Functional Analysis of Antipsychotics in Human iPSC-Based Neural Progenitor 2D and 3D Schizophrenia Models
Abstract
1. Introduction
2. Results
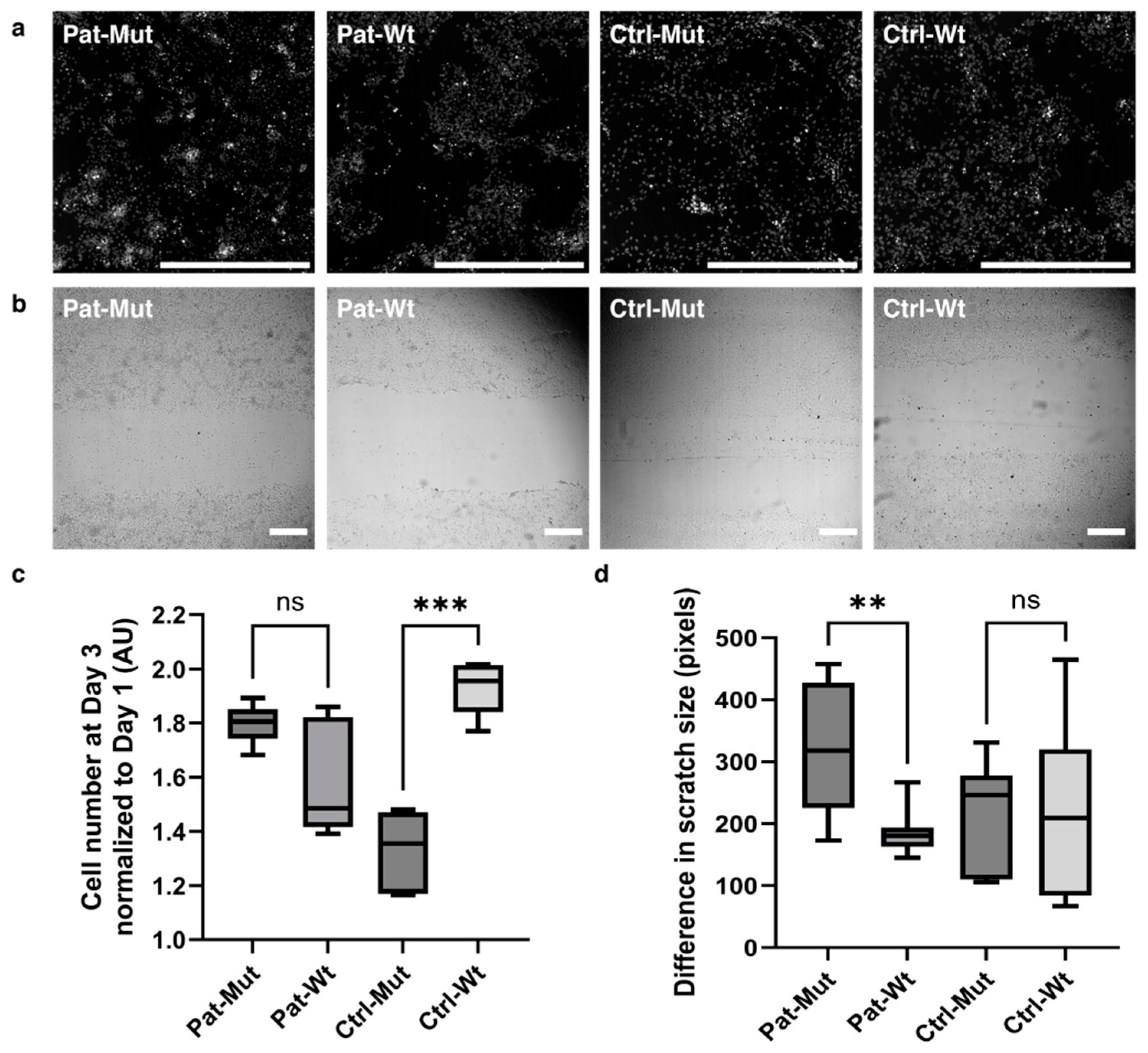
2.1. Characterization of 2D NPCs Differentiated from ZMYND11 Wild Type and Mutant iPSCs
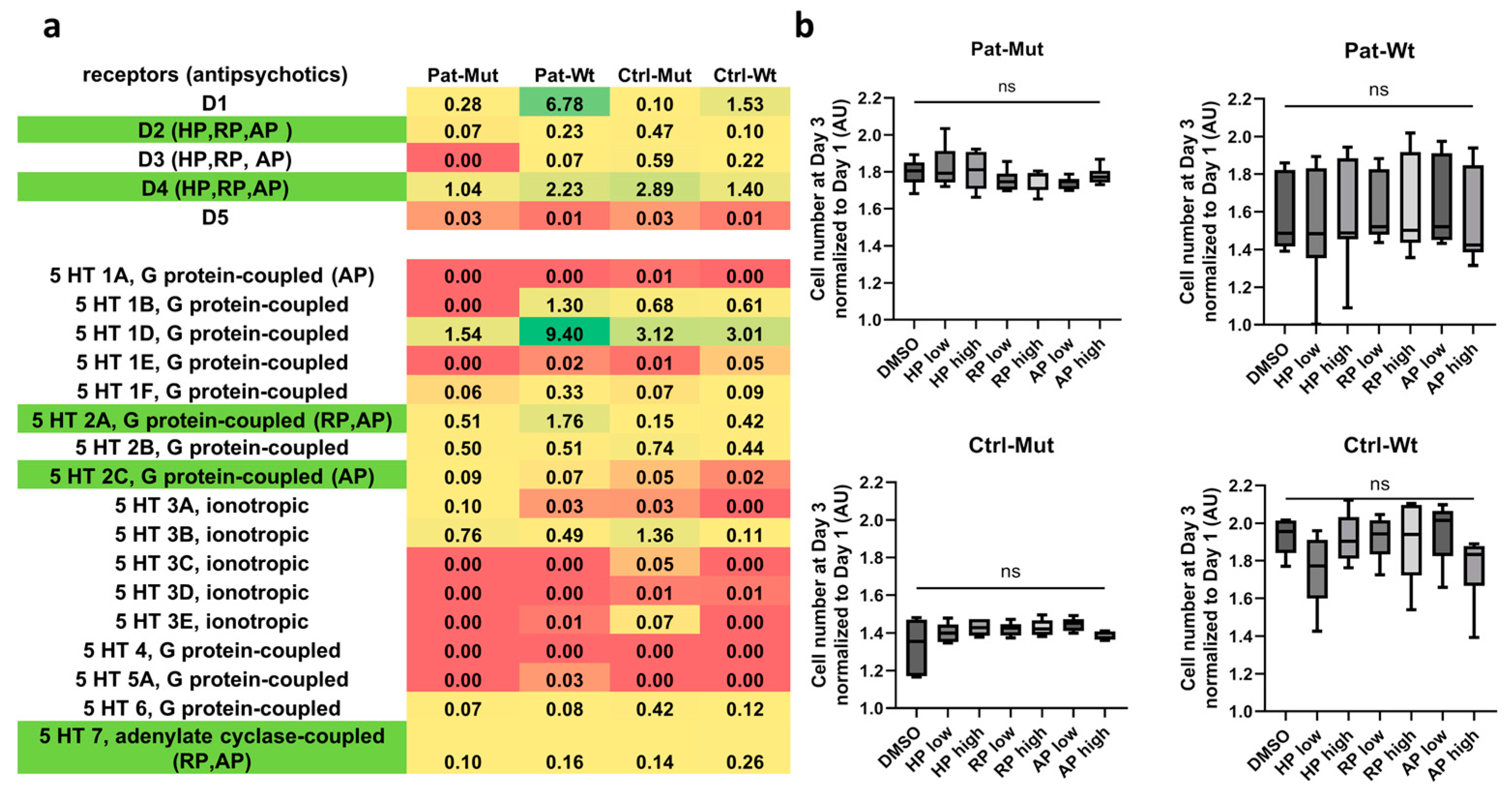
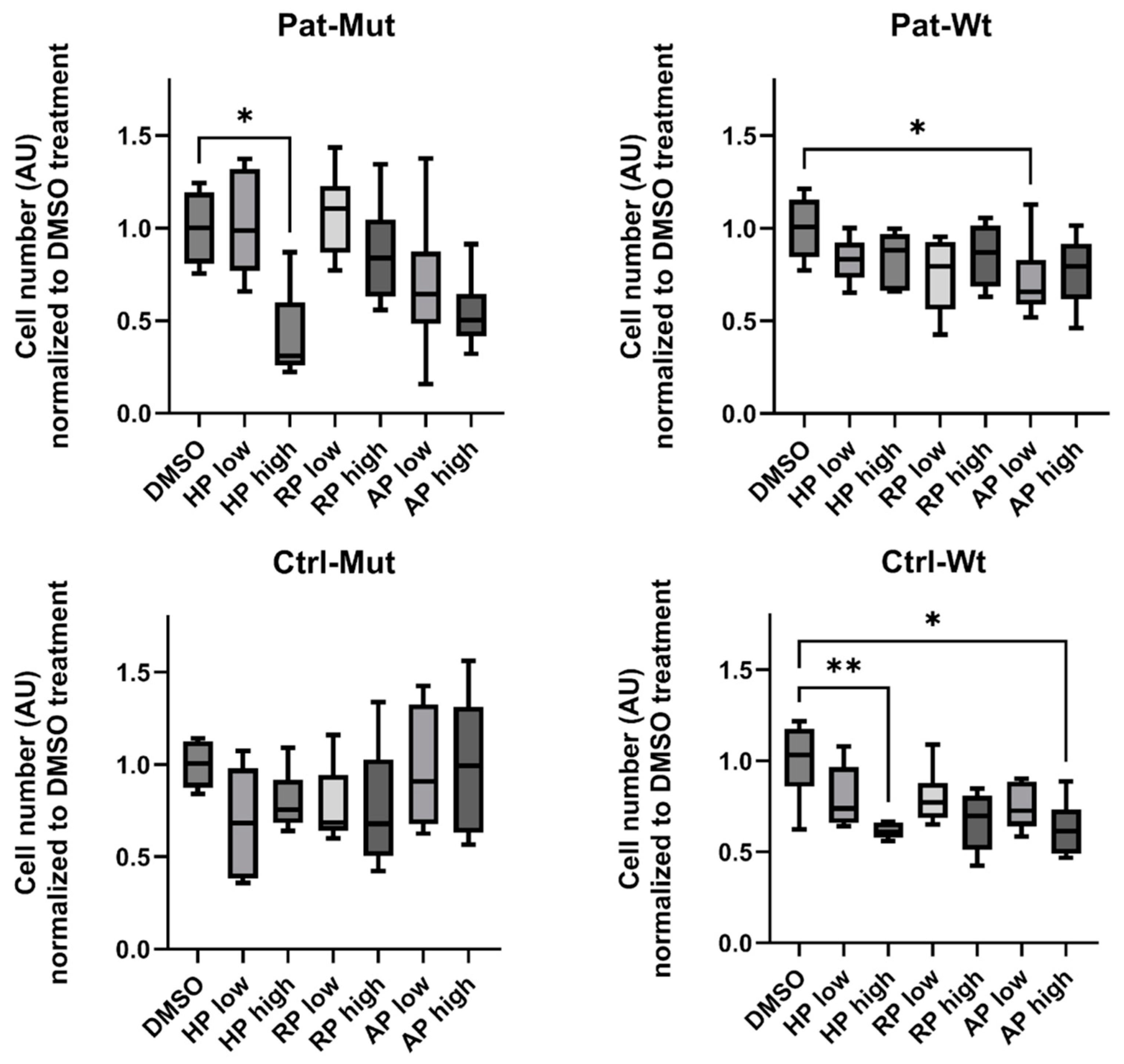
2.2. Receptor Expression and Proliferation of 2D NPCs Differentiated from ZMYND11 Wild Type and Mutant iPSCs After Antipsychotic Treatment
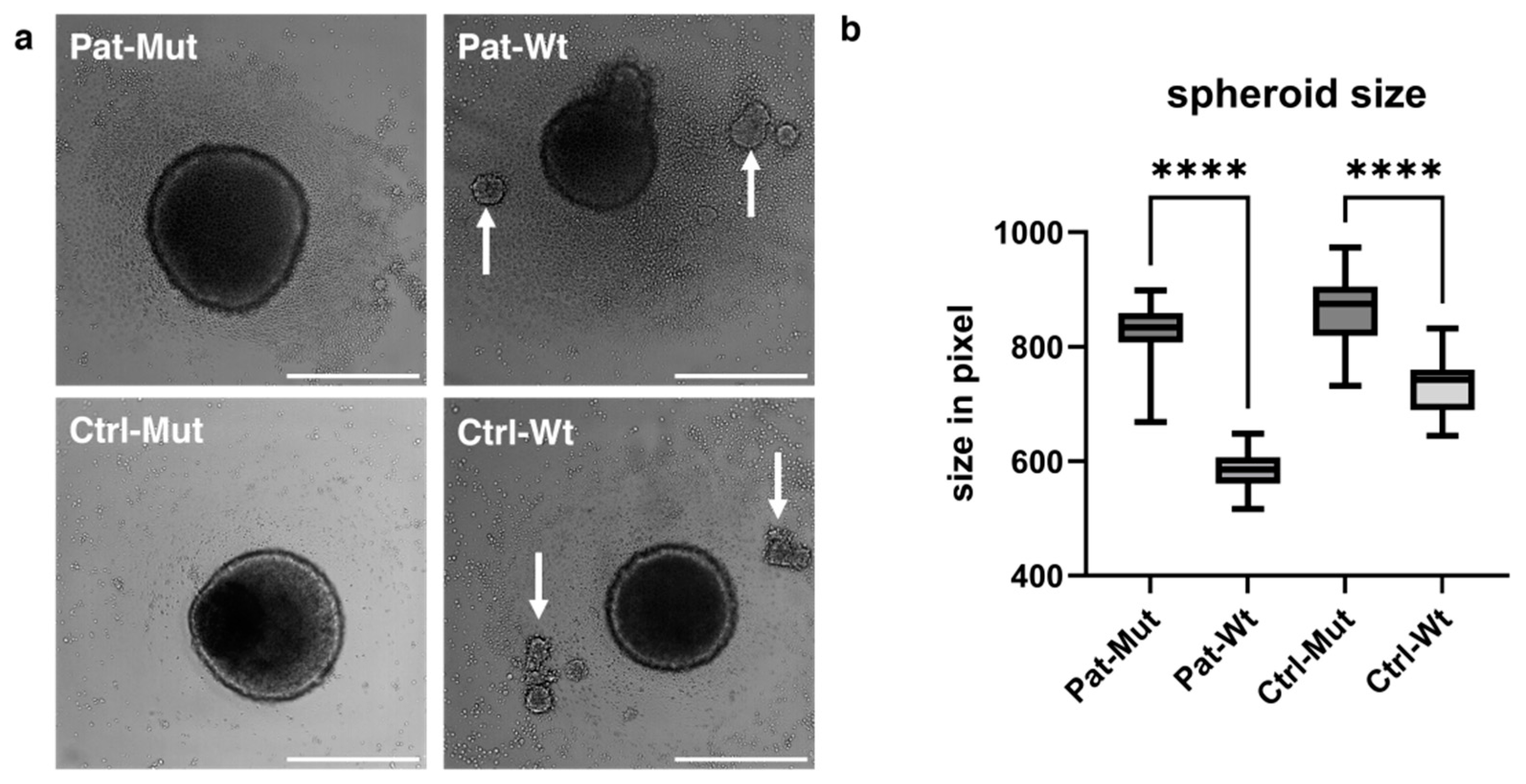
2.3. Spheroid Formation of NPCs Differentiated from ZMYND11 Wild Type and Mutant iPSCs
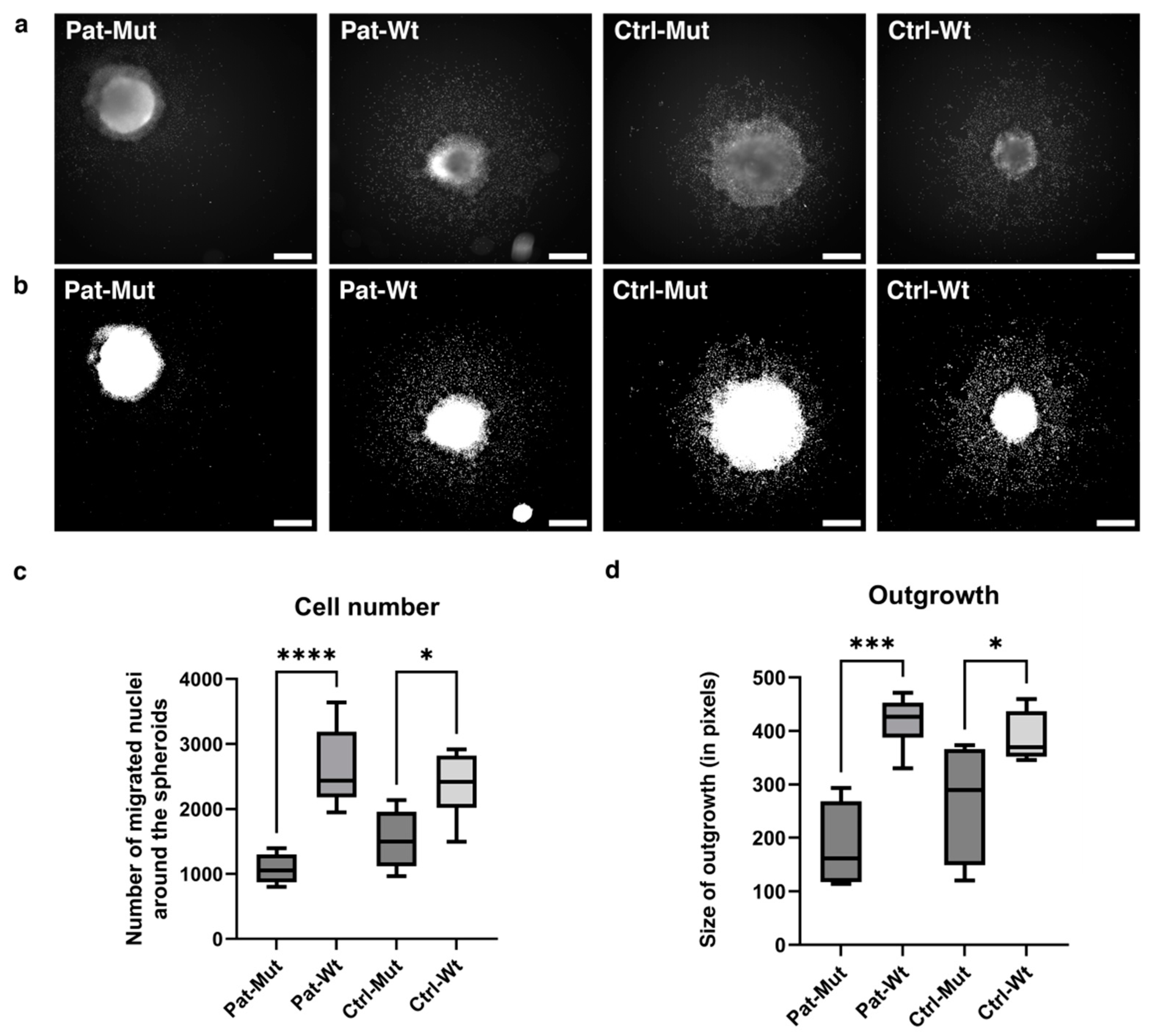
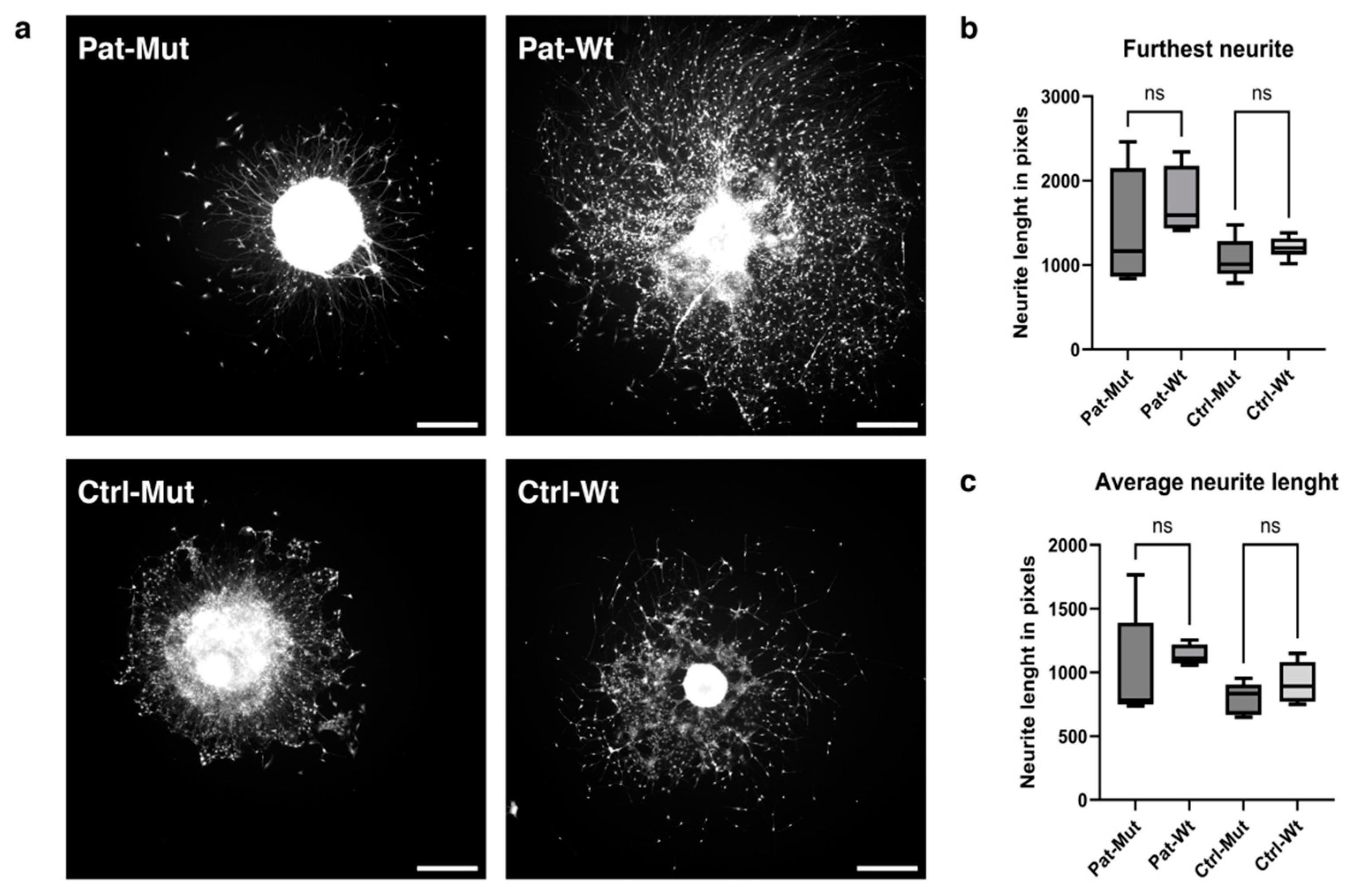
2.4. Spheroid Outgrowth of NPCs Differentiated from ZMYND11 Wild Type and Mutant iPSCs
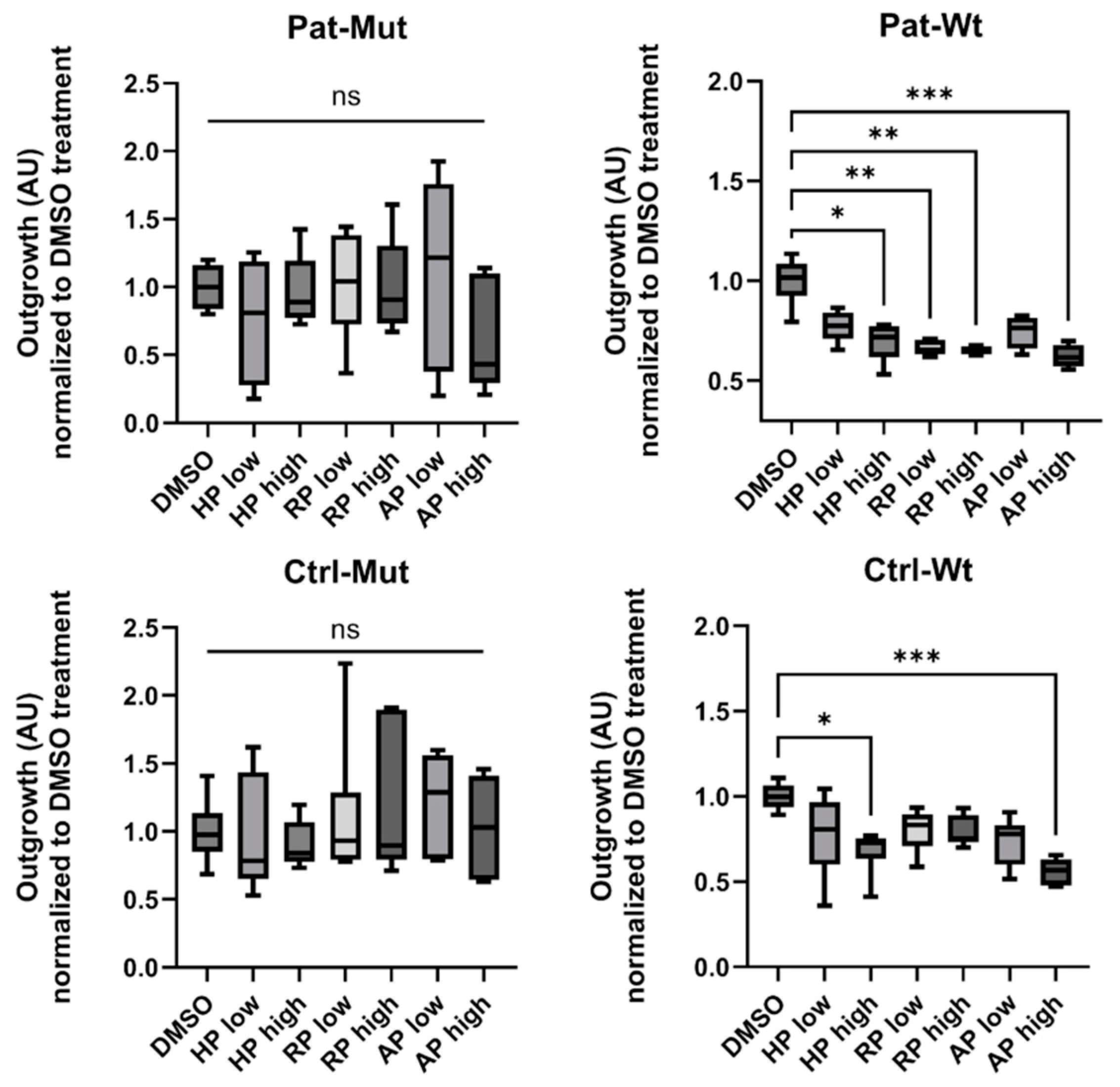
2.5. Effect of Antipsychotic Treatments on Spheroid Outgrowth of NPCs Differentiated from ZMYND11 Wild Type and Mutant iPSCs
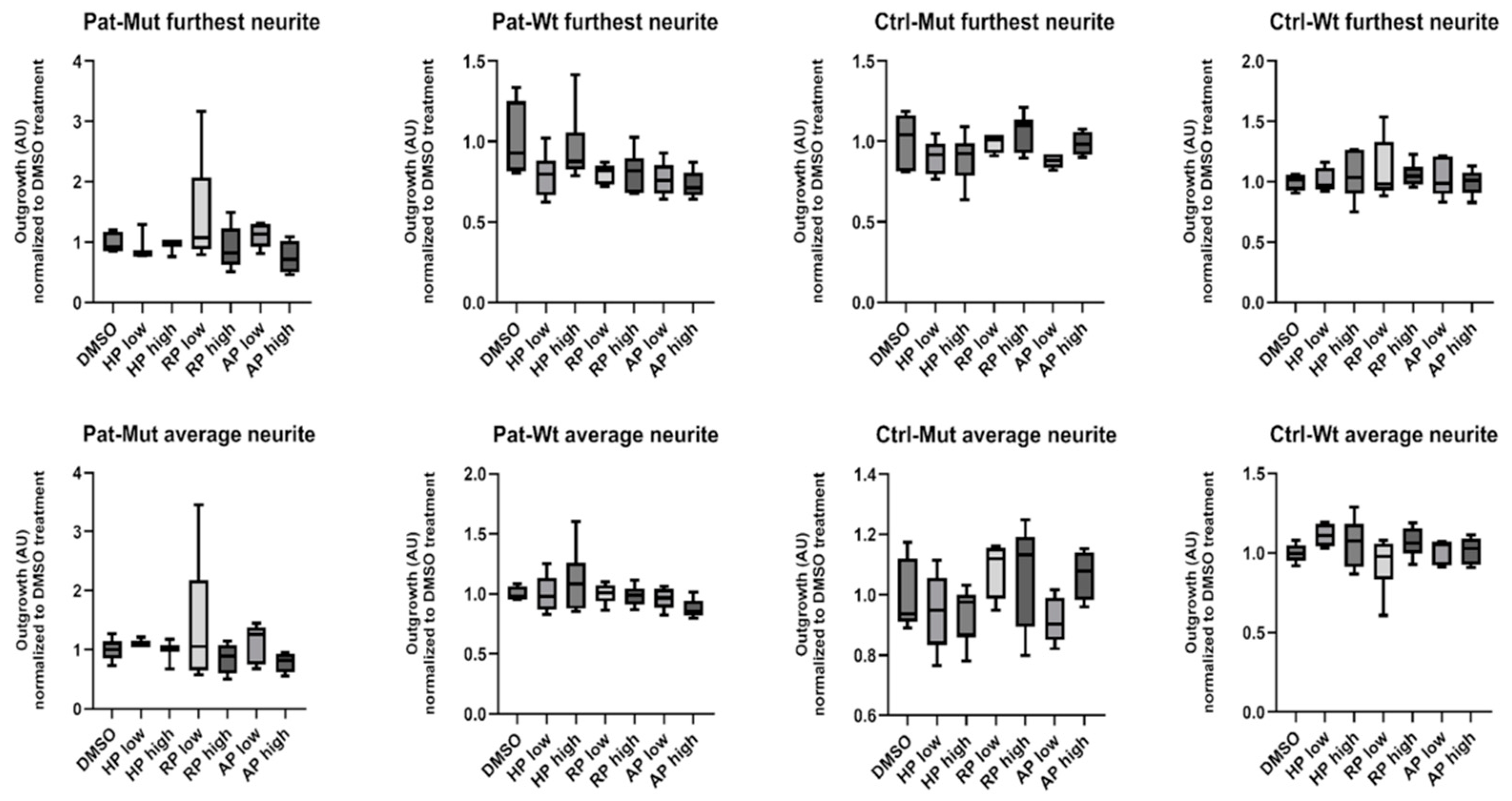
2.6. Spheroid Outgrowth of NPCs During Neural Differentiation and the Effect of Antipsychotic Treatments
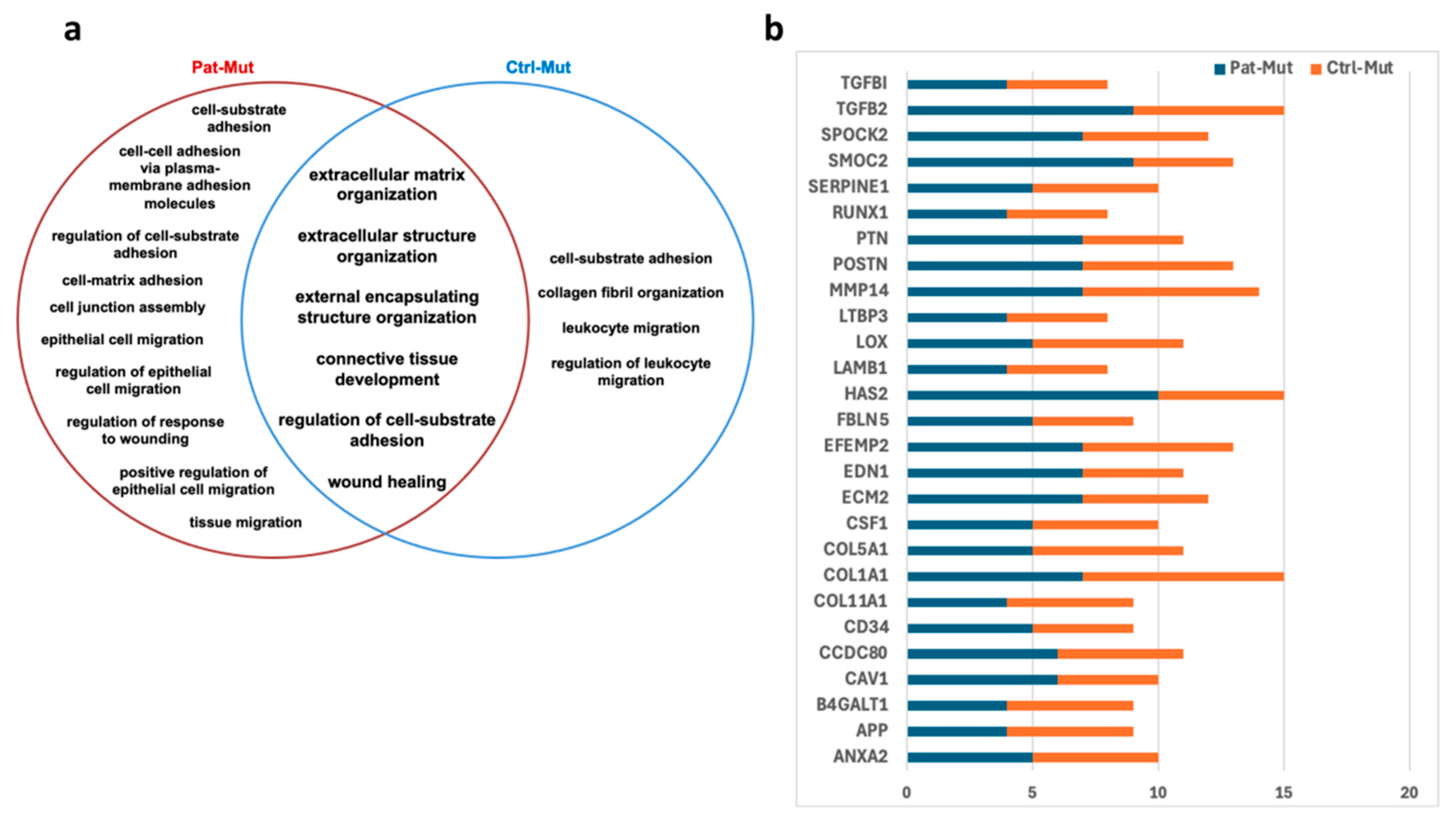
2.7. Characterization of Cell Adhesion Based on mRNA Sequencing Data
3. Discussion
- Limitations of this study
4. Materials and Methods
4.1. Generation of NPCs and Spheroids: Antipsychotic Treatments
4.1.1. Cell Line Selection and Culture
4.1.2. Antipsychotic Treatment of NPCs
4.1.3. Spheroid Formation
4.1.4. Two-Dimensional Proliferation and Scratch Assay
4.2. RNAseq Data Analysis
4.3. DCV and Calcein-AM Staining
4.4. HCS Measurements and Analysis
4.4.1. Two-Dimensional Cell Number Determination
4.4.2. Analysis of the Scratch Assay
4.4.3. Three-Dimensional NPC Number Determination
4.4.4. Three-Dimensional NPC Outgrowth Analysis
4.4.5. Determination of Neurite Outgrowth in 3D Differentiated NPCs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Aripiprazole |
| COL1A1 | Collagen type I alpha 1 chain |
| DCV | Vybrant™ DyeCycle™ Violet |
| DGGC | Dentate gyrus granule cell |
| ECM | Extracellular matrix |
| fMRI | Functional magnetic resonance imaging |
| GO | Gene Ontology |
| ULA | Ultra-low attachment |
| HAS2 | Hyaluronan synthase 2 |
| hiPSC | Human-induced pluripotent stem cell |
| HP | Haloperidol |
| iPSC | Induced pluripotent stem cell |
| LD | Linear dichroism |
| MMP14 | Membrane-type Matrix Metalloproteinase 14 |
| mut | Mutant cell type |
| NPC | Neural progenitor cell |
| PET | Positron emission tomography |
| RP | Risperidone |
| RPKM | Reads Per Kilobase Million |
| SCZ | Schizophrenia |
| TGFB2 | Transforming Growth Factor Beta 2 |
| wt | Wild type |
References
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef]
- Howes, O.D.; Murray, R.M. Schizophrenia: An integrated sociodevelopmental-cognitive model. Lancet 2014, 383, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef]
- Weinberger, D.R.; Berman, K.F.; Suddath, R.; Torrey, E.F. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: A magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am. J. Psychiatry 1992, 149, 890–897. [Google Scholar] [CrossRef]
- Nakahara, S.; Matsumoto, M.; van Erp, T.G.M. Hippocampal subregion abnormalities in schizophrenia: A systematic review of structural and physiological imaging studies. Neuropsychopharmacol. Rep. 2018, 38, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Basu, A.; Benneyworth, M.; Balu, D.; Konopaske, G. Glutamatergic synaptic dysregulation in schizophrenia: Therapeutic implications. Handb. Exp. Pharmacol. 2012, 213, 267–295. [Google Scholar] [CrossRef]
- Harrison, P.J. Postmortem studies in schizophrenia. Dialogues Clin. Neurosci. 2000, 2, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R.; Radulescu, E. Finding the Elusive Psychiatric “Lesion” with 21st-Century Neuroanatomy: A Note of Caution. Am. J. Psychiatry 2016, 173, 27–33. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Tordai, C.; Hathy, E.; Gyergyak, H.; Vincze, K.; Baradits, M.; Koller, J.; Poti, A.; Jezso, B.; Homolya, L.; Molnar, M.J.; et al. Probing the biological consequences of a previously undescribed de novo mutation of ZMYND11 in a schizophrenia patient by CRISPR genome editing and induced pluripotent stem cell based in vitro disease-modeling. Schizophr. Res. 2024, 273, 107–120. [Google Scholar] [CrossRef]
- Yu, D.X.; Di Giorgio, F.P.; Yao, J.; Marchetto, M.C.; Brennand, K.; Wright, R.; Mei, A.; McHenry, L.; Lisuk, D.; Grasmick, J.M.; et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep. 2014, 2, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chai, J.; Wu, H.; Bai, B.; Yang, H.; He, W.; Zhao, Y. New Therapeutic Targets and Drugs for Schizophrenia Beyond Dopamine D2 Receptor Antagonists. Neuropsychiatr. Dis. Treat. 2024, 20, 607–620. [Google Scholar] [CrossRef]
- Jezso, B.; Kalman, S.; Farkas, K.G.; Hathy, E.; Vincze, K.; Kovacs-Schoblocher, D.; Lilienberg, J.; Tordai, C.; Nemoda, Z.; Homolya, L.; et al. Haloperidol, Olanzapine, and Risperidone Induce Morphological Changes in an In Vitro Model of Human Hippocampal Neurogenesis. Biomolecules 2024, 14, 688. [Google Scholar] [CrossRef]
- Panchision, D.M. Concise Review: Progress and Challenges in Using Human Stem Cells for Biological and Therapeutics Discovery: Neuropsychiatric Disorders. Stem Cells 2016, 34, 523–536. [Google Scholar] [CrossRef]
- Wen, Z.; Christian, K.M.; Song, H.; Ming, G.L. Modeling psychiatric disorders with patient-derived iPSCs. Curr. Opin. Neurobiol. 2016, 36, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, N.; Desplan, C. Neuronal differentiation strategies: Insights from single-cell sequencing and machine learning. Development 2020, 147, dev193631. [Google Scholar] [CrossRef]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.M.; Zhou, Y.; Kogan, J.H.; Shin, R.; Webster, M.; Gross, A.K.; Heusner, C.L.; Chen, Q.; Miyake, S.; Tajinda, K.; et al. Detection of an immature dentate gyrus feature in human schizophrenia/bipolar patients. Transl. Psychiatry 2012, 2, e135. [Google Scholar] [CrossRef]
- Reif, A.; Fritzen, S.; Finger, M.; Strobel, A.; Lauer, M.; Schmitt, A.; Lesch, K.P. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 2006, 11, 514–522. [Google Scholar] [CrossRef]
- Steen, R.G.; Mull, C.; McClure, R.; Hamer, R.M.; Lieberman, J.A. Brain volume in first-episode schizophrenia: Systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry 2006, 188, 510–518. [Google Scholar] [CrossRef]
- Dhikav, V.; Anand, K.S. Is hippocampal atrophy a future drug target? Med. Hypotheses 2007, 68, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, H.; Dallmann, A. Antidepressants and Antipsychotics in Human Pregnancy: Transfer Across the Placenta and Opportunities for Modeling Studies. J. Clin. Pharmacol. 2022, 62 (Suppl. 1), S115–S128. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jo, H.; Jeong, Y.D.; Jang, W.; Park, J.; Yim, Y.; Lee, K.; Lee, H.; Lee, S.; Fond, G.; et al. Antipsychotic use during pregnancy and outcomes in pregnant individuals and newborns. J. Affect. Disord. 2025, 373, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Singh, M.K. In utero exposure to atypical antipsychotic drug, risperidone: Effects on fetal neurotoxicity in hippocampal region and cognitive impairment in rat offspring. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 35–44. [Google Scholar] [CrossRef]
- Khan, T.A.; Revah, O.; Gordon, A.; Yoon, S.J.; Krawisz, A.K.; Goold, C.; Sun, Y.; Kim, C.H.; Tian, Y.; Li, M.Y.; et al. Neuronal defects in a human cellular model of 22q11.2 deletion syndrome. Nat. Med. 2020, 26, 1888–1898. [Google Scholar] [CrossRef]
- Kathuria, A.; Lopez-Lengowski, K.; Jagtap, S.S.; McPhie, D.; Perlis, R.H.; Cohen, B.M.; Karmacharya, R. Transcriptomic Landscape and Functional Characterization of Induced Pluripotent Stem Cell-Derived Cerebral Organoids in Schizophrenia. JAMA Psychiatry 2020, 77, 745–754. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Kumar, M.; Nassour-Caswell, L.C.; Alrefai, H.; Anderson, J.C.; Schanel, T.L.; Hicks, P.H.; Cardan, R.; Willey, C.D. A High-Throughput Neurosphere-Based Colony Formation Assay to Test Drug and Radiation Sensitivity of Different Patient-Derived Glioblastoma Lines. Cells 2024, 13, 1995. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Le, M.P.T.; Lee, K.W.; Kim, J.H.; Yoon, H.C.; Pham, H.T.M. Development of a Disease Modeling Framework for Glutamatergic Neurons Derived from Neuroblastoma Cells in 3D Microarrays. Sci. Rep. 2024, 14, 29144. [Google Scholar] [CrossRef]
- Zhang, J.; Medina, A.; Ferrer, M.; Lee, E.M. Generation of Functional Brain Region-Specific Neural Spheroids for High Throughput Screening. Methods Mol. Biol. 2025. [Google Scholar] [CrossRef]
- Strong, C.E.; Zhang, J.; Carrasco, M.; Kundu, S.; Boutin, M.; Vishwasrao, H.D.; Liu, J.; Medina, A.; Chen, Y.C.; Wilson, K.; et al. Functional brain region-specific neural spheroids for modeling neurological diseases and therapeutics screening. Commun. Biol. 2023, 6, 1211. [Google Scholar] [CrossRef]
- Sloan, S.A.; Andersen, J.; Pasca, A.M.; Birey, F.; Pasca, S.P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 2018, 13, 2062–2085. [Google Scholar] [CrossRef] [PubMed]
- Osacka, J.; Kiss, A.; Bacova, Z.; Tillinger, A. Effect of Haloperidol and Olanzapine on Hippocampal Cells’ Proliferation in Animal Model of Schizophrenia. Int. J. Mol. Sci. 2022, 23, 7711. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Ahmadian, E.; Azarmi, Y.; Parvizpur, A.; Hamishehkar, H.; Eghbal, M.A. In vitro/vivo studies towards mechanisms of risperidone-induced oxidative stress and the protective role of coenzyme Q10 and N-acetylcysteine. Toxicol. Mech. Methods 2016, 26, 520–528. [Google Scholar] [CrossRef]
- Dwyer, D.S.; Lu, X.H.; Bradley, R.J. Cytotoxicity of conventional and atypical antipsychotic drugs in relation to glucose metabolism. Brain Res. 2003, 971, 31–39. [Google Scholar] [CrossRef]
- Asada, M.; Mizutani, S.; Takagi, M.; Suzuki, H. Antipsychotics promote neural differentiation of human iPS cell-derived neural stem cells. Biochem. Biophys. Res. Commun. 2016, 480, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 2000, 1, 169–178. [Google Scholar] [CrossRef]
- Liao, J.; Chen, R.; Lin, B.; Deng, R.; Liang, Y.; Zeng, J.; Ma, S.; Qiu, X. Cross-Talk between the TGF-beta and Cell Adhesion Signaling Pathways in Cancer. Int. J. Med. Sci. 2024, 21, 1307–1320. [Google Scholar] [CrossRef]
- Murata, H.; Zhou, L.; Ochoa, S.; Hasan, A.; Badiavas, E.; Falanga, V. TGF-beta3 stimulates and regulates collagen synthesis through TGF-beta1-dependent and independent mechanisms. J. Investig. Dermatol. 1997, 108, 258–262. [Google Scholar] [CrossRef]
- Gutierrez-Fernandez, A.; Soria-Valles, C.; Osorio, F.G.; Gutierrez-Abril, J.; Garabaya, C.; Aguirre, A.; Fueyo, A.; Fernandez-Garcia, M.S.; Puente, X.S.; Lopez-Otin, C. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J. 2015, 34, 1875–1888. [Google Scholar] [CrossRef]
- Spicer, A.P.; Seldin, M.F.; Olsen, A.S.; Brown, N.; Wells, D.E.; Doggett, N.A.; Itano, N.; Kimata, K.; Inazawa, J.; McDonald, J.A. Chromosomal localization of the human and mouse hyaluronan synthase genes. Genomics 1997, 41, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.T.; Nasrallah, H.A. Neuroprotective effects of the second generation antipsychotics. Schizophr. Res. 2019, 208, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhang, Y. Genetic and epigenetic variations in iPSCs: Potential causes and implications for application. Cell Stem Cell 2013, 13, 149–159. [Google Scholar] [CrossRef]
- Kilpinen, H.; Goncalves, A.; Leha, A.; Afzal, V.; Alasoo, K.; Ashford, S.; Bala, S.; Bensaddek, D.; Casale, F.P.; Culley, O.J.; et al. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature 2017, 546, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Hathy, E.; Szabo, E.; Varga, N.; Erdei, Z.; Tordai, C.; Czehlar, B.; Baradits, M.; Jezso, B.; Koller, J.; Nagy, L.; et al. Investigation of de novo mutations in a schizophrenia case-parent trio by induced pluripotent stem cell-based in vitro disease modeling: Convergence of schizophrenia- and autism-related cellular phenotypes. Stem Cell Res. Ther. 2020, 11, 504. [Google Scholar] [CrossRef]
- Riedel, M.; Schwarz, M.J.; Strassnig, M.; Spellmann, I.; Muller-Arends, A.; Weber, K.; Zach, J.; Muller, N.; Moller, H.J. Risperidone plasma levels, clinical response and side-effects. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 255, 261–268. [Google Scholar] [CrossRef]
- Van Putten, T.; Marder, S.R.; Mintz, J.; Poland, R.E. Haloperidol plasma levels and clinical response: A therapeutic window relationship. Am. J. Psychiatry 1992, 149, 500–505. [Google Scholar] [CrossRef]
- Kirschbaum, K.M.; Muller, M.J.; Malevani, J.; Mobascher, A.; Burchardt, C.; Piel, M.; Hiemke, C. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J. Biol. Psychiatry 2008, 9, 212–218. [Google Scholar] [CrossRef]
| Group | Treatment | Concentration | |
|---|---|---|---|
| HPlow | Haloperidol | 10 ng/mL | 0.003 µM |
| HPhigh | 100 ng/mL | 0.03 µM | |
| RPlow | Risperidone | 100 ng/mL | 0.24 µM |
| RPhigh | 1000 ng/mL | 2.4 µM | |
| APhigh | Aripiprazole | 100 ng/mL | 0.22 µM |
| APlow | 1000 ng/mL | 2.2 µM | |
| DMSO | DMSO | 0.2 µL/mL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, K.G.; Vincze, K.; Tordai, C.; Özgen, E.İ.; Gürler, D.; Deli, V.; Lilienberg, J.; Erdei, Z.; Sarkadi, B.; Réthelyi, J.M.; et al. Functional Analysis of Antipsychotics in Human iPSC-Based Neural Progenitor 2D and 3D Schizophrenia Models. Int. J. Mol. Sci. 2025, 26, 4444. https://doi.org/10.3390/ijms26094444
Farkas KG, Vincze K, Tordai C, Özgen Eİ, Gürler D, Deli V, Lilienberg J, Erdei Z, Sarkadi B, Réthelyi JM, et al. Functional Analysis of Antipsychotics in Human iPSC-Based Neural Progenitor 2D and 3D Schizophrenia Models. International Journal of Molecular Sciences. 2025; 26(9):4444. https://doi.org/10.3390/ijms26094444
Chicago/Turabian StyleFarkas, Kiara Gitta, Katalin Vincze, Csongor Tordai, Ece İlay Özgen, Derin Gürler, Vera Deli, Julianna Lilienberg, Zsuzsa Erdei, Balázs Sarkadi, János Miklós Réthelyi, and et al. 2025. "Functional Analysis of Antipsychotics in Human iPSC-Based Neural Progenitor 2D and 3D Schizophrenia Models" International Journal of Molecular Sciences 26, no. 9: 4444. https://doi.org/10.3390/ijms26094444
APA StyleFarkas, K. G., Vincze, K., Tordai, C., Özgen, E. İ., Gürler, D., Deli, V., Lilienberg, J., Erdei, Z., Sarkadi, B., Réthelyi, J. M., & Apáti, Á. (2025). Functional Analysis of Antipsychotics in Human iPSC-Based Neural Progenitor 2D and 3D Schizophrenia Models. International Journal of Molecular Sciences, 26(9), 4444. https://doi.org/10.3390/ijms26094444







