Fluid Biomarkers in Demyelinating Spectrum Disorders: Past, Present, and Prospects
Abstract
1. Introduction
2. Current Biomarkers of Demyelinating Diseases
2.1. OCBs
- -
- Type 1: OCB-negative in both the CSF and serum;
- -
- Type 2: OCB-positive in the CSF, and OCB-negative in the serum;
- -
- Type 3: OCB-positive in both the CSF and serum, with additional bands in the CSF;
- -
- Type 4: identical OCB in both the CSF and serum;
- -
- Type 5: “Monoclonal bands” [27].
2.2. Kappa and Lambda Free Light Chains and Kappa Free Light Chain Index
2.3. Neurofilament Light and Heavy Chain
2.4. AQP4 Antibodies
2.5. Glial Fibrillary Acid Protein (GFAP)
2.6. Calcium Binding Protein S100B
2.7. Chitinase-3-like Protein 1
2.8. CXCL13
2.9. N-Acetyl Aspartate (NAA)
2.10. Galectin-9
2.11. Osteopontin
2.12. Chemokines
2.13. Complement System
2.14. Cytokines
2.15. HERV-W Peptides
2.16. Tau
2.17. Vitamin D
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murray, T.J. The History of Diagnosis and Treatment of MS: A Brief Overview. Curr. Neurol. Neurosci. Rep. 2022, 22, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Höftberger, R.; Lassmann, H. Inflammatory Demyelinating Diseases of the Central Nervous System. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 145, pp. 263–283. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.G.C.; Araújo, S.E.-S.; Alves-Leon, S.V.; Gomes, F.C.A. Central nervous system demyelinating diseases: Glial cells at the hub of pathology. Front. Immunol. 2023, 14, 1135540. [Google Scholar] [CrossRef]
- Sean, Y.S.; Pittock, J. Toward curing neurological autoimmune disorders: Biomarkers, immunological mechanisms, and therapeutic targets. Neuron 2025, 113, 345–379. [Google Scholar]
- Magro, D.; Venezia, M.; Balistreri, C.R. The omics technologies and liquid biopsies: Advantages, limitations, applications. Med. Omics 2024, 11, 100039. [Google Scholar] [CrossRef]
- Wildner, P.; Stasiołek, M.; Matysiak, M. Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Mult. Scler. Relat. Disord. 2020, 37, 101452. [Google Scholar] [CrossRef]
- Świderek-Matysiak, M.; Oset, M.; Domowicz, M.; Galazka, G.; Namiecińska, M.; Stasiołek, M. Cerebrospinal Fluid Biomarkers in Differential Diagnosis of Multiple Sclerosis and Systemic Inflammatory Diseases with Central Nervous System Involvement. Biomedicines 2023, 11, 425. [Google Scholar] [CrossRef]
- Sechi, E.; Cacciaguerra, L.; Chen, J.J.; Mariotto, S.; Fadda, G.; Dinoto, A.; Lopez-Chiriboga, A.S.; Pittock, S.J.; Flanagan, E.P. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front. Neurol. 2022, 13, 885218. [Google Scholar] [CrossRef]
- Maciak, K.; Pietrasik, S.; Dziedzic, A.; Redlicka, J.; Saluk-Bijak, J.; Bijak, M.; Włodarczyk, T.; Miller, E. Th17-Related Cytokines as Potential Discriminatory Markers between Neuromyelitis Optica (Devic’s Disease) and Multiple Sclerosis—A Review. Int. J. Mol. Sci. 2021, 22, 8946. [Google Scholar] [CrossRef]
- Mariano, R.; Messina, S.; Kumar, K.; Kuker, W.; Leite, M.I.; Palace, J. Comparison of Clinical Outcomes of Transverse Myelitis Among Adults With Myelin Oligodendrocyte Glycoprotein Antibody vs. Aquaporin-4 Antibody Disease. JAMA Netw. Open 2019, 2, e1912732. [Google Scholar] [CrossRef]
- Flanagan, E.P.; Cabre, P.; Weinshenker, B.G.; Sauver, J.S.; Jacobson, D.J.; Majed, M.; Lennon, V.A.; Lucchinetti, C.F.; McKeon, A.; Matiello, M.; et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann. Neurol. 2016, 79, 775–783. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.; Hamilton-Shield, A.; Woodhall, M.; Messina, S.; Mariano, R.; Waters, P.; Ramdas, S.; Leite, M.I.; Palace, J. Prevalence and incidence of neuromyelitis optica spectrum disorder, aquaporin-4 antibody-positive NMOSD and MOG antibody-positive disease in Oxfordshire, UK. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- de Mol, C.; Wong, Y.; van Pelt, E.; Wokke, B.; Siepman, T.; Neuteboom, R.; Hamann, D.; Hintzen, R. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult. Scler. J. 2020, 26, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.-W.; Jeong, W.K.; Lee, H.J.; Shin, J.H.; Min, J.-H.; Kim, B.J.; Kim, S.W.; Shin, H.Y.; Oh, J.; Kim, W.; et al. Comparison of MOG and AQP4 antibody seroprevalence in Korean adults with inflammatory demyelinating CNS diseases. Mult. Scler. J. 2021, 27, 964–967. [Google Scholar] [CrossRef]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple Sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499849/ (accessed on 2 February 2025).
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2019, 9, 3116. [Google Scholar] [CrossRef]
- Inojosa, H.; Proschmann, U.; Akgün, K.; Ziemssen, T. A focus on secondary progressive multiple sclerosis (SPMS): Challenges in diagnosis and definition. J. Neurol. 2021, 268, 1210–1221. [Google Scholar] [CrossRef]
- Berger, T.; Reindl, M. Antibody biomarkers in CNS demyelinating diseases—A long and winding road. Eur. J. Neurol. 2015, 22, 1162–1168. [Google Scholar] [CrossRef]
- Papp, V.; Magyari, M.; Aktas, O.; Berger, T.; Broadley, S.A.; Cabre, P.; Jacob, A.; Kira, J.-I.; Leite, M.I.; Marignier, R.; et al. Worldwide Incidence and Prevalence of Neuromyelitis Optica: A Systematic Review. Neurology 2021, 96, 59–77. [Google Scholar] [CrossRef]
- Zelek, W.M.; Fathalla, D.; Morgan, A.; Touchard, S.; Loveless, S.; Tallantyre, E.; Robertson, N.P.; Morgan, B.P. Cerebrospinal fluid complement system biomarkers in demyelinating disease. Mult. Scler. J. 2020, 26, 1929–1937. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Li, R.; Wang, C. Research and progress on biomarkers of neuromyelitis optica spectrum disorders. J. Recept. Signal Transduct. 2021, 41, 417–424. [Google Scholar] [CrossRef]
- O’Connor, K.C.; McLaughlin, K.A.; De Jager, P.L.; Chitnis, T.; Bettelli, E.; Xu, C.; Robinson, W.H.; Cherry, S.V.; Bar-Or, A.; Banwell, B.; et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 2007, 13, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P.; Ramanathan, S.; Waters, P.; Tenembaum, S.; Graves, J.S.; et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Toftegaard, C.; Severinsen, C.M.; Jensen, H.B. Biomarkers Differentiating RRMS and SPMS in Multiple Sclerosis—A Systematic Review. Sclerosis 2024, 2, 166–185. [Google Scholar] [CrossRef]
- Maglio, G.; D’agostino, M.; Caronte, F.P.; Pezone, L.; Casamassimi, A.; Rienzo, M.; Di Zazzo, E.; Nappo, C.; Medici, N.; Molinari, A.M.; et al. Multiple Sclerosis: From the Application of Oligoclonal Bands to Novel Potential Biomarkers. Int. J. Mol. Sci. 2024, 25, 5412. [Google Scholar] [CrossRef]
- Jin, H.; Lu, Q.; Gao, F.; Hao, H. Application of oligoclonal bands and other cerebrospinal fluid variables in multiple sclerosis and other neuroimmunological diseases: A narrative review. Ann. Transl. Med. 2023, 11, 282. [Google Scholar] [CrossRef]
- Forcadela, M.; Birch, K.; Rocchi, C.; Campagna, G.; Jackson, E.; Chadwick, C.; Hamid, S.; Jacob, A.; Huda, S. Do we still need OCBs in MS diagnosis and how many? Mult. Scler. Relat. Disord. 2023, 79, 105035. [Google Scholar] [CrossRef]
- Ziemann, U.; Wahl, M.; Hattingen, E.; Tumani, H. Development of biomarkers for multiple sclerosis as a neurodegenerative disorder. Prog. Neurobiol. 2011, 95, 670–685. [Google Scholar] [CrossRef]
- Karussis, D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: A critical review. J. Autoimmun. 2014, 48, 134–142. [Google Scholar] [CrossRef]
- Jarius, S.; Paul, F.; Franciotta, D.; Ruprecht, K.; Ringelstein, M.; Bergamaschi, R.; Rommer, P.; Kleiter, I.; Stich, O.; Reuss, R.; et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: Results from 211 lumbar punctures. J. Neurol. Sci. 2011, 306, 82–90. [Google Scholar] [CrossRef]
- McDonald Diagnostic Criteria. ECTRIMS. Available online: https://ectrims.eu/mcdonald-diagnostic-criteria/ (accessed on 23 April 2025).
- Konen, F.F.; Wurster, U.; Schwenkenbecher, P.; Gerritzen, A.; Groß, C.C.; Eichhorn, P.; Harrer, A.; Isenmann, S.; Lewczuk, P.; Lewerenz, J.; et al. Oligoclonal bands and kappa free light chains: Competing parameters or complementary biomarkers? Autoimmun. Rev. 2025, 24, 103765. [Google Scholar] [CrossRef]
- Vecchio, D.; Crespi, I.; Virgilio, E.; Naldi, P.; Campisi, M.; Serino, R.; Dianzani, U.; Bellomo, G.; Cantello, R.; Comi, C. Kappa free light chains could predict early disease course in multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 30, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, I.; Axelsson, M.; Novakova, L.; Malmeström, C.; Blennow, K.; Zetterberg, H.; Lycke, J. Intrathecal kappa free light chain synthesis is associated with worse prognosis in relapsing–remitting multiple sclerosis. J. Neurol. 2023, 270, 4800–4811. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Walde, J.; Berek, K.; Arrambide, G.; Gnanapavan, S.; Kaplan, B.; Khalil, M.; Saadeh, R.; Teunissen, C.; Tumani, H.; et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. J. 2023, 29, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Tur, C.; Carbonell-Mirabent, P.; Cobo-Calvo, Á.; Otero-Romero, S.; Arrambide, G.; Midaglia, L.; Castilló, J.; Vidal-Jordana, Á.; Rodríguez-Acevedo, B.; Zabalza, A.; et al. Association of Early Progression Independent of Relapse Activity with Long-term Disability After a First Demyelinating Event in Multiple Sclerosis. JAMA Neurol. 2023, 80, 151–160. [Google Scholar] [CrossRef]
- Rosenstein, I.; Axelsson, M.; Novakova, L.; Rasch, S.; Blennow, K.; Zetterberg, H.; Lycke, J. High levels of kappa free light chain synthesis predict cognitive decline in relapsing-remitting multiple sclerosis. Front. Immunol. 2023, 14, 1106028. [Google Scholar] [CrossRef]
- Ferreira-Atuesta, C.; Reyes, S.; Giovanonni, G.; Gnanapavan, S. The Evolution of Neurofilament Light Chain in Multiple Sclerosis. Front. Neurosci. 2021, 15, 642384. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Kušnierová, P.; Zeman, D.; Hradílek, P.; Čábal, M.; Zapletalová, O. Neurofilament levels in patients with neurological diseases: A comparison of neurofilament light and heavy chain levels. J. Clin. Lab. Anal. 2019, 33, e22948. [Google Scholar] [CrossRef]
- Szczygielski, J.; Kopańska, M.; Wysocka, A.; Oertel, J. Cerebral Microcirculation, Perivascular Unit, and Glymphatic System: Role of Aquaporin-4 as the Gatekeeper for Water Homeostasis. Front. Neurol. 2021, 12, 767470. [Google Scholar] [CrossRef]
- Waters, P.J.; Pittock, S.J.; Bennett, J.L.; Jarius, S.; Weinshenker, B.G.; Wingerchuk, D.M. Evaluation of aquaporin-4 antibody assays. Clin. Exp. Neuroimmunol. 2014, 5, 290–303. [Google Scholar] [CrossRef]
- Li, J.; Bazzi, S.A.; Schmitz, F.; Tanno, H.; McDaniel, J.R.; Lee, C.-H.; Joshi, C.; Kim, J.E.; Monson, N.; Greenberg, B.M.; et al. Molecular Level Characterization of Circulating Aquaporin-4 Antibodies in Neuromyelitis Optica Spectrum Disorder. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1034. [Google Scholar] [CrossRef] [PubMed]
- McKeon, A.; Benarroch, E.E. Glial fibrillary acid protein: Functions and involvement in disease. Neurology 2018, 90, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, M.; Malmeström, C.; Nilsson, S.; Haghighi, S.; Rosengren, L.; Lycke, J. Glial fibrillary acidic protein: A potential biomarker for progression in multiple sclerosis. J. Neurol. 2011, 258, 882–888. [Google Scholar] [CrossRef]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef]
- Michetti, F.; Di Sante, G.; Clementi, M.E.; Sampaolese, B.; Casalbore, P.; Volonté, C.; Spica, V.R.; Parnigotto, P.P.; Di Liddo, R.; Amadio, S.; et al. Growing role of S100B protein as a putative therapeutic target for neurological- and nonneurological-disorders. Neurosci. Biobehav. Rev. 2021, 127, 446–458. [Google Scholar] [CrossRef]
- Barateiro, A.; Afonso, V.; Santos, G.; Cerqueira, J.J.; Brites, D.; van Horssen, J.; Fernandes, A. S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol. Neurobiol. 2016, 53, 3976–3991. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Spica, V.R. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef]
- Yu, J.E.; Yeo, I.J.; Han, S.-B.; Yun, J.; Kim, B.; Yong, Y.J.; Lim, Y.-S.; Kim, T.H.; Son, D.J.; Hong, J.T. Significance of chitinase-3-like protein 1 in the pathogenesis of inflammatory diseases and cancer. Exp. Mol. Med. 2024, 56, 1–18. [Google Scholar] [CrossRef]
- Li, F.; Liu, A.; Zhao, M.; Luo, L. Astrocytic Chitinase-3-like protein 1 in neurological diseases: Potential roles and future perspectives. J. Neurochem. 2023, 165, 772–790. [Google Scholar] [CrossRef]
- Talaat, F.; Abdelatty, S.; Ragaie, C.; Dahshan, A. Chitinase-3-like 1-protein in CSF: A novel biomarker for progression in patients with multiple sclerosis. Neurol. Sci. 2023, 44, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Pachner, A.R.; Pike, S.C.; Smith, A.D.; Gilli, F. The CXCL13 index biomarker predicts success or failure of moderate-efficacy disease-modifying therapies in multiple sclerosis; A real-world study. Mult. Scler. Relat. Disord. 2025, 95, 106303. [Google Scholar] [CrossRef] [PubMed]
- Pachner, A.R.; Pike, S.; Smith, A.D.; Gilli, F. CXCL13 as a Biomarker: Background and Utility in Multiple Sclerosis. Biomolecules 2024, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Piccio, L.; Mikesell, R.J.; Trinkaus, K.; Parks, B.J.; Naismith, R.T.; Cross, A.H. Predicting optimal response to B-cell depletion with rituximab in multiple sclerosis using CXCL13 index, magnetic resonance imaging and clinical measures. Mult. Scler. J. Exp. Transl. Clin. 2015, 1, 2055217315623800. [Google Scholar] [CrossRef]
- DiSano, K.D.; Gilli, F.; Pachner, A.R. Intrathecally produced CXCL13: A predictive biomarker in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320981396. [Google Scholar] [CrossRef]
- Madsen, M.A.; Považan, M.; Wiggermann, V.; Lundell, H.; Blinkenberg, M.; Christensen, J.R.; Sellebjerg, F.; Siebner, H.R. Association of Cortical Lesions with Regional Glutamate, GABA, N-Acetylaspartate, and Myoinositol Levels in Patients with Multiple Sclerosis. Neurology 2024, 103, e209543. [Google Scholar] [CrossRef]
- Saadeh, R.S.; Ramos, P.A.; Algeciras-Schimnich, A.; Flanagan, E.P.; Pittock, S.J.; Willrich, M.A. An Update on Laboratory-Based Diagnostic Biomarkers for Multiple Sclerosis and Beyond. Clin. Chem. 2022, 68, 1134–1150. [Google Scholar] [CrossRef]
- Tortorella, C.; Ruggieri, M.; Di Monte, E.; Ceci, E.; Iaffaldano, P.; Direnzo, V.; Mastrapasqua, M.; Frigeri, A.; Amato, M.P.; Hakiki, B.; et al. Serum and CSF N-acetyl aspartate levels differ in multiple sclerosis and neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1355–1359. [Google Scholar] [CrossRef]
- Stromillo, M.L.; Giorgio, A.; Rossi, F.; Battaglini, M.; Hakiki, B.; Malentacchi, G.; Santangelo, M.; Gasperini, C.; Bartolozzi, M.L.; Portaccio, E.; et al. Brain metabolic changes suggestive of axonal damage in radiologically isolated syndrome. Neurology 2013, 80, 2090–2094. [Google Scholar] [CrossRef]
- Chard, D.T.; Griffin, C.M.; McLean, M.A.; Kapeller, P.; Kapoor, R.; Thompson, A.J.; Miller, D.H. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain 2002, 125, 2342–2352. [Google Scholar] [CrossRef]
- Collorone, S.; Foster, M.A.; Toosy, A.T. Advanced central nervous system imaging biomarkers in radiologically isolated syndrome: A mini review. Front. Neurol. 2023, 14, 1172807. [Google Scholar] [CrossRef] [PubMed]
- Moar, P.; Tandon, R. Galectin-9 as a biomarker of disease severity. Cell. Immunol. 2021, 361, 104287. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.S.; Buttari, F.; Gilio, L.; Iezzi, E.; Galifi, G.; Carbone, F.; Micillo, T.; Dolcetti, E.; Azzolini, F.; Bruno, A.; et al. Osteopontin Is Associated with Multiple Sclerosis Relapses. Biomedicines 2023, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.; Hayden, Z.; Cseh, T.; Berki, T.; Illes, Z. Osteopontin levels are associated with late-time lower regional brain volumes in multiple sclerosis. Sci. Rep. 2021, 11, 23604. [Google Scholar] [CrossRef]
- Marastoni, D.; Magliozzi, R.; Bolzan, A.; Pisani, A.I.; Rossi, S.; Crescenzo, F.; Montemezzi, S.; Pizzini, F.B.; Calabrese, M. CSF Levels of CXCL12 and Osteopontin as Early Markers of Primary Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1083. [Google Scholar] [CrossRef]
- Gębka-Kępińska, B.; Adamczyk, B.; Adamczyk, J.; Czuba, Z.; Gębka-Adamczyk, N.; Szczygieł, J.; Wierzbicki, K.; Adamczyk-Sowa, M. Immunologic analysis of CSF in patients with de novo diagnosed RRMS. The role of chemokines in the early phase of the disease. Mult. Scler. Relat. Disord. 2024, 90, 105800. [Google Scholar] [CrossRef]
- Ransohoff, R.M. The Chemokine System in Neuroinflammation: An Update. J. Infect. Dis. 2002, 186, S152–S156. [Google Scholar] [CrossRef]
- Saez-Calveras, N.; Stuve, O. The role of the complement system in Multiple Sclerosis: A review. Front. Immunol. 2022, 13, 970486. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Dalakas, M.C. The role of complement and complement therapeutics in neuromyelitis optica spectrum disorders. Expert Rev. Clin. Immunol. 2022, 18, 933–945. [Google Scholar] [CrossRef]
- Dinoto, A.; Sechi, E.; Flanagan, E.P.; Ferrari, S.; Solla, P.; Mariotto, S.; Chen, J.J. Serum and Cerebrospinal Fluid Biomarkers in Neuromyelitis Optica Spectrum Disorder and Myelin Oligodendrocyte Glycoprotein Associated Disease. Front. Neurol. 2022, 13, 866824. [Google Scholar] [CrossRef]
- Zhang, C.; Qiu, M.; Fu, H. Oligodendrocytes in central nervous system diseases: The effect of cytokine regulation. Neural Regen. Res. 2024, 19, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Sellner, J.; Sitte, H.H.; Rommer, P.S. Targeting interleukin-6 to treat neuromyelitis optica spectrum disorders: Implications from immunology, the FcRn pathway and clinical experience. Drug Discov. Today 2021, 26, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zheng, T.; Sun, M.; Sun, J.; Wang, M. A meta-analysis to determine the efficacy and safety of tocilizumab in neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2020, 45, 102421. [Google Scholar] [CrossRef] [PubMed]
- Traboulsee, A.; Greenberg, B.M.; Bennett, J.L.; Szczechowski, L.; Fox, E.; Shkrobot, S.; Yamamura, T.; Terada, Y.; Kawata, Y.; Wright, P.; et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: A randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020, 19, 402–412. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Serino, R.; Cantello, R.; Dianzani, U.; Comi, C. Cerebrospinal Tau levels as a predictor of early disability in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103231. [Google Scholar] [CrossRef]
- Hoehne, C.; Stuve, O.; Stopschinski, B.E. Tau in Multiple Sclerosis: A Review of Therapeutic Potential. Curr. Treat. Options Neurol. 2025, 27, 14. [Google Scholar] [CrossRef]
- Islas-Hernandez, A.; Aguilar-Talamantes, H.S.; Bertado-Cortes, B.; Mejia-Delcastillo, G.d.J.; Carrera-Pineda, R.; Cuevas-Garcia, C.F.; Garcia-Delatorre, P. BDNF and Tau as Biomarkers of Severity in Multiple Sclerosis. Biomark. Med. 2018, 12, 717–726. [Google Scholar] [CrossRef]
- Arneth, B.; Kraus, J. Laboratory biomarkers of Multiple Sclerosis (MS). Clin. Biochem. 2022, 99, 1–8. [Google Scholar] [CrossRef]
- Smolders, J.; Torkildsen, Ø.; Camu, W.; Holmøy, T. An Update on Vitamin D and Disease Activity in Multiple Sclerosis. CNS Drugs 2019, 33, 1187–1199. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, X.; Liu, J.; Zhang, C. Biomarkers in autoimmune diseases of the central nervous system. Front. Immunol. 2023, 14, 1111719. [Google Scholar] [CrossRef]
- Rebelos, E.; Daniele, G.; Campi, B.; Saba, A.; Koskensalo, K.; Ihalainen, J.; Saukko, E.; Nuutila, P.; Backes, W.H.; Jansen, J.F.A.; et al. Circulating N-Acetylaspartate does not track brain NAA concentrations, cognitive function or features of small vessel disease in humans. Sci. Rep. 2022, 12, 11530. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Mehan, S.; Das Gupta, G.; Narula, A.S. Immune System Dysregulation in the Progression of Multiple Sclerosis: Molecular Insights and Therapeutic Implications. Neuroscience 2024, 548, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Fortiz, Y.; Boada, M. The role of inflammation in neurological disorders: A brief overview of multiple sclerosis, Alzheimer’s, and Parkinson’s disease’. Front. Neurol. 2024, 15, 1439125. [Google Scholar] [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef]
- Marastoni, D.; Turano, E.; Tamanti, A.; Colato, E.; Pisani, A.I.; Scartezzini, A.; Carotenuto, S.; Mazziotti, V.; Camera, V.; Anni, D.; et al. Association of Levels of CSF Osteopontin with Cortical Atrophy and Disability in Early Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200265. [Google Scholar] [CrossRef]
- Lerch, M.; Schanda, K.; Lafon, E.; Würzner, R.; Mariotto, S.; Dinoto, A.; Wendel, E.M.; Lechner, C.; Hegen, H.; Rostásy, K.; et al. More Efficient Complement Activation by Anti–Aquaporin-4 Compared with Anti–Myelin Oligodendrocyte Glycoprotein Antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200059. [Google Scholar] [CrossRef]
- Pan, Z.; Zhu, T.; Liu, Y.; Zhang, N. Role of the CXCL13/CXCR5 Axis in Autoimmune Diseases. Front. Immunol. 2022, 13, 850998. [Google Scholar] [CrossRef]
- Feki, S.; Damak, M.; Sakka, S.; Ben Ali, Y.; Mejdoub, S.; Bouattour, N.; Hachicha, H.; Mhiri, C.; Masmoudi, H. Intrathecal B cell-related markers for an optimized biological investigation of multiple sclerosis patients. Sci. Rep. 2022, 12, 16425. [Google Scholar] [CrossRef]
- Polacek, H.; Kantorova, E.; Hnilicova, P.; Grendar, M.; Zelenak, K.; Kurca, E. Increased glutamate and deep brain atrophy can predict the severity of multiple sclerosis. Biomed. Pap. 2019, 163, 45–53. [Google Scholar] [CrossRef]
- Shaygannejad, A.; Rafiei, N.; Vaheb, S.; Panah, M.Y.; Shaygannejad, V.; Mirmosayyeb, O. The Role of Glial Fibrillary Acidic Protein as a Biomarker in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 1050. [Google Scholar] [CrossRef] [PubMed]
- Aktas, O.; Smith, M.A.; Marignier, R.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Cutter, G.R.; Green, A.J.; Mealy, M.A.; et al. Serum Glial Fibrillary Acidic Protein: A Neuromyelitis Optica Spectrum Disorder Biomarker. Ann. Neurol. 2021, 89, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Duell, F.; Evertsson, B.; Al Nimer, F.; Sandin, Å.; Olsson, D.; Olsson, T.; Khademi, M.; Hietala, M.A.; Piehl, F.; Hansson, M. Diagnostic accuracy of intrathecal kappa free light chains compared with OCBs in MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e775. [Google Scholar] [CrossRef]
- Tortosa-Carreres, J.; Quiroga-Varela, A.; Castillo-Villalba, J.; Piqueras-Rodríguez, M.; Ramió-Torrenta, L.; Cubas-Núñez, L.; Gasqué-Rubio, R.; Quintanilla-Bordas, C.; Huertas-Pons, J.M.; Miguela, A.; et al. Improving the efficiency of free kappa light chains as diagnostic biomarker of Multiple Sclerosis by using a novel algorithm. Mult. Scler. Relat. Disord. 2023, 79, 104997. [Google Scholar] [CrossRef]
- Jafari, A.; Babajani, A.; Rezaei-Tavirani, M. Multiple Sclerosis Biomarker Discoveries by Proteomics and Metabolomics Approaches. Biomark. Insights 2021, 16, 11772719211013352. [Google Scholar] [CrossRef]
- Zahoor, I.; Rui, B.; Khan, J.; Datta, I.; Giri, S. An emerging potential of metabolomics in multiple sclerosis: A comprehensive overview. Cell. Mol. Life Sci. 2021, 78, 3181–3203. [Google Scholar] [CrossRef]
- Pittock, S.J.; Berthele, A.; Fujihara, K.; Kim, H.J.; Levy, M.; Palace, J.; Nakashima, I.; Terzi, M.; Totolyan, N.; Viswanathan, S.; et al. Eculizumab in Aquaporin-4–Positive Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 2019, 381, 614–625. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Zhukovsky, C.; Herman, S.; Wiberg, A.; Cunningham, J.L.; Kultima, K.; Burman, J. Urokinase, CX3CL1, CCL2, TRAIL and IL-18 induced by interferon-β treatment. Acta Neurol. Scand. 2021, 143, 602–607. [Google Scholar] [CrossRef]
- Levraut, M.; Laurent-Chabalier, S.; Ayrignac, X.; Bigaut, K.; Rival, M.; Squalli, S.; Zéphir, H.; Alberto, T.; Pekar, J.-D.; Ciron, J.; et al. Kappa Free Light Chain Biomarkers Are Efficient for the Diagnosis of Multiple Sclerosis: A Large Multicenter Cohort Study. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200049. [Google Scholar] [CrossRef]
- Ferraro, D.; Bedin, R.; Natali, P.; Franciotta, D.; Smolik, K.; Santangelo, M.; Immovilli, P.; Camera, V.; Vitetta, F.; Gastaldi, M.; et al. Kappa Index versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders. Diagnostics 2020, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Sastre-Garriga, J.; Borras, E.; Villar, L.M.; Saiz, A.; Martínez-Yélamos, S.; García-Merino, J.A.; Pinteac, R.; Fissolo, N.; López, A.J.S.; et al. CSF Chitinase 3–Like 2 Is Associated with Long-term Disability Progression in Patients with Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1082. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, E.-J.; Kim, S.; Choi, L.-K.; Kim, K.; Kim, H.W.; Kim, K.-K.; Lim, Y.-M. Serum biomarkers in myelin oligodendrocyte glycoprotein antibody–associated disease. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e708. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; He, L.; Kuang, Z.; Jian, Y.; Qiu, M.; Liu, Y.; Hu, M.; Ye, Y.; Wu, L. Clinical characteristics, immunological alteration and distinction of MOG-IgG-associated disorders and GFAP-IgG-associated disorders. J. Neuroimmunol. 2024, 393, 578398. [Google Scholar] [CrossRef]
- Demuth, S.; Collongues, N. Disease-modifying treatments for neuromyelitis optica spectrum disorder in the context of a new generation of biotherapies. Rev. Neurol. 2025, 181, 42–51. [Google Scholar] [CrossRef]
- Paul, F.; Marignier, R.; Palace, J.; Arrambide, G.; Asgari, N.; Bennett, J.L.; Cree, B.A.C.; De Sèze, J.; Fujihara, K.; Kim, H.J.; et al. International Delphi Consensus on the Management of AQP4-IgG+ NMOSD: Recommendations for Eculizumab, Inebilizumab, and Satralizumab. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200124. [Google Scholar] [CrossRef]
- Fung, S.; Shirley, M. Satralizumab: A Review in Neuromyelitis Optica Spectrum Disorder. CNS Drugs 2023, 37, 363–370. [Google Scholar] [CrossRef]
- Hartung, H.-P.; Derfuss, T.; Cree, B.A.; Sormani, M.P.; Selmaj, K.; Stutters, J.; Prados, F.; MacManus, D.; Schneble, H.-M.; Lambert, E.; et al. Efficacy and safety of temelimab in multiple sclerosis: Results of a randomized phase 2b and extension study. Mult. Scler. J. 2022, 28, 429–440. [Google Scholar] [CrossRef]
- Giménez-Orenga, K.; Oltra, E. Human Endogenous Retrovirus as Therapeutic Targets in Neurologic Disease. Pharmaceuticals 2021, 14, 495. [Google Scholar] [CrossRef]
- Kornmann, G.; Curtin, F. Temelimab, an IgG4 Anti-Human Endogenous Retrovirus Monoclonal Antibody: An Early Development Safety Review. Drug Saf. 2020, 43, 1287–1296. [Google Scholar] [CrossRef]
- Nishiyama, S.; Seok, J.M.; Wright, A.E.; Lotan, I.; Mikami, T.; Drosu, N.C.; Bobrowski-Khoury, N.; Anderson, M.R.; Bilodeau, P.A.; Schindler, P.; et al. Anti-aquaporin-4 immune complex stimulates complement-dependent Th17 cytokine release in neuromyelitis optica spectrum disorders. Sci. Rep. 2024, 14, 3146. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Mottahedin, A.; Zelco, A.; Lauschke, V.M.; Ek, C.J.; Song, J.; Ardalan, M.; Hua, S.; Zhang, X.; Mallard, C.; et al. Two different isoforms of osteopontin modulate myelination and axonal integrity. FASEB BioAdvances 2023, 5, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, Y.; Liu, N. Osteopontin in autoimmune disorders: Current knowledge and future perspective. Inflammopharmacology 2022, 30, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Rosmus, D.-D.; Lange, C.; Ludwig, F.; Ajami, B.; Wieghofer, P. The Role of Osteopontin in Microglia Biology: Current Concepts and Future Perspectives. Biomedicines 2022, 10, 840. [Google Scholar] [CrossRef]
- Marastoni, D.; Pisani, A.I.; Schiavi, G.; Mazziotti, V.; Castellaro, M.; Tamanti, A.; Bosello, F.; Crescenzo, F.; Ricciardi, G.K.; Montemezzi, S.; et al. CSF TNF and osteopontin levels correlate with the response to dimethyl fumarate in early multiple sclerosis. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221092124. [Google Scholar] [CrossRef]
- Oppong, A.E.; Coelewij, L.; Robertson, G.; Martin-Gutierrez, L.; Waddington, K.E.; Dönnes, P.; Nytrova, P.; Farrell, R.; Pineda-Torra, I.; Jury, E.C. Blood metabolomic and transcriptomic signatures stratify patient subgroups in multiple sclerosis according to disease severity. iScience 2024, 27, 109225. [Google Scholar] [CrossRef]
- Kornilov, S.A.; Price, N.D.; Gelinas, R.; Acosta, J.; Brunkow, M.E.; Gervasi-Follmar, T.; Winger, R.C.; Aldershoff, D.; Lausted, C.; Troisch, P.; et al. Multi-Omic characterization of the effects of Ocrelizumab in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2024, 467, 123303. [Google Scholar] [CrossRef]
- Mohseni, M.; Behzad, G.; Farhadi, A.; Behroozi, J.; Mohseni, H.; Valipour, B. MicroRNA frontiers: Illuminating early detection paths in multiple sclerosis. Mult. Scler. Relat. Disord. 2025, 95, 106237. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, M.; Cui, C.; Zhao, Y.; Sun, X.; Wang, Y.; Liu, C.; Wu, H.; Zhong, X.; et al. Different Exosomal microRNA Profile in Aquaporin-4 Antibody Positive Neuromyelitis Optica Spectrum Disorders. Front. Immunol. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Zabalza, A.; Pappolla, A.; Comabella, M.; Montalban, X.; Malhotra, S. MiRNA-based therapeutic potential in multiple sclerosis. Front. Immunol. 2024, 15, 1441733. [Google Scholar] [CrossRef]
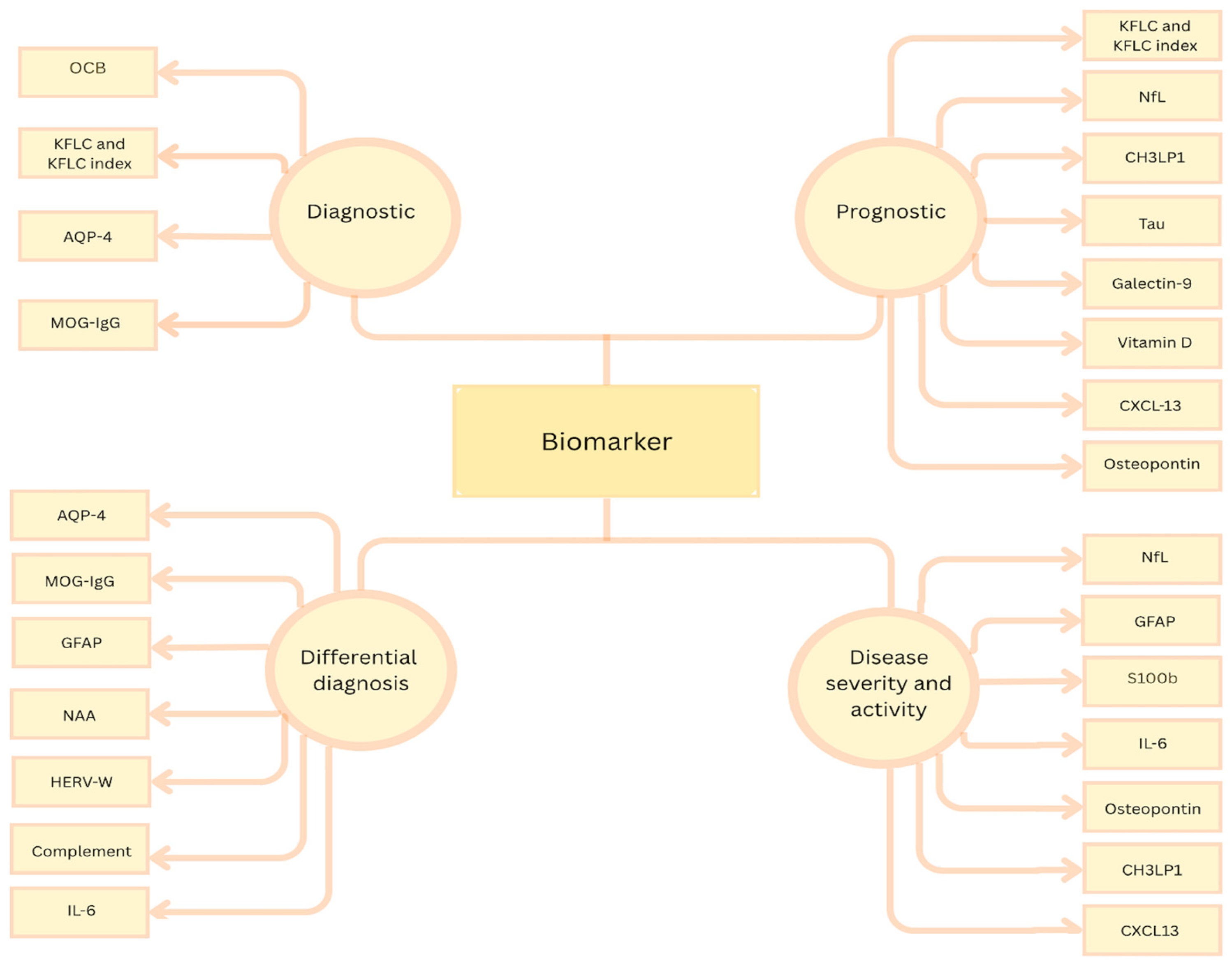
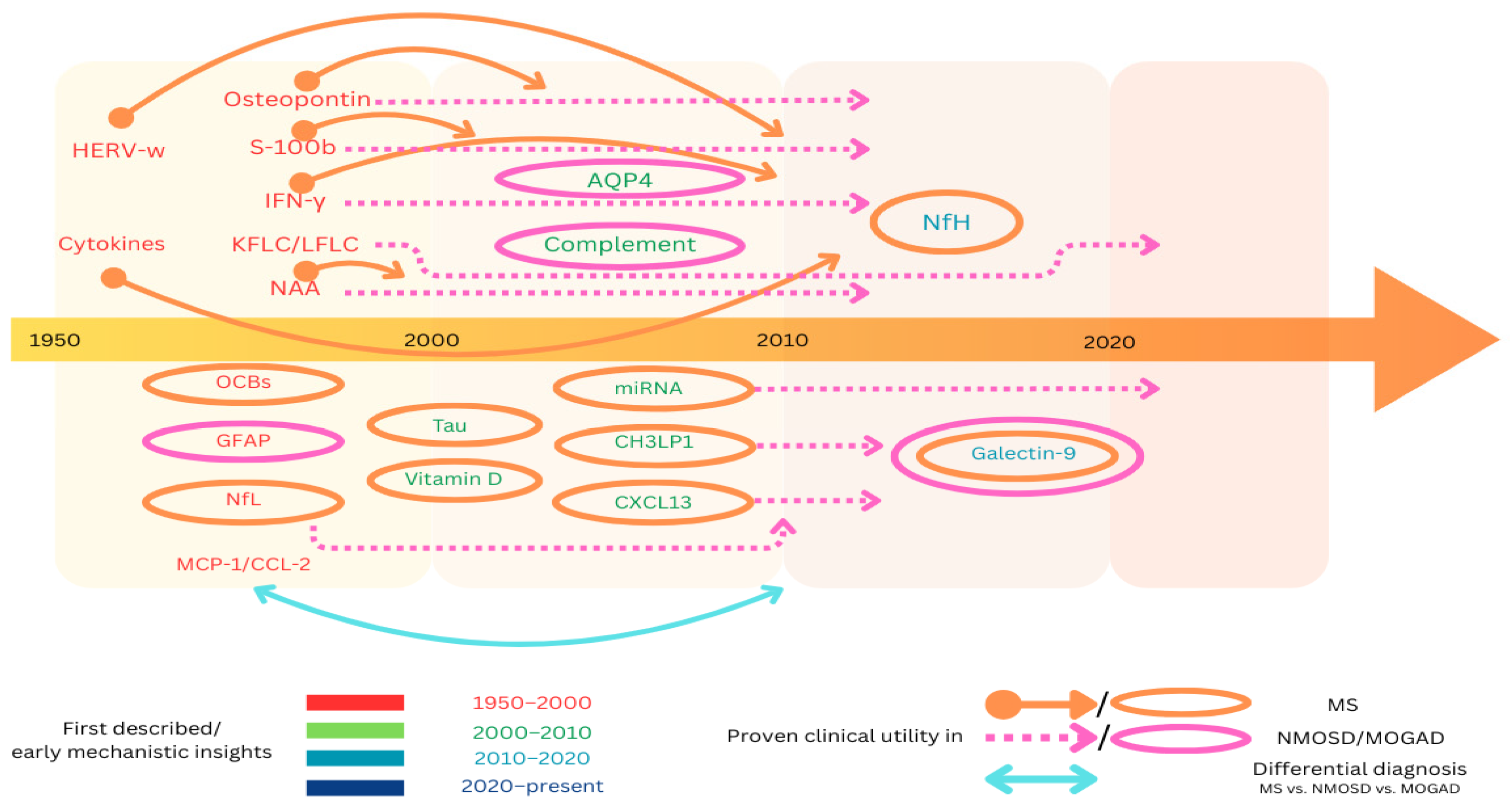
| Multiple Sclerosis (MS) | Neuromyelitis Optica Spectrum Disorder (NMOSD) | Myelin Oligodendrocyte Glycoprotein Antibody Disease (MOGAD) | |
|---|---|---|---|
| Key Pathophysiology | Primarily T cell-mediated autoimmune demyelination in the CNS; B cells also play a role. | Autoimmune astrocytopathy primarily mediated by aquaporin-4 (AQP4) IgG, leading to secondary demyelination. | Autoimmune demyelination mediated by IgG targeting MOG on oligodendrocytes. |
| Typical Age of Onset | 20–40 years (can vary); often a disease of young adults. | 40–60 years, but can vary widely; some patients present later in life. | 20–30 years; children are particularly predisposed. |
| Epidemiology | The female–male ratio is about 2–3:1 (varies by geographic region). | Marked female predominance, up to 9:1. | Nearly equal distribution. |
| Clinical Presentation | Focal motor or sensory deficits; ataxia; unilateral optic neuritis; bladder and bowel dysfunction; sexual dysfunction; cognitive dysfunction. | Severe attacks of optic neuritis, transverse myelitis (frequently longitudinally extensive), or area postrema syndrome. | -Acute disseminated encephalomyelitis (ADEM) -> focal neurological deficits, transverse myelitis, and altered mental status; -Unilateral or bilateral optic neuritis; -Transverse myelitis. |
| MRI Findings | Brain: -Periventricular, juxtacortical, and infratentorial lesions with a typical ovoid aspect and ring enhancement. Spinal cord: -Usually, peripheral cord lesions are limited to short segments. | Brain: -Periventricular white matter lesions, lesions of dorsal medulla, or periependymal surfaces of the third and fourth ventricles; -Long optic nerve lesions are frequently encountered. Spinal cord: -Longitudinally extensive lesions (≥3 vertebral segments). | Brain: -Large, poorly delimited lesions; -Unilateral or bilateral thalamic or basal ganglia involvement; -Longitudinally extensive optic neuritis. Spinal cord: -Lesions can be extensive; “H-sign” or “ventral sagittal line” signs may come across. |
| Serum Biomarker | OCBs in the CSF are supportive, but not definitive. | AQP4-IgG (NMO-IgG)-positive in ~70–80% of NMOSD patients. | MOG-IgG is positive in a significant proportion of patients. |
| Clinical Course | Relapsing–remitting (RR)/secondary progressive (SP)/primary progressive (PP)/progressive relapsing (PR). | Relapsing–remitting, rarely monophasic. | Relapsing–remitting or monophasic. |
| Biomarker | Category | Function/Role | Diseases and Notes |
|---|---|---|---|
| OCBs | Humoral Marker | Indicative of intrathecal IgG (and sometimes IgM) synthesis; crucial for MS diagnosis and prognosis. | MS: Present in over 95% of patients. NMOSD: Low positivity (10–25%). |
| AQP4-IgG | Humoral Marker | Autoantibody against aquaporin-4 water channels on astrocytic endfeet; triggers complement-mediated injury. | NMOSD: Highly specific marker and associated with relapse risk. |
| MOG-IgG | Humoral Marker | Autoantibody targeting MOG; defines a distinct disease entity. | MOGAD: Characteristic marker; also found in AQP4-negative NMOSD cases. |
| HERV-W Peptides | Humoral Marker | Derived from endogenous retroviruses; potential role in disease pathogenesis and differentiation. | MS: High positivity compared to NMOSD and MOGAD, aiding differential diagnosis. |
| KFLC and KFLC Index | Humoral Marker | Measures free light chain synthesis in the CSF; quantifies intrathecal antibody production. | MS: Useful in diagnosis and predicting recurrence; helps differentiate from NMOSD. |
| Galectin-9 | Humoral Marker | Immunomodulatory glycoprotein; elevated levels correlate with increased lesion load and aid in distinguishing secondary progressive (SPMS) from relapsing–remitting MS (RRMS). | MS: Elevated in the CSF, particularly higher in secondary progressive MS compared to relapsing–remitting MS, assisting in phenotypic differentiation. |
| Complement (C4d, C5-C9, C3/C4) | Humoral Marker | Activation cascade triggered by AQP4-IgG binding; mediates complement-dependent cytotoxicity leading to astrocyte, neuron, and myelin damage. | NMOSD: Elevated complement markers (e.g., C4d, C5-C9 complex) compared to MS; the therapeutic target (e.g., eculizumab); differences in C3/C4 levels help differentiate NMOSD from MOGAD. |
| Neurofilaments (NfL, NfH) | CNS-Related Marker | Structural proteins released from axons upon injury; indicate axonal damage and neurodegeneration. | MS: Elevated during relapses and correlates with disability. NMOSD and MOGAD: High levels indicate severe axonal involvement. |
| GFAP | CNS-Related Marker | Astrocytic intermediate filament; marker of astrocyte damage and reactive gliosis. | MS: Associated with disease progression and neurodegeneration. NMOSD: Elevated during attacks (optic neuritis, myelitis). |
| S100B | CNS-Related Marker | Calcium-binding protein; supports astrocyte proliferation at physiological levels but is neurotoxic at high concentrations. | MS and NMOSD: Elevated during acute phases/relapses, contributing to neuroinflammation. |
| CH3LP1 (Chitinase-3-Like Protein 1) | CNS-Related Marker | Secreted by activated astrocytes; correlates with lesion load, cognitive impairment, and progression. | MS: Serves as a diagnostic/prognostic marker; higher levels indicate clinical progression. |
| NAA (N-Acetyl Aspartate) | CNS-Related Marker | Neuronal metabolite reflects neuronal and axonal integrity; reduction indicates neuronal loss and demyelination. | MS: Reduced in lesions; differential levels help distinguish MS from NMOSD. |
| Tau | CNS-Related Marker | Microtubule-associated protein released upon axonal and neuronal injury; reflects neurodegeneration. | MS: CSF total tau is elevated, correlates with disease progression. |
| CXCL13 and Related Chemokines (e.g., CCL11, CXCL10) | Cell Marker | Chemokines that mediate immune cell recruitment (e.g., CXCL13 recruits B cells) and promote inflammation. | MS: Elevated levels correlate with disease activity, lesion burden, and treatment response. |
| Osteopontin | Cell Marker | Proinflammatory glycoprotein secreted by various immune cells; involved in active lesion formation and secondary neurodegeneration. | MS: Elevated during relapses. NMOSD: Increased but less specific for phenotype differentiation. |
| IL-6 | Cytokine | Pro-inflammatory cytokine; drives immune activation and modulates relapse risk and severity. | NMOSD: High IL-6 levels are linked to relapse risk and higher disability scores; also implicated in MOGAD. |
| Vitamin D | Nutritional/immunological biomarker | Lipid-soluble vitamin, exerts hormone-like effects. | MS: Low vitamin D levels in early RRMS predict a greater risk of exacerbations and MRI activity. |
| Biomarker | Application | Limitations | Accessibility |
|---|---|---|---|
| OCBs | Incorporated into McDonald criteria as a diagnostic substitute for dissemination in time; routine CSF test in MS. | Low specificity (present in other neuroinflammatory diseases); transient or low-level positivity in NMOSD; requires lumbar puncture. | Widely available. |
| KFLC and KFLC Index | Included in 2024 McDonald criteria as alternative evidence of intrathecal Ig synthesis; emerging prognostic marker. | Lack of universally accepted cut-offs; serum elevations (e.g., renal impairment) can confound; steroid treatment may affect levels; limited specificity outside MS. | Available in many immunology labs; faster and less labor-intensive than OCBs. |
| Neurofilaments (NfL, NfH) | Serum NfL increasingly used to monitor disease activity and treatment response. | Low specificity (elevated in various neurodegenerative and acute CNS injuries); neurofilament heavy chains (NfHs) are less clinically validated. | Requires ultra-sensitive assays; available in reference laboratories. |
| AQP4 IgG | Gold-standard diagnostic biomarker for NMOSD. | Titer does not reliably reflect disease activity or severity; double-seronegative cases remain challenging. | Widely available. |
| MOG-IgG | Diagnostic marker for MOGAD; distinguishes MOGAD from AQP4-NMOSD and MS. | Variable assay sensitivity and specificity across platforms; seronegative cases possible. | Available in specialized/reference labs; less widespread than AQP4-IgG. |
| GFAP | Research marker of astrocytic damage; potential differentiation between NMOSD, MOGAD, and MS phenotypes. | Age-dependent expression; multiple isoforms; overlap between diseases; not used clinically. | Measured in research settings; not routine. |
| S100B | Research marker of acute astrocyte activation in relapses; correlates with lesion severity. | Low disease specificity (elevated in many CNS pathologies); biphasic effects (neurotrophic vs. toxic); not used clinically. | Measured in research settings. |
| CH3LP1 | Research diagnostic/prognostic marker in MS; correlates with lesion load, cognitive decline, and progression. | Inconsistent therapeutic-monitoring data; overlap with other neuroinflammatory conditions; not used clinically. | Measured in research settings. |
| CXCL13 and Related Chemokines | Research tool to predict B cell–driven activity and treatment response in MS. | Short half-life; influenced by concurrent infections; lack of normative ranges; not in guidelines. | Measured in reference labs. |
| NAA (N-Acetyl Aspartate) | Research imaging biomarker via spectroscopy for neuronal integrity; potential in differential diagnosis (MS vs. NMOSD). | Requires specialized MR spectroscopy hardware and expertise; influenced by scanner field strength; not routine. | Available at centers with spectroscopy capability. |
| Galectin-9 | Research marker to distinguish SPMS from RRMS; correlates with lesion burden. | Limited longitudinal data; overlap with other autoimmune diseases; not validated clinically. | Measured in research settings. |
| Osteopontin | Research marker of acute relapses and lesion activity in MS and NMOSD; potential treatment-response indicator. | Elevated in both RRMS and SPMS (limited phenotypic specificity); influenced by systemic inflammation; not clinical. | Measured in research settings. |
| Complement Components (C4d, C5-C9) | Research differentiation of NMOSD vs. MS/MOGAD; therapeutic target. | Complement activation is common to many conditions; requires CSF/serum; dynamic and labile. | Measured by specialized immunoassays in a few labs. |
| IL-6 | Research marker of relapse risk in NMOSD; guides anti-IL-6 therapies. | Levels vary with systemic inflammation; pre-analytical instability. | Available in clinical immunology labs. |
| HERV-W | Research marker to differentiate MS from NMOSD/MOGAD; potential therapeutic target. | Novel; assays not standardized; unclear pathophysiological specificity. | Measured in research settings. |
| Tau | Research prognostic marker of early disability accumulation in MS; reflects axonal injury. | Low specificity (elevated in various neurodegenerative diseases); dynamic relationship with inflammation; not in MS protocols. | Not routine for MS; serum assays experimental. |
| Vitamin D | Routinely measured as an add-on to monitor supplementation in MS. | Not diagnostic. | Widely available in clinical labs; serum 25-OH vitamin D assay routine. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, A.-M.; Neațu, M.; Luca, D.-G.; Davidescu, E.I.; Popescu, B.-O. Fluid Biomarkers in Demyelinating Spectrum Disorders: Past, Present, and Prospects. Int. J. Mol. Sci. 2025, 26, 4455. https://doi.org/10.3390/ijms26094455
Florea A-M, Neațu M, Luca D-G, Davidescu EI, Popescu B-O. Fluid Biomarkers in Demyelinating Spectrum Disorders: Past, Present, and Prospects. International Journal of Molecular Sciences. 2025; 26(9):4455. https://doi.org/10.3390/ijms26094455
Chicago/Turabian StyleFlorea, Anca-Maria, Monica Neațu, Dimela-Gabriela Luca, Eugenia Irene Davidescu, and Bogdan-Ovidiu Popescu. 2025. "Fluid Biomarkers in Demyelinating Spectrum Disorders: Past, Present, and Prospects" International Journal of Molecular Sciences 26, no. 9: 4455. https://doi.org/10.3390/ijms26094455
APA StyleFlorea, A.-M., Neațu, M., Luca, D.-G., Davidescu, E. I., & Popescu, B.-O. (2025). Fluid Biomarkers in Demyelinating Spectrum Disorders: Past, Present, and Prospects. International Journal of Molecular Sciences, 26(9), 4455. https://doi.org/10.3390/ijms26094455








