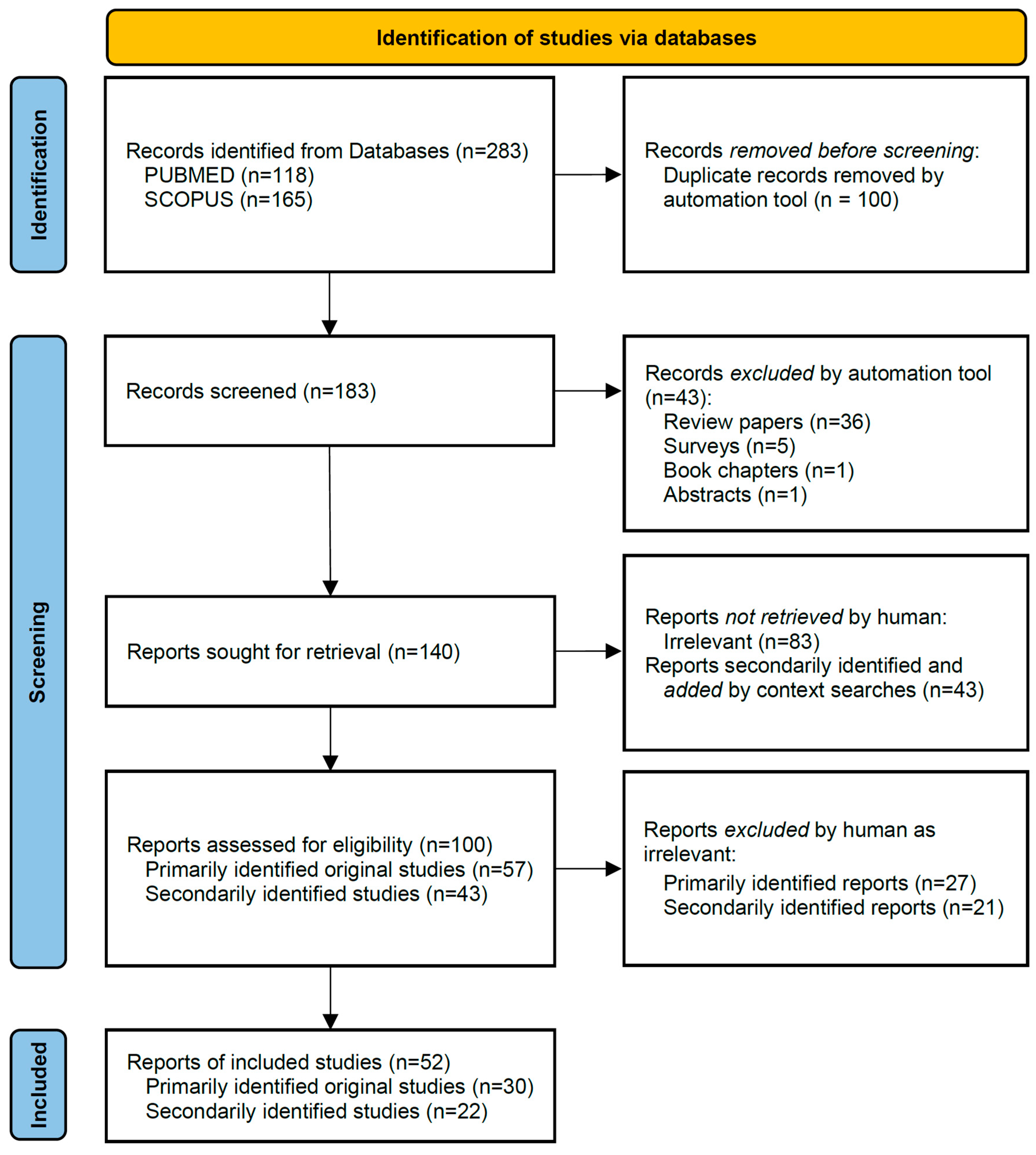
2. Materials and Methods
3. Results and Discussion
3.1. Overview of Articles Included in This Review
3.2. Globins as Overriding Determinants of SCD Severity
3.2.1. The Influence of γ-Globin and α-Globin
3.2.2. The Influence of HBB Alleles
3.3. Genetic Modifiers Associated with Pain in Vaso-Occlusive Crisis as the Main Phenotype
3.4. Genetic Modifiers Associated with Acute Chest Syndrome
3.5. Genetic Modifiers Associated with Pain in Priapism
3.6. Genetic Modifiers Associated with Avascular Necrosis
3.7. Genetic Modifiers Associated with Pain in Other or Mixed SCD Phenotypes
3.8. Network and Enrichment Analysis
| GDM | SNV(s) 1 | Phenotype 2 | Hospitalization | Effect | p-Value | Cohort | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOC | Dactylitis | AVN | ACS | Priapism | Diseased Group Size | Diseased Group Age (Age Range) | Control Group Size | Control Group Age (Age Range) | ||||||
| ADRB2 | rs1042713 | nt | nt | nt | nt | nt | ✓ | Unclear if increased or decreased | <0.001 | 436 | 16 (5–54) | 105 | Age-matched | [30] |
| ANXA2 | rs7163836, hCV11770326, rs7170178, rs1033028, hCV26910500, hCV1571628 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | <0.001–0.034 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| APOL1 | G1/G2 | nt | nt | nt | nt | nt | ✓ | Increased hospitalization | 0.048 | 436 | 16 (5–54) | 105 | Age-matched | [30] |
| AQP1 | rs10244884 | nt | nt | nt | nt | ✓ | nt | Association with occurrence of priapism for the C allele based on FDR alpha of 0.1 | 0.08959 | 190 | Not stated | / | / | [82] |
| AVPR1A | rs10877969 | ✓ | nt | nt | nt | nt | nt | Increased acute care, in particular for CT genotype | 0.02 | 107 | 35.2 ± 12.0 | / | / | [106] |
| BCL11A | not specified | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] |
| rs4671393 | nt | nt | nt | nt | nt | ✓ | HbF-promoting variants correlated with decreased hospitalization | 0.026 | 436 | 16 (5–54) | 105 | Age-matched | [30] | |
| rs1427407 rs7557939 rs1186868 | ✓ | ✓ | nt | ns | nt | nt | HbF-promoting variants correlated with fewer pain (and dactylitis, p not given) episodes with placebo [and HU] treatment | 0.0066 [<0.0001] 0.0003 [0.0005] 0.0006 [0.0005] | 190 [94 HU, 96 PB] | 14 months, 9–18 months | / | Age-matched | [33] | |
| rs4671393 | nt | nt | nt | ns | nt | [✓] | Association with increased infection-related [but not pain-related] hospitalization | 0.05 | 250 | 8.86 ± 0.19 (5–16) | / | / | [77] | |
| BMP6 | rs270393, rs267196, rs267201, rs449853, rs1225934 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.001–0.012 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| rs3812163 | nt | nt | ✓ | nt | nt | nt | Case-control study showing close association of the minor BMP6 allele with AVN | 0.021 | 39 | 38.82 (19–65) | 205 | 35.9 (15–84) | [84] | |
| CACNA2D3 | rs6777055 | ✓ | nt | nt | nt | nt | nt | Unclear if increased or decreased | 0.025 | 436 | 16 (5–54) | 105 | Age-matched | [30] |
| COMMD7 | rs6141803 | nt | nt | nt | ✓ | nt | nt | Increased risk for ACS | < 0.0001 | 1514 | 14.2 ± 11.9 | / | / | [55] |
| COMT | rs4680 | ✓ | nt | nt | nt | nt | nt | Higher chance of VOC for rare Met allele Higher rate for Met over Val homozygotes | 0.020 0.010 | 113 | Overall 34.78 ± 11.35 | 17 | Overall 34.78 ± 11.35 | [60] |
| not specified | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] | |
| rs4633, rs165599 (ATCAA haplotype) | nt | nt | nt | nt | nt | ✓ | Increased frequency of pain-related ER visits, profoundly in women | 0.0004 | 438 | 36 ± 12.5 | / | / | [58] | |
| rs6269 | nt | nt | nt | nt | nt | ✓ | Unclear if increased or decreased | 0.027 | 436 | 16 (5–54) | 105 | Age-matched | [30] | |
| DRD2 | rs4274224 | ✓ | nt | nt | nt | nt | nt | Unclear if increased or decreased | 0.037 | 436 | 16 (5–54) | 105 | Age-matched | [30] |
| DRD3 | rs6280 | ✓ | nt | nt | nt | nt | nt | Absence or fewer VOC for heterozygotes (n = 52/n = 13) vs. homozygote (n = 61/n = 4) | 0.035 | 113 | Overall 34.78 ± 11.35 | 17 | Overall 34.78 ± 11.35 | [60] |
| ECE1 | rs212527 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | <0.001 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| ERG | rs979091, rs2836430 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.005–0.014 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| EDN1 | rs5369, hCV7464888 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.001 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| T8002 | nt | nt | nt | ✓ | nt | nt | Increased risk of ACS | 0.039 | 95 | 11.2 ± 4.2 | 62 | 10.6 ± 4.8 | [74] | |
| FAAH | rs4141964 | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] |
| rs4141964 | nt | nt | nt | nt | nt | ✓ | Unclear if increased or decreased | 0.003 | 436 | 16 (5–54) | 105 | Age-matched | [30] | |
| G6PD | various | ✓ | nt | nt | nt | nt | nt | G6PD deficiency correlated with fewer pain episodes for placebo [but not for HU] treatment | 0.0010 [0.26] | 190 [94 HU, 96 PB] | 14 months, 9–18 months | / | Age-matched | [33] |
| GCH1 | rs8007267 [rs2878172] | ✓ | nt | nt | nt | nt | nt | Association with severe VOC for two cohorts, discovery cohort (155 vs. 73)|CSSCD cohort (313 vs. 200) for rs8007267 at FDR ≤ 0.05; [for rs2878172 at FDR ≤ 0.1] | 0.02|0.004 [0.01] | 155|313 | 32.4 ± 10.0|n/a | 73|200 | 34.9 ± 13.6|n/a | [71] |
| rs3783641 haplotype rs3783641-rs8007267 | ✓ | nt | nt | nt | nt | nt | rs3783641A associated with higher VOC rate (additive|recessive model) Haplotype rs3783641T-rs8007267C with lower VOC rate | 0.024|0.018 0.001 | 131 | 34.3 ± 11.8 | n/a | n/a | [70] | |
| GSTM1 | not specified | ✓ | nt | nt | nt | nt | nt | Increased risk of severe VOC | 0.005 | 50 | 10.1 ± 4.7 | 50 | Age-matched | [65] |
| HBA1/HBA2 | various | ✓ | nt | nt | nt | nt | nt | For patients under HU treatment, ATT correlated with fewer pain episodes | 0.033 | 190 [94 HU, 96 PB] | 14 months, 9–18 months | / | Age-matched | [33] |
| -α3.7k (type I) IthaID: 300 | ✓ | nt | nt | nt | nt | nt | Increased frequency of VOC | 0.002 | 436 | 16 (5–54) | 105 | age-matched | [30] | |
| -α3.7k (type I) IthaID: 300 | ✓ | nt | nt | nt | nt | nt | Enriched for VOC (allele frequency 0.34 VOC vs. 0.1–0.28 Others) | <0.001 | 127 | Not stated | 187 | Not stated | [26] | |
| various | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.03 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] | |
| -α/-α | nt | nt | nt | ✓ | nt | nt | UK SCA cohort with significant decrease in occurrence of ACS for -α/-α homozygotes (7 ACS+, 37 ACS−) compared to αα/αα (30 ACS+, 58 ACS−) | 0.016 | 44 | Not stated | 88 | Age-matched | [27] | |
| various | nt | nt | ✓ | nt | nt | nt | US SCA cohort with significant increase in occurrence of AVN for α-thal (24 AVN+, 14 AVN−) compared to non-α-thal (20 AVN+, 42 AVN−) | 0.00152 | 24 | Overall 34.8 (15–54) | 83 | Overall 34.8 (15–54) | [35] | |
| HBB haplotype | Combined rs7482144, rs2070972, rs2844105, rs10128556, rs968857, rs10768683 | ✓ | nt | nt | nt | nt | nt | Favorable haplotypes correlated with (unexpectedly) more pain episodes for placebo [and not for HU] treatment | 0.0201 [0.35] | 190 [94 HU, 96 PB] | 14 months, 9–18 months | / | Age-matched | [33] |
| Combined rs11036351, rs4320977, rs16912210, rs2855039 rs7482144 | nt | nt | nt | ✓ | nt | nt | Lower ACS rate for H1/H3 and H3/H3 haplotypes vs. H1/H1 | 0.02 | 99 | 8.5 (5–14) (across the study) | 410 | Age-matched | [50] | |
| Senegal/Benin, Benin/Benin, CAR/Benin haplotypes | ✓ | nt | ✓ | nt | ✓ | ✓ | Lower hospitalization, VOC, AVN, and priapism rates for Senegal/Benin haplotype | 0.002 0.002 0.00001 0.025 | 28 | Not stated | 139 | Not stated | [41] | |
| HbF-related PTS SNVs | BCL11A [rs1427407, rs7606173] HBSL1-MYB [ rs6940878, rs9389269, rs114398597] HBBP1 [rs10128556] | ns | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of HbF-related PTS association with ACS rate | 0.0004 | 1271 | Not stated | n/a | n/a | [78] |
| HBG2 | rs7482144 (XmnI) | ✓ | ✓ | nt | nt | nt | nt | High-HbF SNV correlated with (unexpectedly) more pain episodes for placebo [and not for HU] treatment | 0.0047 [0.35] | 190 [94 HU, 96 PB] | 14 months, 9–18 months | / | Age-matched | [33] |
| HPA genes ITGA2 | 3a/3b & 3b/3b | ✓ | nt | nt | nt | nt | ✓ | Both associated with increased need for hospitalization; the latter also associated with increased VOC frequency | 0.002 & 0.006; 0.005 | 127 | 15.0 ± 8.4 | 130 | 16.0 ± 9.2 | [66] |
| 5a/5b & 5b/5b ITGA2 | ✓ | nt | nt | nt | nt | nt | VOC risk greatly increased in the presence of the HPA-5b allele | 0.0002 | 34 | 29.4 (16–48) | 63 | 27.8, 14–51 | [67] | |
| IL1A | not specified | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] |
| ITGAV | rs10244884 | nt | nt | nt | nt | ✓ | nt | Association with occurrence of priapism for the G allele based on FDR alpha of 0.1 | 0.08959 | 190 | Not stated | / | / | [82] |
| KCNS1 | rs734784 | ✓ | nt | nt | nt | nt | nt | Unclear if increased or decreased | 0.01 | 436 | 16 (5–54) | 105 | Age-matched | [30] |
| KCNJ6 | not specified | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] |
| KLOTHO | rs480780, rs211235, s2149860, s685417, rs516306, rs565587, rs211239, rs211234, rs2238166, s499091, rs576404 | nt | nt | ✓ | nt | nt | nt | CSSCD-based case-control study of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.001–0.046 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| rs2249358 rs211239 combined rs211234/rs211239 haplotype | nt | nt | nt | nt | ✓ | nt | CSSCD case-control study of genotype enrichment for priapism+ (n = 148) vs. priapism− (n = 529) patients (p-values based on OR and CI [107]) | 0.0062 0.0072 7.61 × 10−5 | 148 | 25.9 ± 11.6 | 529 | 21.1 ± 11.6 | [29] | |
| MBL2 | rs7096206, rs10031251 | ✓ | nt | nt | nt | nt | nt | Low MBL level association with VOC frequency | 0.0229 | 117 | 5 | / | / | [52] |
| rs5030737 (R52C), rs1800450 (G54N), rs1800451) (G57E) | ✓ | nt | nt | nt | nt | nt | Association of AO + OO haplotype with VOC+ group (n = 48, 27 AA) vs. VOC- group (n = 39, 31 AA) | 0.039 | 48 | 2.5 median (0–5) (given for 87 total) | 39 | 2.5 median (0–5) (given for 87 total) | [53] | |
| XY variant [-221G>C] AO haplotype [rs5030737 (R52C), rs1800450 (G54N), rs1800451) (G57E)] | ✓ | nt | nt | nt | nt | nt | Association of conflated “intermediate/low” (YA/XA, XA/XA, YA/YO) haplotypes [vs. haplotypes “high” (YA/YA)] with high VOC frequency | 0.0188 | 39 | 3.41 ± 1.58 | 48 | 3.52 ± 1.42 | [54] | |
| MTHFR | C677T and FVLG1691A variants | ✓ | nt | nt | nt | nt | nt | Increased frequency of acute painful crises | 0.003 | 82 | 12.8 ± 8.4 | 70 | 20.06 ± 12.15 | [62] |
| rs1801133 [C677T] | nt | nt | ✓ | nt | nt | nt | US SCA cohort with significant increase for MTHFR CT&TT (16 AVN+, 8 AVN−) compared to MTHFR CC (29 AVN+, 54 AVN−) of occurrence of AVN | 0.00367 | 24 | Overall 34.8, (15–54) | 83 | Overall 34.8 (15–54) | [35] | |
| rs1801133 [C677T] | nt | nt | ✓ | nt | nt | nt | Brazilian mixed SCA & HbSC cohort with significant association of MTHFR CT&TT with a combination of AVN, stroke, and retinopathy | 0.047 | 19 | Overall 32.8 (13–72) | 34 | Overall 32.8 (13–72) | [43] | |
| MYB | not specified | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] |
| rs28384513 | nt | nt | nt | nt | nt | ✓ | HBS1L-MYB HbF-promoting loci variants correlated with decreased hospitalization | 0.01 | 436 | 16 (5–54) | 105 | Age-matched | [30] | |
| rs9399137 rs28384513 | ✓ | ✓ | nt | nt | nt | nt | High-HbF SNV correlated with fewer episodes differentially: rs9399137 for pain {and dactylitis} for placebo, and rs28384513 for [HU] treatment | 0.0001 {0.0149} [0.22] 0.31 [<0.0001] | 190 [94 HU, 96 PB] | 14 months, 9–18 months | / | Age-matched | [33] | |
| NOS1 | AAT repeats | nt | nt | nt | ✓ | nt | nt | ACS risk in patients without physician-diagnosed asthma | 0.001 | 86 | 12.6 ± 4.64 | 48 | 14 ± 8.9 | [72] |
| NOS3 | C-786 | nt | nt | nt | ✓ | nt | nt | Decreased risk of ACS | 0.021 | 104 | 11.2 ± 3.1 | 55 | 9.9 ± 4.8 | [74] |
| C-786 | nt | nt | nt | ✓ | nt | nt | Increased risk of ACS in females | 0.0061 | 41 | 36 | 46 | 31 | [79] | |
| VNTR (intron 4a vs. 4b polymorphisms with 4 vs. 5 × 27-bp repeat) | ✓ | nt | nt | nt | nt | nt | Increased frequency of VOCs for aa/ab (n = 28) compared to bb (n = 23) genotype within SCD population | 0.017 | 28 | 11.2 ± 3.9 | 23 | 10.6 ± 3.4 | [75] | |
| NR3C1 | not specified | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] |
| rs33389 rs2963155 rs9324918 | ✓ | nt | nt | nt | nt | nt | Higher VOC rate for rs33389T (additive|recessive model) Ditto for rs2963155G (additive|dominant|recessive model) Ditto for rs9324918C (additive model) | 0.014|0.011 <0.001|0.021|< 0.001 0.021 | 136 | 34.0 ± 11.7 | / | / | [56] | |
| OPRM1 | rs1799971 | nt | nt | nt | nt | nt | ✓ | Unclear if increased or decreased | 0.031 | 436 | 16 (5–54) | 105 | Age-matched | [30] |
| PDS5B | hCV3118898, hCV11710292 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.001–0.014 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| PKLR | rs2071053, rs8177970, rs116244351, rs114455416, rs12741350, rs3020781, rs8177964 | nt | nt | nt | nt | nt | ✓ | Increased hospitalization | 0.0071 | 242, 977 | 33.05 ± 11.26, 8.98 ± 2.43 | / | / | [55] |
| PLSCR4 | not specified | ✓ | nt | nt | nt | nt | nt | High fold change in the comparison of VOC to steady-state patients | <0.01 | 10 | 33 ± 10.82 | 8 | Age-matched | [64] |
| PNMT | rs876493, rs2934965, rs2941523 | ✓ | nt | nt | nt | nt | nt | Lower VOC rate for rs876493A, rs2934965T, rs2941523G | 0.012, 0.044, 0.017 | 131 | 34.3 ± 11.8 | / | / | [108] |
| PROZ promoter | rs3024718, rs3024719, rs3024731, rs3024735; GGTG, AGTG | ✓ | nt | nt | nt | nt | nt | Higher frequency in VOC compared to steady-state patients | 0.028, 0.045; 0.018, 0.001 | 239 | 11.9 ± 7.1 | 138 | 14.2 ± 10.5 | [68] |
| PTS region HMIP-2A | rs7776196, rs9399137, rs35786788, rs796983051, rs79651256 | nt | nt | nt | ✓ | nt | ✓ | Association of rs7776196 with pain-related hospitalization and of [rs9399137] with ACS | 0.04 [0.005] | 250 | 8.86 ± 0.19 (5–16) | / | / | [77] |
| PTS region HMIP-2B | rs9402686, rs4895441, rs9494145 | nt | nt | nt | ✓ | nt | ns | Association of rs9402686 with ACS | 0.01 | 250 | 8.86 ± 0.19 (5–16) | / | / | [77] |
| STARD13 | rs538874, rs475303, rs648464 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.001–0.029 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| TBC1D1 | not specified | ✓ | nt | nt | nt | nt | nt | Unweighted polygenic score showed high association with VOC | <0.0001 | 722 | >11.5 | / | / | [55] |
| TGFBR2 | rs1019856, rs934328 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | <0.001–0.023 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| TGFBR3 | rs284157 | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | <0.001 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
| rs7526590 | nt | nt | nt | nt | ✓ | nt | Association with occurrence of priapism for the T allele based on FDR alpha of 0.1 | 0.08959 | 190 | Not stated | / | / | [82] | |
| TRPA1 | rs920829 CGAGG haplotype | ✓ | nt | nt | nt | nt | nt | Lower VOC rate for rs920829 GG genotype Higher VOC count for CGAGG haplotype | 0.008 0.009 | 132 | 34.2 ± 11.8 | n/a | n/a | [109] |
| UGT2B7 | rs7438135 | nt | nt | nt | nt | nt | ✓ | Unclear if increased or decreased | 0.037 | 436 | 16 (5–54) | 105 | Age-matched | [30] |
| VEGFA | rs3025020 | nt | nt | nt | ✓ | nt | nt | Homozygosity for the minor -583T allele increases risk for ACS according to univariate [and multivariate] analysis | 0.013 [0.019] | 90 | 13 (2–16) | 261 | 12, 3–16 | [80] |
| Male/female | n/a | nt | nt | ✓ | nt | nt | nt | CSSCD cohort analysis of genotype enrichment for AVN+ (n = 442) vs. AVN− (n = 455) patients | 0.02 | 442 | Not stated | 455 | >20, 6 years older than diseased group | [83] |
3.9. Beyond GWAS and Genetics
3.10. The Influence of Environmental Factors
3.11. Areas for Exploration of Genetic Modifiers: Complex Factors, Co-Morbidities, and Linked Phenomena
3.12. Limitations of the Present Article
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bender, M.A.; Carlberg, K. Sickle Cell Disease. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2023. [Google Scholar]
- Zaidi, A.U.; Glaros, A.K.; Lee, S.; Wang, T.; Bhojwani, R.; Morris, E.; Donohue, B.; Paulose, J.; Iorga, Ş.R.; Nellesen, D. A Systematic Literature Review of Frequency of Vaso-Occlusive Crises in Sickle Cell Disease. Orphanet J. Rare Dis. 2021, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- McClish, D.K.; Smith, W.R.; Dahman, B.A.; Levenson, J.L.; Roberts, J.D.; Penberthy, L.T.; Aisiku, I.P.; Roseff, S.D.; Bovbjerg, V.E. Pain Site Frequency and Location in Sickle Cell Disease: The PiSCES Project. PAIN 2009, 145, 246. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.E.V.; Stotts, N.A.; Humphreys, J.; Treadwell, M.J.; Miaskowski, C. A Review of the Literature on the Multiple Dimensions of Chronic Pain in Adults with Sickle Cell Disease. J. Pain Symptom Manag. 2010, 40, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Klings, E.S.; Steinberg, M.H. Acute Chest Syndrome of Sickle Cell Disease: Genetics, Risk Factors, Prognosis, and Management. Expert. Rev. Hematol. 2022, 15, 117–125. [Google Scholar] [CrossRef]
- Levasseur, D.N.; Ryan, T.M.; Reilly, M.P.; McCune, S.L.; Asakura, T.; Townes, T.M. A Recombinant Human Hemoglobin with Anti-Sickling Properties Greater than Fetal Hemoglobin. J. Biol. Chem. 2004, 279, 27518–27524. [Google Scholar] [CrossRef]
- Nitric Oxide Effects in Sickle Cell Disease. |Blood|American Society of Hematology. Available online: https://ashpublications.org/blood/article/112/11/sci-48/74923/Nitric-Oxide-Effects-in-Sickle-Cell-Disease (accessed on 9 December 2024).
- Lopes, F.C.M.; Traina, F.; Almeida, C.B.; Leonardo, F.C.; Franco-Penteado, C.F.; Garrido, V.T.; Colella, M.P.; Soares, R.; Olalla-Saad, S.T.; Costa, F.F.; et al. Key Endothelial Cell Angiogenic Mechanisms Are Stimulated by the Circulating Milieu in Sickle Cell Disease and Attenuated by Hydroxyurea. Haematologica 2015, 100, 730–739. [Google Scholar] [CrossRef]
- Satterwhite, E.; Sonoki, T.; Willis, T.G.; Harder, L.; Nowak, R.; Arriola, E.L.; Liu, H.; Price, H.P.; Gesk, S.; Steinemann, D.; et al. The BCL11 Gene Family: Involvement of BCL11A in Lymphoid Malignancies. Blood 2001, 98, 3413–3420. [Google Scholar] [CrossRef]
- Uda, M.; Galanello, R.; Sanna, S.; Lettre, G.; Sankaran, V.G.; Chen, W.; Usala, G.; Busonero, F.; Maschio, A.; Albai, G.; et al. Genome-Wide Association Study Shows BCL11A Associated with Persistent Fetal Hemoglobin and Amelioration of the Phenotype of Beta-Thalassemia. Proc. Natl. Acad. Sci. USA 2008, 105, 1620–1625. [Google Scholar] [CrossRef]
- Davey Smith, G. Commentary: Known Knowns and Known Unknowns in Medical Research: James Mackenzie Meets Donald Rumsfeld. Int. J. Epidemiol. 2016, 45, 1747–1748. [Google Scholar] [CrossRef]
- PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation|Annals of Internal Medicine. Available online: https://www.acpjournals.org/doi/10.7326/M18-0850 (accessed on 8 December 2024).
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J. Grad. Med. Educ. 2022, 14, 565–567. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING Database in 2025: Protein Networks with Directionality of Regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, C.; Menzel, S.; Philipsen, S.; Kountouris, P. Genetic Polymorphisms Associated with Fetal Hemoglobin (HbF) Levels and F-Cell Numbers: A Systematic Review of Genome-Wide Association Studies. Int. J. Mol. Sci. 2024, 25, 11408. [Google Scholar] [CrossRef]
- Stephanou, C.; Tamana, S.; Minaidou, A.; Papasavva, P.; Kleanthous, M.; Kountouris, P. Genetic Modifiers at the Crossroads of Personalised Medicine for Haemoglobinopathies. J. Clin. Med. 2019, 8, 1927. [Google Scholar] [CrossRef]
- Cato, L.D.; Li, R.; Lu, H.Y.; Yu, F.; Wissman, M.; Mkumbe, B.S.; Ekwattanakit, S.; Deelen, P.; Mwita, L.; Sangeda, R.; et al. Genetic Regulation of Fetal Hemoglobin across Global Populations. medRxiv 2023, 2023.03.24.23287659. [Google Scholar] [CrossRef]
- Bou-Fakhredin, R.; De Franceschi, L.; Motta, I.; Cappellini, M.D.; Taher, A.T. Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective. Pharmaceuticals 2022, 15, 753. [Google Scholar] [CrossRef]
- Ting, P.Y.; Borikar, S.; Kerrigan, J.R.; Thomsen, N.M.; Aghania, E.; Hinman, A.E.; Pizzato, N.; Reyes, A.; Fodor, B.D.; Wu, F.; et al. Targeted Degradation of the Wiz Transcription Factor for Gamma Globin De-Repression. Blood 2023, 142, 2. [Google Scholar] [CrossRef]
- Barak, M.; Hu, C.; Matthews, A.; Fortenberry, Y.M. Current and Future Therapeutics for Treating Patients with Sickle Cell Disease. Cells 2024, 13, 848. [Google Scholar] [CrossRef]
- Kountouris, P.; Stephanou, C.; Archer, N.; Bonifazi, F.; Giannuzzi, V.; Kuo, K.H.M.; Maggio, A.; Makani, J.; Mañú-Pereira, M.d.M.; Michailidou, K.; et al. The International Hemoglobinopathy Research Network (INHERENT): An International Initiative to Study the Role of Genetic Modifiers in Hemoglobinopathies. Am. J. Hematol. 2021, 96, E416–E420. [Google Scholar] [CrossRef]
- Traeger-Synodinos, J.; Vrettou, C.; Sofocleous, C.; Zurlo, M.; Finotti, A.; Gambari, R. Impact of α-Globin Gene Expression and α-Globin Modifiers on the Phenotype of β-Thalassemia and Other Hemoglobinopathies: Implications for Patient Management. Int. J. Mol. Sci. 2024, 25, 3400. [Google Scholar] [CrossRef]
- Mensah, C.; Sheth, S. Optimal Strategies for Carrier Screening and Prenatal Diagnosis of α- and β-Thalassemia. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 607–613. [Google Scholar] [CrossRef]
- Steinberg, M.H.; Adewoye, A.H. Modifier Genes and Sickle Cell Anemia. Curr. Opin. Hematol. 2006, 13, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Bonello-Palot, N.; Badens, C.; Pissard, S.; Chamouine, A.; Bernaudin, F.; Bertrand, Y.; Connes, P.; Renoux, C. HbF-Promoting Polymorphisms May Specifically Reduce the Residual Risk of Cerebral Vasculopathy in SCA Children with Alpha-Thalassemia. Clin. Hemorheol. Microcirc. 2021, 77, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Higgs, D.R.; Aldridge, B.E.; Lamb, J.; Clegg, J.B.; Weatherall, D.J.; Hayes, R.J.; Grandison, Y.; Lowrie, Y.; Mason, K.P.; Serjeant, B.E.; et al. The Interaction of Alpha-Thalassemia and Homozygous Sickle-Cell Disease. N. Engl. J. Med. 1982, 306, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Gaston, M.; Smith, J.; Gallagher, D.; Flournoy-Gill, Z.; West, S.; Bellevue, R.; Farber, M.; Grover, R.; Koshy, M.; Ritchey, A.K. Recruitment in the Cooperative Study of Sickle Cell Disease (CSSCD). Control Clin. Trials 1987, 8, 131S–140S. [Google Scholar] [CrossRef]
- Nolan, V.G.; Baldwin, C.; Ma, Q.; Wyszynski, D.F.; Amirault, Y.; Farrell, J.J.; Bisbee, A.; Embury, S.H.; Farrer, L.A.; Steinberg, M.H. Association of Single Nucleotide Polymorphisms in Klotho with Priapism in Sickle Cell Anaemia. Br. J. Haematol. 2005, 128, 266–272. [Google Scholar] [CrossRef]
- Wonkam, A.; Mnika, K.; Ngo Bitoungui, V.J.; Chetcha Chemegni, B.; Chimusa, E.R.; Dandara, C.; Kengne, A.P. Clinical and Genetic Factors Are Associated with Pain and Hospitalisation Rates in Sickle Cell Anaemia in Cameroon. Br. J. Haematol. 2018, 180, 134–146. [Google Scholar] [CrossRef]
- Rumaney, M.B.; Ngo Bitoungui, V.J.; Vorster, A.A.; Ramesar, R.; Kengne, A.P.; Ngogang, J.; Wonkam, A. The Co-Inheritance of Alpha-Thalassemia and Sickle Cell Anemia Is Associated with Better Hematological Indices and Lower Consultations Rate in Cameroonian Patients and Could Improve Their Survival. PLoS ONE 2014, 9, e100516. [Google Scholar] [CrossRef]
- Adekile, A.D.; Azab, A.F. The Sickle β-Thalassemia Phenotype. J. Pediatr. Hematol. Oncol. 2017, 39, 327–331. [Google Scholar] [CrossRef]
- Sheehan, V.A.; Luo, Z.; Flanagan, J.M.; Howard, T.A.; Thompson, B.W.; Wang, W.C.; Kutlar, A.; Ware, R.E.; BABY HUG Investigators. Genetic Modifiers of Sickle Cell Anemia in the BABY HUG Cohort: Influence on Laboratory and Clinical Phenotypes. Am. J. Hematol. 2013, 88, 571–576. [Google Scholar] [CrossRef]
- Alsultan, A.; Aleem, A.; Ghabbour, H.; AlGahtani, F.H.; Al-Shehri, A.; Osman, M.E.; Kurban, K.; Alsultan, M.S.; Bahakim, H.; Al-Momen, A.M. Sickle Cell Disease Subphenotypes in Patients From Southwestern Province of Saudi Arabia. J. Pediatr. Hematol. /Oncol. 2012, 34, 79–84. [Google Scholar] [CrossRef]
- Kutlar, A.; Kutlar, F.; Turker, I.; Tural, C. The Methylene Tetrahydrofolate Reductase (C677T) Mutation as a Potential Risk Factor for Avascular Necrosis in Sickle Cell Disease. Hemoglobin 2001, 25, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Ozahata, M.C.; Page, G.P.; Guo, Y.; Ferreira, J.E.; Dinardo, C.L.; Carneiro-Proietti, A.B.F.; Loureiro, P.; Mota, R.A.; Rodrigues, D.O.W.; Belisario, A.R.; et al. Clinical and Genetic Predictors of Priapism in Sickle Cell Disease: Results from the Recipient Epidemiology and Donor Evaluation Study III Brazil Cohort Study. J. Sex. Med. 2013, 16, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Meliti, A.H.N.; Muftah, S.; Saleh, D. Compound Heterogeneous Sickle Cell-B+ Thalassemia Incidentally Discovered Through Cytological Examination of a Fine-Needle Aspiration Specimen from an Aneurysmal Bone Cyst in a Young Child: A Case Report. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Cancio, M.I.; Aygun, B.; Chui, D.H.K.; Rothman, J.A.; Scott, J.P.; Estepp, J.H.; Hankins, J.S. The Clinical Severity of Hemoglobin S/Black (AΓδβ)0-thalassemia. Pediatr. Blood Cancer 2017, 64, e26596. [Google Scholar] [CrossRef]
- Zimmerman, S.A.; O’Branski, E.E.; Rosse, W.F.; Ware, R.E. Hemoglobin S/OARAB: Thirteen New Cases and Review of the Literature. Am. J. Hematol. 1999, 60, 279–284. [Google Scholar] [CrossRef]
- Goode, E.; Boruchov, D.; Oliveira, J.L.; Lau, C.C. Hemoglobin S/Hemoglobin Quebec-Chori Presenting as Sickle Cell Disease: A Case Report. J. Pediatr. Hematol./Oncol. 2020, 42, e775–e777. [Google Scholar] [CrossRef]
- Powars, D.R.; Chan, L.; Schroeder, W.A. Beta S-Gene-Cluster Haplotypes in Sickle Cell Anemia: Clinical Implications. Am. J. Pediatr. Hematol. Oncol. 1990, 12, 367–374. [Google Scholar] [CrossRef]
- Rezende, P.V.; Santos, M.V.; Campos, G.F.; Vieira, L.L.M.; Souza, M.B.; Belisário, A.R.; Silva, C.M.; Viana, M.B. Clinical and Hematological Profile in a Newborn Cohort with Hemoglobin SC. J. Pediatr. 2018, 94, 666–672. [Google Scholar] [CrossRef]
- Moreira Neto, F.; Lourenço, D.M.; Noguti, M.A.E.; Morelli, V.M.; Gil, I.C.P.; Beltrão, A.C.S.; Figueiredo, M.S. The Clinical Impact of MTHFR Polymorphism on the Vascular Complications of Sickle Cell Disease. Braz. J. Med. Biol. Res. 2006, 39, 1291–1295. [Google Scholar] [CrossRef]
- Filho, I.L.D.S.; Ribeiro, G.S.; Moura, P.G.; Vechi, M.L.; Cavalcante, A.C.; Andrada-Serpa, M.J.D. Sickle Cell Disease: Acute Clinical Manifestations in Early Childhood and Molecular Characteristics in a Group of Children in Rio de Janeiro. Rev. Bras. Hematol. Hemoter. 2012, 34, 196–201. [Google Scholar] [CrossRef]
- Armenis, I.; Kalotychou, V.; Tzanetea, R.; Kollia, P.; Kontogeorgiou, Z.; Anastasopoulou, D.; Mantzourani, M.; Samarkos, M.; Pantos, K.; Konstantopoulos, K.; et al. Prognostic Value of T786C and G894T eNOS Polymorphisms in Sickle Cell Disease. Nitric Oxide 2017, 62, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.S.; Okumura, J.V.; Belini-Júnior, É.; Oliveira, R.G.; Nascimento, P.P.; Silva, D.G.H.; Lobo, C.L.C.; Oliani, S.M.; Bonini-Domingos, C.R. Phenotypic Diversity of Sickle Cell Disease in Patients with a Double Heterozygosity for Hb S and Hb D-Punjab. Hemoglobin 2016, 40, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Patel, S.; Mashon, R.S.; Dash, P.M.; Mukherjee, M.B. Diverse Phenotypic Expression of Sickle Cell Hemoglobin C Disease in an Indian Family. Ann. Hematol. 2011, 90, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Warthe, V.; Dayama, P.; Sarate, D.; Colah, R.; Mehta, P.; Serjeant, G. Sickle Cell Disease in Central India: A Potentially Severe Syndrome. Indian J. Pediatr. 2016, 83, 1071–1076. [Google Scholar] [CrossRef]
- Adekile, A.D.; Haider, M.Z. Haptoglobin Gene Polymorphisms in Sickle Cell Disease Patients with Different βS-Globin Gene Haplotypes. Med. Princ. Pract. 2010, 19, 447–450. [Google Scholar] [CrossRef]
- Bean, C.J.; Boulet, S.L.; Yang, G.; Payne, A.B.; Ghaji, N.; Pyle, M.E.; Hooper, W.C.; Bhatnagar, P.; Keefer, J.; Barron-Casella, E.A.; et al. Acute Chest Syndrome Is Associated with Single Nucleotide Polymorphism-Defined Beta Globin Cluster Haplotype in Children with Sickle Cell Anaemia. Br. J. Haematol. 2013, 163, 268–276. [Google Scholar] [CrossRef]
- Chang, Y.-P.C.; Maier-Redelsperger, M.; Smith, K.D.; Contu, L.; Ducrocq, R. The Relative Importance of the X-Linked FCP Locus and β-Globin Haplotypes in Determining Haemoglobin F Levels: A Study of SS Patients Homozygous for bS Haplotypes. Br. J. Haematol. 1997, 96, 806–814. [Google Scholar] [CrossRef]
- Medeiros, F.S.; de Mendonça, T.F.; Lopes, K.A.d.M.; França, L.M.d.C.; Silva, d.A.S.; Vasconcelos, L.R.S.; Oliveira, M.d.C.V.C.; Anjos, d.A.C.M.; Hatzlhofer, B.L.D.; Bezerra, M.A.C.; et al. Combined Genotypes of the MBL2 Gene Related to Low Mannose-Binding Lectin Levels Are Associated with Vaso-Occlusive Events in Children with Sickle Cell Anemia. Genet. Mol. Biol. 2017, 40, 600–603. [Google Scholar] [CrossRef]
- Oliveira, M.C.V.C.; Mendonça, T.F.; Vasconcelos, L.R.S.; Moura, P.; Bezerra, M.A.C.; Santos, M.N.N.; Araújo, A.S.; Cavalcanti, M.S.M. Association of the MBL2 Gene EXON1 Polymorphism and Vasoocclusive Crisis in Patients with Sickle Cell Anemia. Acta Haematol. 2009, 121, 212–215. [Google Scholar] [CrossRef]
- Mendonça, T.F.; Oliveira, M.C.V.C.; Vasconcelos, L.R.S.; Pereira, L.M.M.B.; Moura, P.; Bezerra, M.A.C.; Santos, M.N.N.; Araújo, A.S.; Cavalcanti, M.S.M. Association of Variant Alleles of MBL2 Gene with Vasoocclusive Crisis in Children with Sickle Cell Anemia. Blood Cells Mol. Dis. 2010, 44, 224–228. [Google Scholar] [CrossRef]
- Rampersaud, E.; Kang, G.; Palmer, L.E.; Rashkin, S.R.; Wang, S.; Bi, W.; Alberts, N.M.; Anghelescu, D.; Barton, M.; Birch, K.; et al. A Polygenic Score for Acute Vaso-Occlusive Pain in Pediatric Sickle Cell Disease. Blood Adv. 2021, 5, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Jhun, E.H.; Sadhu, N.; Yao, Y.; He, Y.; Molokie, R.E.; Wilkie, D.J.; Wang, Z.J. Glucocorticoid Receptor Single Nucleotide Polymorphisms Are Associated with Acute Crisis Pain in Sickle Cell Disease. Pharmacogenomics 2018, 19, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Allard, P.; Alhaj, N.; Lobitz, S.; Cario, H.; Jarisch, A.; Grosse, R.; Oevermann, L.; Hakimeh, D.; Tagliaferri, L.; Kohne, E.; et al. Genetic Modifiers of Fetal Hemoglobin Affect the Course of Sickle Cell Disease in Patients Treated with Hydroxyurea. Haematol 2021, 107, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Belfer, I.; Nouraie, M.; Zeng, Q.; Goel, R.; Chu, Y.; Krasiy, I.; Krishnamurti, L. Association of Genetic Variation in COMT Gene with Pain Related to Sickle Cell Disease in Patients from the Walk-PHaSST Study. J. Pain Res. 2018, 11, 537–543. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Bhatnagar, P.; Bean, C.J.; Steinberg, M.H.; Milton, J.N.; Casella, J.F.; Barron-Casella, E.; Arking, D.E.; DeBaun, M.R. Genome-Wide Association Study to Identify Variants Associated with Acute Severe Vaso-Occlusive Pain in Sickle Cell Anemia. Blood 2017, 130, 686–688. [Google Scholar] [CrossRef]
- Jhun, E.; He, Y.; Yao, Y.; Molokie, R.E.; Wilkie, D.J.; Wang, Z.J. Dopamine D3 Receptor Ser9Gly and Catechol-o-Methyltransferase Val158Met Polymorphisms and Acute Pain in Sickle Cell Disease. Anesth. Analg. 2014, 119, 1201–1207. [Google Scholar] [CrossRef]
- Wang, X.; Gardner, K.; Tegegn, M.B.; Dalgard, C.L.; Alba, C.; Menzel, S.; Patel, H.; Pirooznia, M.; Fu, Y.-P.; Seifuddin, F.T.; et al. Genetic Variants of PKLR Are Associated with Acute Pain in Sickle Cell Disease. Blood Adv. 2022, 6, 3535–3540. [Google Scholar] [CrossRef]
- Sokkar, M.F.; Kamal, L.; Salama, N.; Hamdy, M. Thrombophilic Mutations and Risk of Vascular Complications in Sickle Cell Disease. Gene Rep. 2022, 27, 101595. [Google Scholar] [CrossRef]
- Nishank, S.S.; Singh, M.P.S.S.; Yadav, R. Clinical Impact of Factor V Leiden, Prothrombin G20210A, and MTHFR C677T Mutations among Sickle Cell Disease Patients of Central India. Eur. J. Haematol. 2013, 91, 462–466. [Google Scholar] [CrossRef]
- Abdulwahab, H.; Aljishi, M.; Sultan, A.; Al-Kafaji, G.; Sridharan, K.; Bakhiet, M.; Taha, S. Whole Blood Transcriptomic Analysis Reveals PLSCR4 as a Potential Marker for Vaso-Occlusive Crises in Sickle Cell Disease. Sci. Rep. 2021, 11, 22199. [Google Scholar] [CrossRef]
- Shiba, H.F.; El-Ghamrawy, M.K.; Shaheen, I.A.E.-M.; Ali, R.A.E.-G.; Mousa, S.M. Glutathione S-Transferase Gene Polymorphisms (GSTM1, GSTT1, and GSTP1) in Egyptian Pediatric Patients with Sickle Cell Disease. Pediatr. Dev. Pathol. 2014, 17, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Al-Subaie, A.M.; Fawaz, N.A.; Mahdi, N.; Al-Absi, I.K.; Al-Ola, K.; Ameen, G.; Almawi, W.Y. Human Platelet Alloantigens (HPA) 1, HPA2, HPA3, HPA4, and HPA5 Polymorphisms in Sickle Cell Anemia Patients with Vaso-occlusive Crisis. Eur. J. Haematol. 2009, 83, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Alberto, F.L.; Costa, R.N.P.; Lepikson-Neto, J.; Gualandro, S.F.M.; Figueiredo, M.S.; Annichino-Bizzacchi, J.M.; Saad, S.T.O.; Costa, F.F. Polymorphism of the Human Platelet Antigen-5 System Is a Risk Factor for Occlusive Vascular Complications in Patients with Sickle Cell Anemia. Vox Sang. 2004, 87, 118–123. [Google Scholar] [CrossRef]
- Mahdi, N.; Abu-Hijleh, T.M.; Abu-Hijleh, F.M.; Sater, M.S.; Al-Ola, K.; Almawi, W.Y. Protein Z Polymorphisms Associated with Vaso-Occlusive Crisis in Young Sickle Cell Disease Patients. Ann. Hematol. 2012, 91, 1215–1220. [Google Scholar] [CrossRef]
- Yousry, S.M.; Ellithy, H.N.; Shahin, G.H. Endothelial Nitric Oxide Synthase Gene Polymorphisms and the Risk of Vasculopathy in Sickle Cell Disease. Hematology 2016, 21, 359–367. [Google Scholar] [CrossRef]
- Sadhu, N.; Jhun, E.H.; Yao, Y.; He, Y.; Molokie, R.E.; Wilkie, D.J.; Wang, Z.J. Genetic Variants of GCH1 Associate with Chronic and Acute Crisis Pain in African Americans with Sickle Cell Disease. Exp. Hematol. 2018, 66, 42–49. [Google Scholar] [CrossRef]
- Belfer, I.; Youngblood, V.; Darbari, D.S.; Wang, Z.; Diaw, L.; Freeman, L.; Desai, K.; Dizon, M.; Allen, D.; Cunnington, C.; et al. A GCH1 Haplotype Confers Sex-Specific Susceptibility to Pain Crises and Altered Endothelial Function in Adults with Sickle Cell Anemia. Am. J. Hematol. 2014, 89, 187–193. [Google Scholar] [CrossRef]
- Duckworth, L.; Hsu, L.; Feng, H.; Wang, J.; Sylvester, J.E.; Kissoon, N.; Sandler, E.; Lima, J.J. Physician-diagnosed Asthma and Acute Chest Syndrome: Associations with NOS Polymorphisms. Pediatr. Pulmonol. 2007, 42, 332–338. [Google Scholar] [CrossRef]
- Bean, C.J.; Boulet, S.L.; Ellingsen, D.; Pyle, M.E.; Barron-Casella, E.A.; Casella, J.F.; Payne, A.B.; Driggers, J.; Trau, H.A.; Yang, G.; et al. Heme Oxygenase-1 Gene Promoter Polymorphism Is Associated with Reduced Incidence of Acute Chest Syndrome among Children with Sickle Cell Disease. Blood 2012, 120, 3822–3828. [Google Scholar] [CrossRef][Green Version]
- Chaar, V.; Tarer, V.; Etienne-Julan, M.; Diara, J.P.; Elion, J.; Romana, M. ET-1 and ecNOS Gene Polymorphisms and Susceptibility to Acute Chest Syndrome and Painful Vaso-Occlusive Crises in Children with Sickle Cell Anemia. Haematologica 2006, 91, 1277–1278. [Google Scholar]
- Tantawy, A.A.G.; Adly, A.A.M.; Ismail, E.A.R.; Aly, S.H. Endothelial Nitric Oxide Synthase Gene Intron 4 VNTR Polymorphism in Sickle Cell Disease: Relation to Vasculopathy and Disease Severity. Pediatr. Blood Cancer 2015, 62, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, G.; Coady, S.; Garrett, M.E.; Jeffries, N.; Puggal, M.; Paltoo, D.; Soldano, K.; Guasch, A.; Ashley-Koch, A.E.; Telen, M.J.; et al. Gene-Centric Association Study of Acute Chest Syndrome and Painful Crisis in Sickle Cell Disease Patients. Blood 2013, 122, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sales, R.R.; Belisário, A.R.; Faria, G.; Mendes, F.; Luizon, M.R.; Viana, M.B. Functional Polymorphisms of BCL11A and HBS1L-MYB Genes Affect Both Fetal Hemoglobin Level and Clinical Outcomes in a Cohort of Children with Sickle Cell Anemia. Ann. Hematol. 2020, 99, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Pincez, T.; Lo, K.S.; D’Orengiani, A.-L.P.H.d.; Garrett, M.E.; Brugnara, C.; Ashley-Koch, A.E.; Telen, M.J.; Galacteros, F.; Joly, P.; Bartolucci, P.; et al. Variation and Impact of Polygenic Hematologic Traits in Monogenic Sickle Cell Disease. Haematologica 2023, 108, 870–881. [Google Scholar] [CrossRef]
- Sharan, K.K.M.; Surrey, S.; Ballas, S.; Borowski, M.; Devoto, M.; Wang, K.F.; Sandler, E. Association of T-786C eNOS Gene Polymorphism with Increased Susceptibility to Acute Chest Syndrome in Females with Sickle Cell Disease. Br. J. Haematol. 2004, 124, 240–243. [Google Scholar] [CrossRef]
- Redha, N.A.; Mahdi, N.; Al-Habboubi, H.H.; Almawi, W.Y. Impact of VEGFA −583C > T Polymorphism on Serum VEGF Levels and the Susceptibility to Acute Chest Syndrome in Pediatric Patients with Sickle Cell Disease. Pediatr. Blood Cancer 2014, 61, 2310–2312. [Google Scholar] [CrossRef]
- Figueiredo, C.V.B.; Santiago, R.P.; Da Guarda, C.C.; Oliveira, R.M.; Fiuza, L.M.; Yahouédéhou, S.C.M.A.; Carvalho, S.P.; Neres, J.S.D.S.; Oliveira, A.M.D.J.; Fonseca, C.A.; et al. Priapism in Sickle Cell Disease: Associations between NOS3 and EDN1 Genetic Polymorphisms and Laboratory Biomarkers. PLoS ONE 2021, 16, e0246067. [Google Scholar] [CrossRef]
- Elliott, L.; Ashley-Koch, A.E.; Castro, L.D.; Jonassaint, J.; Price, J.; Ataga, K.I.; Levesque, M.C.; Brice Weinberg, J.; Eckman, J.R.; Orringer, E.P.; et al. Genetic Polymorphisms Associated with Priapism in Sickle Cell Disease. Br. J. Haematol. 2007, 137, 262–267. [Google Scholar] [CrossRef]
- Baldwin, C.; Nolan, V.G.; Wyszynski, D.F.; Ma, Q.-L.; Sebastiani, P.; Embury, S.H.; Bisbee, A.; Farrell, J.; Farrer, L.; Steinberg, M.H. Association of Klotho, Bone Morphogenic Protein 6, and Annexin A2 Polymorphisms with Sickle Cell Osteonecrosis. Blood 2005, 106, 372–375. [Google Scholar] [CrossRef]
- Ulug, P.; Vasavda, N.; Awogbade, M.; Cunningham, J.; Menzel, S.; Thein, S.L. Association of Sickle Avascular Necrosis with Bone Morphogenic Protein 6. Ann. Hematol. 2009, 88, 803–805. [Google Scholar] [CrossRef]
- Zhang, X.; Shah, B.N.; Zhang, W.; Saraf, S.L.; Nouraie, M.; Nekhai, S.; Machado, R.F.; Gladwin, M.T.; Gordeuk, V.R. S100B Has Pleiotropic Effects on Vaso-occlusive Manifestations in Sickle Cell Disease. Am. J. Hematol. 2020, 95, E62. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.F.; Barst, R.J.; Yovetich, N.A.; Hassell, K.L.; Kato, G.J.; Gordeuk, V.R.; Gibbs, J.S.R.; Little, J.A.; Schraufnagel, D.E.; Krishnamurti, L.; et al. Hospitalization for Pain in Patients with Sickle Cell Disease Treated with Sildenafil for Elevated TRV and Low Exercise Capacity. Blood 2011, 118, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Gordeuk, V.R.; Minniti, C.P.; Nouraie, M.; Campbell, A.D.; Rana, S.R.; Luchtman-Jones, L.; Sable, C.; Dham, N.; Ensing, G.; Prchal, J.T.; et al. Elevated Tricuspid Regurgitation Velocity and Decline in Exercise Capacity over 22 Months of Follow up in Children and Adolescents with Sickle Cell Anemia. Haematologica 2011, 96, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Ma, S.-F.; Desai, A.A.; Saraf, S.; Miasniakova, G.; Sergueeva, A.; Ammosova, T.; Xu, M.; Nekhai, S.; et al. Hypoxic Response Contributes to Altered Gene Expression and Precapillary Pulmonary Hypertension in Patients with Sickle Cell Disease. Circulation 2014, 129, 1650–1658. [Google Scholar] [CrossRef]
- Desai, A.A.; Zhou, T.; Ahmad, H.; Zhang, W.; Mu, W.; Trevino, S.; Wade, M.S.; Raghavachari, N.; Kato, G.J.; Peters-Lawrence, M.H.; et al. A Novel Molecular Signature for Elevated Tricuspid Regurgitation Velocity in Sickle Cell Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 359–368. [Google Scholar] [CrossRef]
- Saraf, S.L.; Zhang, X.; Kanias, T.; Lash, J.P.; Molokie, R.E.; Oza, B.; Lai, C.; Rowe, J.H.; Gowhari, M.; Hassan, J.; et al. Haemoglobinuria Is Associated with Chronic Kidney Disease and Its Progression in Patients with Sickle Cell Anaemia. Br. J. Haematol. 2014, 164, 729–739. [Google Scholar] [CrossRef]
- Orolu, A.K.; Ahmad, S.F.; Oluwaseun, O.T. Splenomegaly and Splenic Pseudocyst in a Female Teenage Patient with Sickle Cell Anemia-A Case Report. Niger. J. Clin. Pract. 2023, 26, 844–846. [Google Scholar] [CrossRef]
- Barrett-Connor, E. Cholelithiasis in Sickle Cell Anemia. Am. J. Med. 1968, 45, 889–898. [Google Scholar] [CrossRef]
- Girish, G.; Xiang, B.; Hsu, L.L. A 21-Year-Old Woman with Sickle Cell Disease and Vaso-Occlusive Pain Associated with Using an Electronic Nicotine Dispensing System (E-Cigarette or Vape)—A Case Report. Am. J. Case Rep. 2023, 24, e941268. [Google Scholar] [CrossRef]
- Benkerrou, M.; Alberti, C.; Couque, N.; Haouari, Z.; Ba, A.; Missud, F.; Boizeau, P.; Holvoet, L.; Ithier, G.; Elion, J.; et al. Impact of Glucose-6-phosphate Dehydrogenase Deficiency on Sickle Cell Anaemia Expression in Infancy and Early Childhood: A Prospective Study. Br. J. Haematol. 2013, 163, 646–654. [Google Scholar] [CrossRef]
- Khamees, I.; Ata, F.; Choudry, H.; Soliman, A.T.; De Sanctis, V.; Yassin, M.A. Manifestations of HbSE Sickle Cell Disease: A Systematic Review. J. Transl. Med. 2021, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- Passon, R.G.; Howard, T.A.; Zimmerman, S.A.; Schultz, W.H.; Ware, R.E. Influence of Bilirubin Uridine Diphosphate– Glucuronosyltransferase 1A Promoter Polymorphisms on Serum Bilirubin Levels and Cholelithiasis in Children With Sickle Cell Anemia. J. Pediatr. Hematol./Oncol. 2001, 23, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Vasavda, N.; Menzel, S.; Kondaveeti, S.; Maytham, E.; Awogbade, M.; Bannister, S.; Cunningham, J.; Eichholz, A.; Daniel, Y.; Okpala, I.; et al. The Linear Effects of Alpha-Thalassaemia, the UGT1A1 and HMOX1 Polymorphisms on Cholelithiasis in Sickle Cell Disease. Br. J. Haematol. 2007, 138, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chaar, V.; Kéclard, L.; Diara, J.P.; Leturdu, C.; Elion, J.; Krishnamoorthy, R.; Clayton, J.; Romana, M. Association of UGT1A1 Polymorphism with Prevalence and Age at Onset of Cholelithiasis in Sickle Cell Anemia. Haematologica 2005, 90, 188–199. [Google Scholar]
- Kumar, R.; Yadav, R.; Mishra, S.; Singh, M.P.S.S.; Gwal, A.; Bharti, P.K.; Rajasubramaniam, S. Krüppel-like Factor 1 (KLF1) Gene Single Nucleotide Polymorphisms in Sickle Cell Disease and Its Association with Disease-Related Morbidities. Ann. Hematol. 2021, 100, 365–373. [Google Scholar] [CrossRef]
- El-Hazmi, M.A.F. Heterogeneity and Variation of Clinical and Haematological Expression of Haemoglobin S in Saudi Arabs. Acta Haematol. 1992, 88, 67–71. [Google Scholar] [CrossRef]
- Nishank, S.S.; Singh, M.P.S.S.; Yadav, R.; Gupta, R.B.; Gadge, V.S.; Gwal, A. Endothelial Nitric Oxide Synthase Gene Polymorphism Is Associated with Sickle Cell Disease Patients in India. J. Hum. Genet. 2013, 58, 775–779. [Google Scholar] [CrossRef]
- Lakkakula, B.V.K.S.; Pattnaik, S. IL1RN VNTR Polymorphism and Kidney Damage in Sickle Cell Anemia Patients. J. Nephropharmacol. 2021, 12, e10437. [Google Scholar] [CrossRef]
- Domingos, I.F.; Pereira-Martins, D.A.; Sobreira, M.J.V.C.; Oliveira, R.T.D.; Alagbe, A.E.; Lanaro, C.; Albuquerque, D.M.; Blotta, M.H.S.L.; Araujo, A.S.; Costa, F.F.; et al. High Levels of Proinflammatory Cytokines IL-6 and IL-8 Are Associated with a Poor Clinical Outcome in Sickle Cell Anemia. Ann. Hematol. 2020, 99, 947–953. [Google Scholar] [CrossRef]
- Belisário, A.R.; Mendes-Oliveira, F.; de Souza, V.R.; Bolina-Santos, E.; Mendes, F.G.; Moreno, E.C.; Franca, A.T.; Sabino, E.C.; Otta, D.A.; de Faria, E.S.; et al. Association between Inflammatory Molecules, Nitric Oxide Metabolites and Leg Ulcers in Individuals with Sickle Cell Anemia. Hematol. Transfus. Cell Ther. 2022, 44, 169–176. [Google Scholar] [CrossRef]
- Rosenberg, J.B.; Hutcheson, K.A. Pediatric Sickle Cell Retinopathy: Correlation with Clinical Factors. J. AAPOS 2011, 15, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Powell-Roach, K.L.; Yao, Y.; Jhun, E.H.; He, Y.; Suarez, M.L.; Ezenwa, M.O.; Molokie, R.E.; Wang, Z.J.; Wilkie, D.J. Vasopressin SNP Pain Factors and Stress in Sickle Cell Disease. PLoS ONE 2019, 14, e0224886. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. How to Obtain the Confidence Interval from a P Value. BMJ 2011, 343, d2090. [Google Scholar] [CrossRef]
- Sadhu, N.; Jhun, E.H.; Posen, A.; Yao, Y.; He, Y.; Molokie, R.E.; Wilkie, D.J.; Wang, Z.J. Phenylethanolamine N-Methyltransferase Gene Polymorphisms Associate with Crisis Pain in Sickle Cell Disease Patients. Pharmacogenomics 2020, 21, 269–278. [Google Scholar] [CrossRef]
- Jhun, E.H.; Hu, X.; Sadhu, N.; Yao, Y.; He, Y.; Wilkie, D.J.; Molokie, R.E.; Wang, Z.J. Transient Receptor Potential Polymorphism and Haplotype Associate with Crisis Pain in Sickle Cell Disease. Pharmacogenomics 2018, 19, 401–411. [Google Scholar] [CrossRef]
- Diamantidis, M.D.; Ikonomou, G.; Argyrakouli, I.; Pantelidou, D.; Delicou, S. Genetic Modifiers of Hemoglobin Expression from a Clinical Perspective in Hemoglobinopathy Patients with Beta Thalassemia and Sickle Cell Disease. Int. J. Mol. Sci. 2024, 25, 11886. [Google Scholar] [CrossRef]
- Labarque, V.; Okocha, E.C. Systematic Review of Genetic Modifiers Associated with the Development and/or Progression of Nephropathy in Patients with Sickle Cell Disease. Int. J. Mol. Sci. 2024, 25, 5427. [Google Scholar] [CrossRef]
- Oni, M.O.; Brito, M.; Rotman, C.; Archer, N.M. Genetic Modifiers of Stroke in Patients with Sickle Cell Disease—A Scoping Review. Int. J. Mol. Sci. 2024, 25, 6317. [Google Scholar] [CrossRef]
- Chatzidavid, S.; Flevari, P.; Tombrou, I.; Anastasiadis, G.; Dimopoulou, M. Pulmonary Hypertension in Sickle Cell Disease: Novel Findings of Gene Polymorphisms Related to Pathophysiology. Int. J. Mol. Sci. 2024, 25, 4792. [Google Scholar] [CrossRef]
- Evangelidis, P.; Venou, T.-M.; Fani, B.; Vlachaki, E.; Gavriilaki, E. Endocrinopathies in Hemoglobinopathies: What Is the Role of Iron? Int. J. Mol. Sci. 2023, 24, 16263. [Google Scholar] [CrossRef]
- Gambari, R.; Waziri, A.D.; Goonasekera, H.; Peprah, E. Pharmacogenomics of Drugs Used in β-Thalassemia and Sickle-Cell Disease: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 4263. [Google Scholar] [CrossRef] [PubMed]
- Harteveld, C.L.; Achour, A.; Fairuz Mohd Hasan, N.F.; Legebeke, J.; Arkesteijn, S.J.G.; Huurne, J.t.; Verschuren, M.; Bhagwandien-Bisoen, S.; Schaap, R.; Vijfhuizen, L.; et al. Loss-of-Function Variants in SUPT5H as Modifying Factors in Beta-Thalassemia. Int. J. Mol. Sci. 2024, 25, 8928. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.; Sabir, A.; Rao, L.; Baker, B.; Balasa, V.; Sathi, B.K. SARS-CoV-2 Infection Presenting as Acute Chest Syndrome in a Child With Hemoglobin SD-Los Angeles Disease: A Case Report and Review of Literature. J. Pediatr. Hematol./Oncol. 2023, 45, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.Y.; Margulies, S.; Speller-Brown, B.; Majumdar, S.; Darbari, D.S.; Campbell, A.D. The Evolution of the COVID-19 Pandemic in Paediatric Patients with Sickle Cell Disease: From Alpha to Omicron. Br. J. Haematol. 2023, 202, 479–484. [Google Scholar] [CrossRef]
- Sepulveda, K.; Issa, T.; Dubrocq, G. Cerebral Fat Embolism Syndrome in a Patient with Homozygous Sickle Cell Disease in the Setting of Multisystem Inflammatory Syndrome in Children. In Proceedings of the Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2023; Volume 36, pp. 266–268. [Google Scholar] [CrossRef]
- Pessoa, N.L.; Diniz, L.M.; Andrade, A.D.; Kroon, E.G.; Bentes, A.A.; Campos, M.A. Children with Sickle Cell Disease and Severe COVID-19 Presenting Single Nucleotide Polymorphisms in Innate Immune Response Genes—A Case Report. EJHaem 2022, 3, 199–202. [Google Scholar] [CrossRef]
- Kaye, A.D.; Garcia, A.J.; Hall, O.M.; Jeha, G.M.; Cramer, K.D.; Granier, A.L.; Kallurkar, A.; Cornett, E.M.; Urman, R.D. Update on the Pharmacogenomics of Pain Management. Pharmgenom. Pers. Med. 2019, 12, 125–143. [Google Scholar] [CrossRef]
- Sabrie, M.; Cannas, G.; Tazarourte, K.; Poutrel, S.; Connes, P.; Hot, A.; Renoux, C.; Fattoum, J.; Joly, P. Drepa-Opia: A Pilot Study to Determine the Predictive Factors of Morphine Use and Consumption in Hospitalized Adult Patients with Sickle Cell Disease. Hemoglobin 2018, 42, 217–224. [Google Scholar] [CrossRef]
- Osunkwo, I. An Update on the Recent Literature on Sickle Cell Bone Disease. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 539–546. [Google Scholar] [CrossRef]
- Nordness, M.E.; Lynn, J.; Zacharisen, M.C.; Scott, P.J.; Kelly, K.J. Asthma Is a Risk Factor for Acute Chest Syndrome and Cerebral Vascular Accidents in Children with Sickle Cell Disease. Clin. Mol. Allergy 2005, 3, 2. [Google Scholar] [CrossRef]
- Knight-Madden, J.M.; Forrester, T.S.; Lewis, N.A.; Greenough, A. Asthma in Children with Sickle Cell Disease and Its Association with Acute Chest Syndrome. Thorax 2005, 60, 206–210. [Google Scholar] [CrossRef]
- Gupta, K.; Gupta, P.; Solovey, A.; Hebbel, R.P. Mechanism of Interaction of Thrombospondin with Human Endothelium and Inhibition of Sickle Erythrocyte Adhesion to Human Endothelial Cells by Heparin. Biochim. Biophys. Acta 1999, 1453, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wick, T.M.; Moake, J.L.; Udden, M.M.; McIntire, L.V. Unusually Large von Willebrand Factor Multimers Preferentially Promote Young Sickle and Nonsickle Erythrocyte Adhesion to Endothelial Cells. Am. J. Hematol. 1993, 42, 284–292. [Google Scholar] [CrossRef]
- Wick, T.M.; Moake, J.L.; Udden, M.M.; Eskin, S.G.; Sears, D.A.; McIntire, L.V. Unusually Large von Willebrand Factor Multimers Increase Adhesion of Sickle Erythrocytes to Human Endothelial Cells under Controlled Flow. J. Clin. Investig. 1987, 80, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Gee, B.E.; Platt, O.S. Sickle Reticulocytes Adhere to VCAM-1. Blood 1995, 85, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Eckmam, J.R.; Swerlick, R.A.; Wick, T.M. Phorbol Ester Stimulation Increases Sickle Erythrocyte Adherence to Endothelium: A Novel Pathway Involving Alpha 4 Beta 1 Integrin Receptors on Sickle Reticulocytes and Fibronectin. Blood 1996, 88, 4348–4358. [Google Scholar] [CrossRef]
- Kato, G.J.; Martyr, S.; Blackwelder, W.C.; Nichols, J.S.; Coles, W.A.; Hunter, L.A.; Brennan, M.-L.; Hazen, S.L.; Gladwin, M.T. Levels of Soluble Endothelium-Derived Adhesion Molecules in Patients with Sickle Cell Disease Are Associated with Pulmonary Hypertension, Organ Dysfunction, and Mortality. Br. J. Haematol. 2005, 130, 943–953. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef]
- Karkoska, K.A.; Jacob, S.A.; McGann, P.T. Accelerated Drug Approvals and Patient Trust: Impact of Voxelotor & Crizanlizumab for Sickle Cell Disease. Blood Adv. 2025, bloodadvances.2025015822. [Google Scholar] [CrossRef]
- Zennadi, R.; Hines, P.C.; De Castro, L.M.; Cartron, J.-P.; Parise, L.V.; Telen, M.J. Epinephrine Acts through Erythroid Signaling Pathways to Activate Sickle Cell Adhesion to Endothelium via LW-Alphavbeta3 Interactions. Blood 2004, 104, 3774–3781. [Google Scholar] [CrossRef]
- Kucukal, E.; Man, Y.; Quinn, E.; Tewari, N.; An, R.; Ilich, A.; Key, N.S.; Little, J.A.; Gurkan, U.A. Red Blood Cell Adhesion to ICAM-1 Is Mediated by Fibrinogen and Is Associated with Right-to-Left Shunts in Sickle Cell Disease. Blood Adv. 2020, 4, 3688–3698. [Google Scholar] [CrossRef]
- Esposito, E.; Napolitano, G.; Pescatore, A.; Calculli, G.; Incoronato, M.R.; Leonardi, A.; Ursini, M.V. COMMD7 as a Novel NEMO Interacting Protein Involved in the Termination of NF-κB Signaling. J. Cell Physiol. 2016, 231, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Wang, Q. Anti-Inflammatory Role of the Klotho Protein and Relevance to Aging. Cells 2024, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Knight-Perry, J.; DeBaun, M.R.; Strunk, R.C.; Field, J.J. Leukotriene Pathway in Sickle Cell Disease: A Potential Target for Directed Therapy. Expert. Rev. Hematol. 2009, 2, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ballas, S.K.; Files, B.; Luchtman-Jones, L.; Benjamin, L.; Swerdlow, P.; Hilliard, L.; Coates, T.; Abboud, M.; Wojtowicz-Praga, S.; Kuypers, F.A.; et al. Secretory Phospholipase A2 Levels in Patients with Sickle Cell Disease and Acute Chest Syndrome. Hemoglobin 2006, 30, 165–170. [Google Scholar] [CrossRef]
- Styles, L.A.; Schalkwijk, C.G.; Aarsman, A.J.; Vichinsky, E.P.; Lubin, B.H.; Kuypers, F.A. Phospholipase A2 Levels in Acute Chest Syndrome of Sickle Cell Disease. Blood 1996, 87, 2573–2578. [Google Scholar] [CrossRef]
- Styles, L.A.; Aarsman, A.J.; Vichinsky, E.P.; Kuypers, F.A. Secretory Phospholipase A(2) Predicts Impending Acute Chest Syndrome in Sickle Cell Disease. Blood 2000, 96, 3276–3278. [Google Scholar] [CrossRef]
- Smith, W.R.; Scherer, M. Sickle-Cell Pain: Advances in Epidemiology and Etiology. Hematology 2010, 2010, 409–415. [Google Scholar] [CrossRef]
- Gehling, G.M.; Powell-Roach, K.; Wilkie, D.J.; Dungan, J.R. Single Nucleotide Polymorphisms and Sickle Cell Disease-Related Pain: A Systematic Review. Front. Pain Res. 2023, 4, 1223309. [Google Scholar] [CrossRef]
- Ezenwa, M.O.; Molokie, R.E.; Wang, Z.J.; Yao, Y.; Suarez, M.L.; Angulo, V.; Wilkie, D.J. Outpatient Pain Predicts Subsequent One-Year Acute Health Care Utilization among Adults with Sickle Cell Disease. J. Pain Symptom Manag. 2014, 48, 65–74. [Google Scholar] [CrossRef]
- Melzack, R. The McGill Pain Questionnaire: Major Properties and Scoring Methods. Pain 1975, 1, 277–299. [Google Scholar] [CrossRef]
- Ojinnaka, U.; Ahmed, Z.; Kannan, A.; Quadir, H.; Hakobyan, K.; Gaddam, M.; Mostafa, J.A. A Traditional Review of Sickle Cell Disease and the Associated Onset of Dementia: Hematological and Neurocognitive Crossroads. Cureus 2021, 13, e18906. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; Moscou-Jackson, G.; Carroll, C.P.; Kiley, K.; Haywood, C.; Lanzkron, S.; Hand, M.; Edwards, R.R.; Haythornthwaite, J.A. An Evaluation of Central Sensitization in Patients with Sickle Cell Disease. J. Pain 2016, 17, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Jhun, E.H.; Sadhu, N.; Hu, X.; Yao, Y.; He, Y.; Wilkie, D.J.; Molokie, R.E.; Wang, Z.J. Beta2-Adrenergic Receptor Polymorphisms and Haplotypes Associate With Chronic Pain in Sickle Cell Disease. Front. Pharmacol. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Karlson, C.; Dickens, H.; Williams-Kirkwood, W.; Mascaro, M.; Jackson, E.; Carullo, V.; McNaull, M.; Morris, M.C. Temporal Summation of Pain in Sickle Cell Disease: Comparison of Adolescents and Young Adults with Chronic vs. Infrequent Pain. J. Pediatr. Psychol. 2024, 49, 882–890. [Google Scholar] [CrossRef]
- Jonassaint, C.R.; Parchuri, E.; O’Brien, J.A.; Lalama, C.M.; Lin, J.; Badawy, S.M.; Hamm, M.E.; Stinson, J.; Lalloo, C.; Carroll, C.P.; et al. Mental Health, Pain and Likelihood of Opioid Misuse among Adults with Sickle Cell Disease. Br. J. Haematol. 2024, 204, 1029–1038. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sophocleous, F.; Archer, N.M.; Lederer, C.W., on behalf of the International Hemoglobinopathy Research Network (INHERENT). Genetic Modifiers Associated with Vaso-Occlusive Crises and Acute Pain Phenomena in Sickle Cell Disease: A Scoping Review. Int. J. Mol. Sci. 2025, 26, 4456. https://doi.org/10.3390/ijms26094456
Sophocleous F, Archer NM, Lederer CW on behalf of the International Hemoglobinopathy Research Network (INHERENT). Genetic Modifiers Associated with Vaso-Occlusive Crises and Acute Pain Phenomena in Sickle Cell Disease: A Scoping Review. International Journal of Molecular Sciences. 2025; 26(9):4456. https://doi.org/10.3390/ijms26094456
Chicago/Turabian StyleSophocleous, Froso, Natasha M. Archer, and Carsten W. Lederer on behalf of the International Hemoglobinopathy Research Network (INHERENT). 2025. "Genetic Modifiers Associated with Vaso-Occlusive Crises and Acute Pain Phenomena in Sickle Cell Disease: A Scoping Review" International Journal of Molecular Sciences 26, no. 9: 4456. https://doi.org/10.3390/ijms26094456
APA StyleSophocleous, F., Archer, N. M., & Lederer, C. W., on behalf of the International Hemoglobinopathy Research Network (INHERENT). (2025). Genetic Modifiers Associated with Vaso-Occlusive Crises and Acute Pain Phenomena in Sickle Cell Disease: A Scoping Review. International Journal of Molecular Sciences, 26(9), 4456. https://doi.org/10.3390/ijms26094456







