CRISPR/Cas9 Delivery Systems to Enhance Gene Editing Efficiency
Abstract
1. Introduction
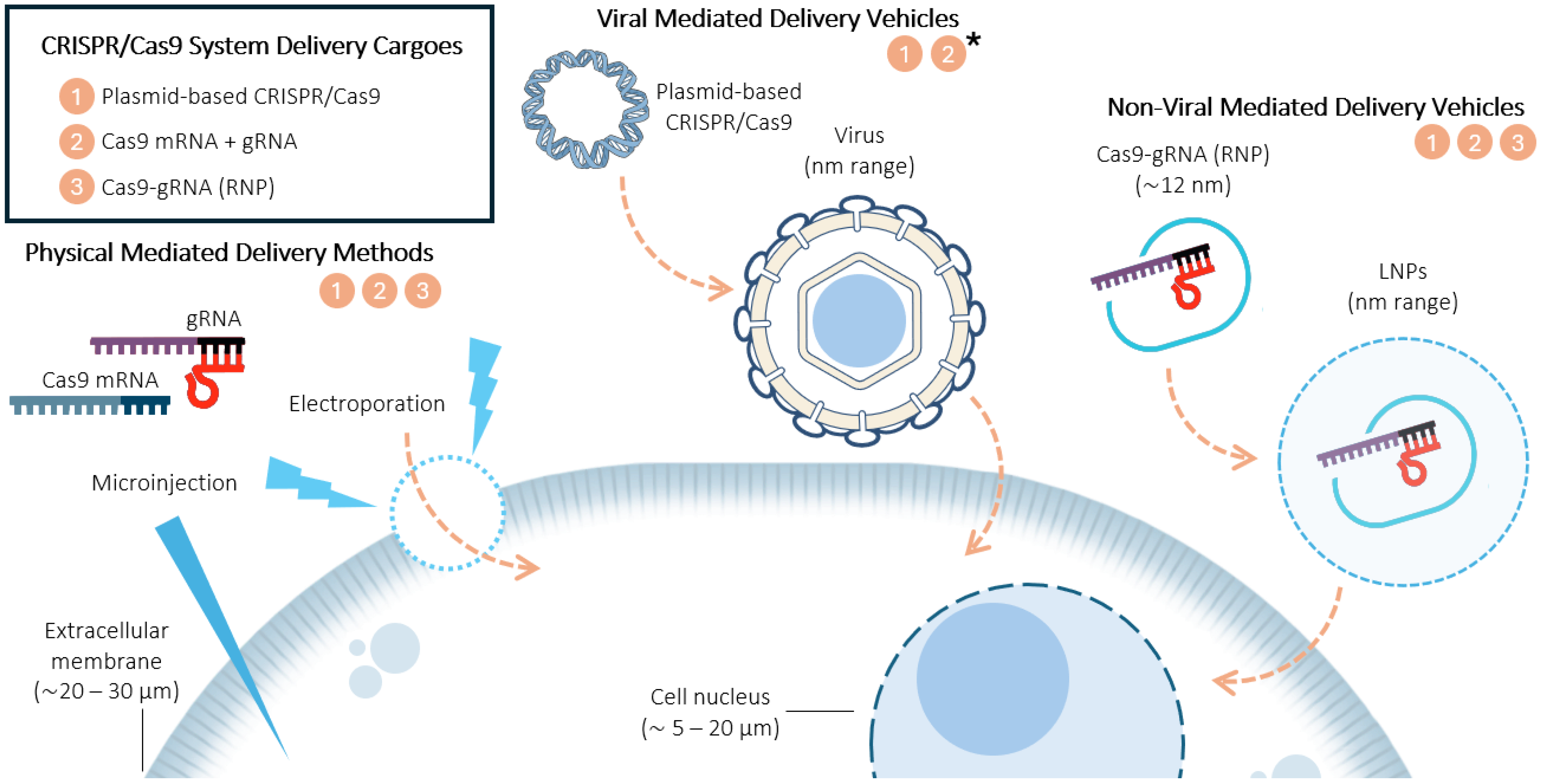
2. CRISPR/Cas9 Delivery Systems
2.1. CRISPR/Cas9 System Delivery Cargoes
- Plasmid-based CRISPR/Cas9. This system is widely used due to its simplicity and low-cost manipulation [18]. The moderate toxicity reported in certain cell lines could limit its application as the optimal lipid composition may be different [19]. In addition, both the large size of Cas9 and the need for nuclear entry limit its genome editing efficiency [8,18]. gRNA can be encoded within the plasmid alongside Cas9 or introduced separately as a synthetic gRNA for more precise control over the editing efficiency [20]. Viral vector cargoes might also be included in this category, as they typically deliver DNA or RNA encoding both Cas9 and gRNA, rather than introducing them separately, leading to either genome integration or transient or episomal expression [7].
- Cas9 mRNA coupled with gRNA. This method offers fast and low-toxicity genome editing, making it ideal for sensitive cells. Liu et al. [21] clearly demonstrated its biocompatibility and high genome editing efficacy using bioreducible LNPs for the simultaneous delivery of Cas9 mRNA and gRNA. This system decreases off-target editing events, making it suitable for the transient expression of Cas9 [18]. The gRNA can either be co-transcribed within the Cas9 mRNA or delivered separately as an independent molecule to optimize stability and efficiency. Viral vector cargoes could also be included in this category when delivering RNA encoding Cas9 and gRNA without genome integration.
- RNP complexes. RNPs are composed of a Cas9 protein and gRNA, and they offer the highest gene editing efficiency and specificity [20]. Wei et al. demonstrated that lipid nanoparticles encapsulating RNPs exhibited tissue-specific gene editing in mice’s lungs and liver [22]. Moreover, this system also minimizes off-target effects and toxicity [18,22].
2.2. Types of Delivery Vehicles
| Delivery Method | Details | Editing Efficiency | Ref. |
|---|---|---|---|
| Physically mediated | Microinjection used to insert green fluorescent protein into HepG2 cells | Around 40% | [24] |
| Electroporation in mouse zygotes | Reported as being highly efficient (no quantitative data provided) | [25] | |
| Electroporation of Cas9 RNP in HSPCs (CASGEVYTM—ex vivo) | Up to 90% indels in BCL11A enhancer | [9] | |
| Electroporation of Cas9 in somatic cells (in vitro) | High editing efficiency reported (no quantitative data provided) | [26] | |
| Virally mediated vehicles | Use of AAVs to specifically edit cell lines in mouse nervous system | Reported as being efficient (no quantitative data provided) | [27] |
| Use of engineered AAVs with capsid modifications to enhance transduction | Improved editing reported in muscle and neural tissues (no quantitative data provided) | [28,29] | |
| Use of lentiviral vectors optimized for stable genome editing in hematopoietic and liver cells | High editing efficiency and long-term expression reported (no quantitative data provided) | [30] | |
| Non-virally mediated vehicles | Bioreducible LNPs | Up to 90% in cultured cells and up to 80% in vivo | [21] |
| LNPs used to deliver RNPs into cells and edit tissues such as muscles, brain, liver, and lungs | High editing efficiency reported (no quantitative data provided) | [22] | |
| Bioreducible LNPs with negative charge used in mammalian cells and rodent brain | Approximately 70% | [31] | |
| Nanoparticles with polyglutamic acid used in different types of T cells | Knockin efficiency of up to >50% | [13] | |
| LNPs delivering iGeoCas9 RNP (engineered thermostable Cas9) to liver and lung in vivo (mouse) | 16–37% in liver and 19% in lung | [32] | |
| Polyplex micelles (PEG–PLL) used for Cas9 mRNA/sgRNA delivery to mouse brain (intraparenchymal) | ~20% | [33] | |
| C14–PEI micelleplexes (non-coated) used for Cas9 mRNA/sgRNA delivery to KRAS-mutant lung cells | ~60% (T7EI), 48.5% (ddPCR) | [34] | |
| PEG–PLE/C14–PEI micelleplexes used for Cas9 mRNA/sgRNA delivery to KRAS-mutant lung cells | Up to 69% | [35] | |
| Mini enveloped delivery vehicles (EDVs) | Up to ~85% indels in HeLa and U2OS cells | [26] | |
| Gold nanoparticles used to correct mutations of Duchenne muscular dystrophy in different cell types | 5.4% of gene edited to wild type | [36] | |
| Nanoscale ZIFs | 37% reduction in gene expression | [37] |
2.2.1. Physically Mediated Delivery Methods
2.2.2. Virally Mediated Delivery Vehicles
2.2.3. Non-Virally Mediated Delivery Vehicles
3. Aggregation Behavior of Cas9 Protein
4. Encapsulation Efficiency of Cas9 Protein
| Delivery System | Cas9 Encapsulation Efficiency | Particle Size (Hydrodynamic) | Gene Editing Efficiency/Therapeutic Outcome | Ref. |
|---|---|---|---|---|
| Exosomes (native) | ~1% (low stochastic loading) | Not specified | Poor delivery, no editing data | [72] |
| Cas9 conjugated to a 12 nm gold nanoparticle | ~45 Cas9 proteins per particle (~6%) | ~23 ± 5 nm | Comparable to electroporation in reported assays | [74] |
| LNPs | Not reported numerically; varied with RNP ratio | ~100–200 nm (est.) | Up to 45.2% indels in IL-10 gene | [75] |
| LNPs | Not quantified | Not specified | ~3–3.5% HDR integration; 80% restoration of cystic fibrosis transmembrane conductance regulator chloride channel function | [77] |
| LNPs | Not reported (mRNA and sgRNA co-delivered) | 81–99 nm (PDI of 0.19–0.22) | 39.1% editing in liver; phenylalanine levels normalized within 48 h | [78] |
| LNPs | Not specified | Not reported | Exon skipping of ~90% in APP; ~70% reduction in Aβ42 in vivo | [79] |
| LNPs | Up to 98% depending on formulation | 112–176 nm (PDI of ~0.10–0.17) | 37% in liver, 19% in lung (in vivo); >90% in vitro (NPCs, HEK) | [32] |
| Virus-like particles | Not specified, but functional loading confirmed | ~100–200 nm | Knockout efficiency of 70–90% in vitro and 60–70% in primary human T cells | [76] |
| Gold nanoparticle aggregates | Surface clustering observed (variable density) | >40 nm in aggregated states | Decreased nuclear entry and editing when aggregation was not controlled | [74] |
| PEG–PLL polyplex micelles | Co-encapsulation of Cas9 mRNA and sgRNA confirmed; no quantitative encapsulation efficiency reported | ~30–35 nm | ~20% editing in neurons, astrocytes, and microglia after brain injection | [33] |
| C14–PEI micelleplexes | Encapsulation stability confirmed; some aggregation | ~140 nm | 60% (T7EI) adn 48.5% (ddPCR) in KRAS-mutant lung cells | [34] |
| PEG–PLE/C14–PEI micelleplexes | High stability, PEG shield reduced non-specific interactions | ~120 nm | Up to 69% indels; improved lung delivery | [35] |
| EDVs | ~35% of Cas9 remained intact post-packaging | ~120–140 nm (estimated) | Accelerated editing kinetics | [26] |
5. Clinical Translation Barriers: Manufacturing, Scalability, and Regulatory Considerations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAVs | Adeno-associated viruses |
| ABE | Adenine Base Editor |
| ADGNs | Artificially Designed Grafting Nanoparticles |
| ATMPs | Advanced Therapy Medicinal Products |
| Cas9 | CRISPR-associated protein 9 |
| CDK11 | Cyclin-dependent kinase 11 |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CPP | Cell-Penetrating Peptide |
| CRISPR | Clustered regulatory interspaced short palindromic repeats |
| ddPCR | Droplet digital PCR |
| DLS | Dynamic light scattering |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphorylethanolamine |
| EDVs | Mini enveloped delivery vehicles |
| EMA | European Medicines Agency |
| FCS | Fluorescence correlation spectroscopy |
| FDA | Food and Drug Administration |
| gRNA | Guide RNA |
| HDR | Homology-Directed Repair |
| iPSCs | Induced pluripotent stem cells |
| LNPs | Lipid nanoparticles |
| mRNA | Messenger RNA |
| NPCs | Neural Progenitor Cells |
| PBS | Phosphate-Buffered Saline |
| PDI | Polydispersity Index |
| pDNA | Plasmid DNA |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PMs | Polyplex micelles |
| RNP | Ribonucleoprotein |
| SORT | Selective Organ Targeting |
| ZIFs | Zeolitic imidazolate frameworks |
References
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA Interrogation by the CRISPR RNA-Guided Endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Feng, Q.; Wang, N.; Chen, Z.; Huang, Y.; Zheng, W.; Jiang, X. Lipid Nanoparticle-Mediated Efficient Delivery of CRISPR/Cas9 for Tumor Therapy. NPG Asia Mater. 2017, 9, e441. [Google Scholar] [CrossRef]
- Du, Y.; Liu, Y.; Hu, J.; Peng, X.; Liu, Z. CRISPR/Cas9 Systems: Delivery Technologies and Biomedical Applications. Asian J. Pharm. Sci. 2023, 18, 100854. [Google Scholar] [CrossRef]
- Sinclair, F.; Begum, A.A.; Dai, C.C.; Toth, I.; Moyle, P.M. Recent Advances in the Delivery and Applications of Nonviral CRISPR/Cas9 Gene Editing. Drug Deliv. Transl. Res. 2023, 13, 1500–1519. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, P.; Yu, S.-Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle Delivery of CRISPR/Cas9 for Genome Editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; De La Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Singh, A.; Irfan, H.; Fatima, E.; Nazir, Z.; Verma, A.; Akilimali, A. Revolutionary Breakthrough: FDA Approves CASGEVY, the First CRISPR/Cas9 Gene Therapy for Sickle Cell Disease. Ann. Med. Surg. 2024, 86, 4555–4559. [Google Scholar] [CrossRef]
- Toral, M.A.; Charlesworth, C.T.; Ng, B.; Chemudupati, T.; Homma, S.; Nakauchi, H.; Bassuk, A.G.; Porteus, M.H.; Mahajan, V.B. Investigation of Cas9 Antibodies in the Human Eye. Nat. Commun. 2022, 13, 1053. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, C.T.; Deshpande, P.S.; Dever, D.P.; Camarena, J.; Lemgart, V.T.; Cromer, M.K.; Vakulskas, C.A.; Collingwood, M.A.; Zhang, L.; Bode, N.M.; et al. Identification of Preexisting Adaptive Immunity to Cas9 Proteins in Humans. Nat. Med. 2019, 25, 249–254. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Roth, T.L.; Li, P.J.; Chen, P.A.; Apathy, R.; Mamedov, M.R.; Vo, L.T.; Tobin, V.R.; Goodman, D.; Shifrut, E.; et al. Polymer-Stabilized Cas9 Nanoparticles and Modified Repair Templates Increase Genome Editing Efficiency. Nat. Biotechnol. 2020, 38, 44–49. [Google Scholar] [CrossRef]
- den Engelsman, J.; Garidel, P.; Smulders, R.; Koll, H.; Smith, B.; Bassarab, S.; Seidl, A.; Hainzl, O.; Jiskoot, W. Strategies for the Assessment of Protein Aggregates in Pharmaceutical Biotech Product Development. Pharm. Res. 2011, 28, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Pukala, T.L. Mass Spectrometric Insights into Protein Aggregation. Essays Biochem. 2023, 67, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Wu, Q.; Gong, C. Nanotechnology-Based CRISPR/Cas9 Delivery System for Genome Editing in Cancer Treatment. MedComm—Biomater. Appl. 2024, 3, e70. [Google Scholar] [CrossRef]
- Li, L.; Hu, S.; Chen, X. Non-Viral Delivery Systems for CRISPR/Cas9-Based Genome Editing: Challenges and Opportunities. Biomaterials 2018, 171, 207–218. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Myhre, J.L.; Chen, S.; Tam, Y.Y.C.; Danescu, A.; Richman, J.M.; Cullis, P.R. Design of Lipid Nanoparticles for in Vitro and in Vivo Delivery of Plasmid DNA. Nanomedicine 2017, 13, 1377–1387. [Google Scholar] [CrossRef]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.-Y.; Liu, D.R. Cationic Lipid-Mediated Delivery of Proteins Enables Efficient Protein-Based Genome Editing in Vitro and in Vivo. Nat. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Liu, J.; Chang, J.; Jiang, Y.; Meng, X.; Sun, T.; Mao, L.; Xu, Q.; Wang, M. Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles. Adv. Mater. 2019, 31, e1902575. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Cheng, Q.; Min, Y.-L.; Olson, E.N.; Siegwart, D.J. Systemic Nanoparticle Delivery of CRISPR-Cas9 Ribonucleoproteins for Effective Tissue Specific Genome Editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef]
- Lotfi, M.; Morshedi Rad, D.; Mashhadi, S.S.; Ashouri, A.; Mojarrad, M.; Mozaffari-Jovin, S.; Farrokhi, S.; Hashemi, M.; Lotfi, M.; Ebrahimi Warkiani, M.; et al. Recent Advances in CRISPR/Cas9 Delivery Approaches for Therapeutic Gene Editing of Stem Cells. Stem Cell Rev. Rep. 2023, 19, 2576–2596. [Google Scholar] [CrossRef]
- Chen, S.; Jiao, Y.; Pan, F.; Guan, Z.; Cheng, S.H.; Sun, D. Knock-In of a Large Reporter Gene via the High-Throughput Microinjection of the CRISPR/Cas9 System. IEEE Trans. Biomed. Eng. 2022, 69, 2524–2532. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takemoto, T. Electroporation Enables the Efficient mRNA Delivery into the Mouse Zygotes and Facilitates CRISPR/Cas9-Based Genome Editing. Sci. Rep. 2015, 5, 11315. [Google Scholar] [CrossRef]
- Karp, H.; Zoltek, M.; Wasko, K.; Vazquez, A.L.; Brim, J.; Ngo, W.; Schepartz, A.; Doudna, J.A. Packaged Delivery of CRISPR–Cas9 Ribonucleoproteins Accelerates Genome Editing. Nucleic Acids Res. 2025, 56, gkaf105. [Google Scholar] [CrossRef] [PubMed]
- Moffa, J.C.; Bland, I.N.; Tooley, J.R.; Kalyanaraman, V.; Heitmeier, M.; Creed, M.C.; Copits, B.A. Cell Specific Single Viral Vector CRISPR/Cas9 Editing and Genetically Encoded Tool Delivery in the Central and Peripheral Nervous Systems. bioRxiv 2023. [Google Scholar] [CrossRef]
- Waehler, R.; Russell, S.J.; Curiel, D.T. Engineering Targeted Viral Vectors for Gene Therapy. Nat. Rev. Genet. 2007, 8, 573–587. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering Adeno-Associated Virus Vectors for Gene Therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Follenzi, A.; Sabatino, G.; Lombardo, A.; Boccaccio, C.; Naldini, L. Efficient Gene Delivery and Targeted Expression to Hepatocytes In Vivo by Improved Lentiviral Vectors. Hum. Gene Ther. 2002, 13, 243–260. [Google Scholar] [CrossRef]
- Wang, M.; Zuris, J.A.; Meng, F.; Rees, H.; Sun, S.; Deng, P.; Han, Y.; Gao, X.; Pouli, D.; Wu, Q.; et al. Efficient Delivery of Genome-Editing Proteins Using Bioreducible Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 2868–2873. [Google Scholar] [CrossRef]
- Chen, K.; Han, H.; Zhao, S.; Xu, B.; Yin, B.; Lawanprasert, A.; Trinidad, M.; Burgstone, B.W.; Murthy, N.; Doudna, J.A. Lung and Liver Editing by Lipid Nanoparticle Delivery of a Stable CRISPR–Cas9 Ribonucleoprotein. Nat. Biotechnol. 2024, ahead of print. [Google Scholar] [CrossRef]
- Abbasi, S.; Uchida, S.; Toh, K.; Tockary, T.A.; Dirisala, A.; Hayashi, K.; Fukushima, S.; Kataoka, K. Co-Encapsulation of Cas9 mRNA and Guide RNA in Polyplex Micelles Enables Genome Editing in Mouse Brain. J. Control. Release 2021, 332, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Triki, M.; Pinto Carneiro, S.; Merkel, O.M. A Novel Micelleplex for Tumour-Targeted Delivery of CRISPR-Cas9 against KRAS-Mutated Lung Cancer. Nanoscale 2025, 17, 6604–6619. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pinto Carneiro, S.; Merkel, O.M. Anionic Polymer Coating for Enhanced Delivery of Cas9 mRNA and sgRNA Nanoplexes. Biomater. Sci. 2025, 11, 659–676. [Google Scholar] [CrossRef]
- Lee, K.; Conboy, M.; Park, H.M.; Jiang, F.; Kim, H.J.; Dewitt, M.A.; Mackley, V.A.; Chang, K.; Rao, A.; Skinner, C.; et al. Nanoparticle Delivery of Cas9 Ribonucleoprotein and Donor DNA in Vivo Induces Homology-Directed DNA Repair. Nat. Biomed. Eng. 2017, 1, 889–901. [Google Scholar] [CrossRef]
- Alsaiari, S.K.; Patil, S.; Alyami, M.; Alamoudi, K.O.; Aleisa, F.A.; Merzaban, J.S.; Li, M.; Khashab, N.M. Endosomal Escape and Delivery of CRISPR/Cas9 Genome Editing Machinery Enabled by Nanoscale Zeolitic Imidazolate Framework. J. Am. Chem. Soc. 2018, 140, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.A.; Permyakov, O.A.; Grigorieva, O.O.; Starshin, A.S.; Mazur, A.M.; Prokhortchouk, E.B.; Dontsova, O.A.; Sergiev, P.V. Comparative Analysis of Genome Editors Efficiency on a Model of Mice Zygotes Microinjection. Int. J. Mol. Sci. 2021, 22, 10221. [Google Scholar] [CrossRef]
- Chen, L.-N.; Fan, X.-Y.; Liu, Y.-T.; Chen, S.-Q.; Xie, F.-Y.; Zeng, L.; Wen, J.; Li, J.; Ma, J.-Y.; Ou, X.-H.; et al. High-Survival Rate After Microinjection of Mouse Oocytes and Early Embryos With mRNA by Combining a Tip Pipette and Piezoelectric-Assisted Micromanipulator. Front. Cell Dev. Biol. 2021, 9, 735971. [Google Scholar] [CrossRef]
- Niola, F.; Dagnæs-Hansen, F.; Frödin, M. In Vivo Editing of the Adult Mouse Liver Using CRISPR/Cas9 and Hydrodynamic Tail Vein Injection. Methods Mol. Biol. 2019, 1961, 329–341. [Google Scholar] [CrossRef]
- Kanefuji, T.; Yokoo, T.; Suda, T.; Abe, H.; Kamimura, K.; Liu, D. Hemodynamics of a Hydrodynamic Injection. Mol. Ther.- Methods Clin. Dev. 2014, 1, 14029. [Google Scholar] [CrossRef]
- Goodwin, T.; Huang, L. Nonviral Vectors: We Have Come a Long Way. Adv. Genet. 2014, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Gao, C.; Zheng, Y.-M.; Yi, L.; Lu, J.-C.; Huang, X.-Y.; Cai, J.-B.; Zhang, P.-F.; Cui, Y.-H.; Ke, A.-W. Current Applications and Future Perspective of CRISPR/Cas9 Gene Editing in Cancer. Mol. Cancer 2022, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Breyer, B.; Jiang, W.; Cheng, H.; Haydon, R.; Zhou, L.; Feng, T.; Ishikawa, A.; He, T.-C. Development and Use of Viral Vectors for Gene Transfer: Lessons from Their Applications in Gene Therapy. Anat. Rec. 2001, 263, 4–17. [Google Scholar]
- Walther, W.; Stein, U. Viral Vectors for Gene Transfer. A Rev. Their Use Treat. Hum. Dis. Drugs 2000, 60, 249–271. [Google Scholar] [CrossRef]
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef]
- Warrington, K.H.; Herzog, R.W. Treatment of Human Disease by Adeno-Associated Viral Gene Transfer. Hum. Genet. 2006, 119, 571–603. [Google Scholar] [CrossRef]
- Schauber, C.A.; Tuerk, M.J.; Pacheco, C.D.; Escarpe, P.A.; Veres, G. Lentiviral Vectors Pseudotyped with Baculovirus Gp64 Efficiently Transduce Mouse Cells in Vivo and Show Tropism Restriction against Hematopoietic Cell Types in Vitro. Gene Ther. 2004, 11, 266–275. [Google Scholar] [CrossRef]
- Coroadinha, A.S. Host Cell Restriction Factors Blocking Efficient Vector Transduction: Challenges in Lentiviral and Adeno-Associated Vector Based Gene Therapies. Cells 2023, 12, 732. [Google Scholar] [CrossRef]
- Xie, L.; Han, Y.; Liu, Y.; Zhou, Y.; Yu, J.; Von Brunn, A.; Lei, J. Viral Vector-based Cancer Treatment and Current Clinical Applications. MedComm–Oncology 2023, 2, e55. [Google Scholar] [CrossRef]
- Nasimuzzaman, M. Viral Vectors for Gene Therapy of Genetic Diseases: Challenges and Prospects. J. Hum. Virol. Retrovirol. 2014, 2, 00048. [Google Scholar] [CrossRef][Green Version]
- Miao, C.H.; Nakai, H.; Thompson, A.R.; Storm, T.A.; Chiu, W.; Snyder, R.O.; Kay, M.A. Nonrandom Transduction of Recombinant Adeno-Associated Virus Vectors in Mouse Hepatocytes In Vivo: Cell Cycling Does Not Influence Hepatocyte Transduction. J. Virol. 2000, 74, 3793–3803. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Pan, Y.-C.; Hsiao, Y.-H.; Lim, S.-K.; Cheng, T.-W.; Huang, S.-W.; Wu, S.M.-Y.; Sun, C.-P.; Tao, M.-H.; Mou, K.Y. Improvement of Gene Delivery by Minimal Bacteriophage Particles. ACS Nano 2023, 17, 14532–14544. [Google Scholar] [CrossRef] [PubMed]
- Bezeljak, U. Cancer Gene Therapy Goes Viral: Viral Vector Platforms Come of Age. Radiol. Oncol. 2022, 56, 1–13. [Google Scholar] [CrossRef]
- Dudek, A.M.; Porteus, M.H. Answered and Unanswered Questions in Early-Stage Viral Vector Transduction Biology and Innate Primary Cell Toxicity for Ex-Vivo Gene Editing. Front. Immunol. 2021, 12, 660302. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of Lipid-Based Nanoparticles for Delivery of Therapeutic Nucleic Acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, Y.; Lim, S.; Jang, H.-K.; Kim, H.-O. Advances in Nanoparticles as Non-Viral Vectors for Efficient Delivery of CRISPR/Cas9. Pharmaceutics 2024, 16, 1197. [Google Scholar] [CrossRef] [PubMed]
- Miteva, M.; Kirkbride, K.C.; Kilchrist, K.V.; Werfel, T.A.; Li, H.; Nelson, C.E.; Gupta, M.K.; Giorgio, T.D.; Duvall, C.L. Tuning PEGylation of Mixed Micelles to Overcome Intracellular and Systemic siRNA Delivery Barriers. Biomaterials 2015, 38, 97–107. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Sun, Y.; Yu, X.; Lee, S.M.; Cheng, Q.; Wei, T.; Gong, J.; Robinson, J.; Zhang, D.; et al. Preparation of Selective Organ-Targeting (SORT) Lipid Nanoparticles (LNPs) Using Multiple Technical Methods for Tissue-Specific mRNA Delivery. Nat. Protoc. 2023, 18, 265–291. [Google Scholar] [CrossRef]
- Su, K.; Shi, L.; Sheng, T.; Yan, X.; Lin, L.; Meng, C.; Wu, S.; Chen, Y.; Zhang, Y.; Wang, C.; et al. Reformulating Lipid Nanoparticles for Organ-Targeted mRNA Accumulation and Translation. Nat. Commun. 2024, 15, 5659. [Google Scholar] [CrossRef]
- Guzman Gonzalez, V.; Grunenberger, A.; Nicoud, O.; Czuba, E.; Vollaire, J.; Josserand, V.; Le Guével, X.; Desai, N.; Coll, J.-L.; Divita, G.; et al. Enhanced CRISPR-Cas9 RNA System Delivery Using Cell Penetrating Peptides-Based Nanoparticles for Efficient in Vitro and in Vivo Applications. J. Control. Release 2024, 376, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-Y.; He, X.-Y.; Zhuo, R.-X.; Cheng, S.-X. Tumor Targeted Genome Editing Mediated by a Multi-Functional Gene Vector for Regulating Cell Behaviors. J. Control. Release 2018, 291, 90–98. [Google Scholar] [CrossRef]
- Sun, W.; Ji, W.; Hall, J.M.; Hu, Q.; Wang, C.; Beisel, C.L.; Gu, Z. Self-Assembled DNA Nanoclews for the Efficient Delivery of CRISPR-Cas9 for Genome Editing. Angew. Chem. Int. Ed. Engl. 2015, 54, 12029–12033. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-C.; Samanta, B.; Agasti, S.S.; Jeong, Y.; Zhu, Z.-J.; Rana, S.; Miranda, O.R.; Rotello, V.M. Drug Delivery Using Nanoparticle-Stabilized Nanocapsules. Angew. Chem. Int. Ed. Engl. 2011, 50, 477–481. [Google Scholar] [CrossRef]
- Alyami, M.Z.; Alsaiari, S.K.; Li, Y.; Qutub, S.S.; Aleisa, F.A.; Sougrat, R.; Merzaban, J.S.; Khashab, N.M. Cell-Type-Specific CRISPR/Cas9 Delivery by Biomimetic Metal Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Xu, L.; Iqbal, Z.; Ouyang, K.; Zhang, H.; Wen, C.; Duan, L.; Xia, J. Chondrocyte-Specific Genomic Editing Enabled by Hybrid Exosomes for Osteoarthritis Treatment. Theranostics 2022, 67, 4866–4878. [Google Scholar] [CrossRef]
- Bzhilyanskaya, V.; Ma, L.; Liu, S.; Fox, L.R.; Whittaker, M.N.; Meis, R.J.; Choi, U.; Lawson, A.; Ma, M.; Theobald, N.; et al. High-Fidelity PAMless Base Editing of Hematopoietic Stem Cells to Treat Chronic Granulomatous Disease. Sci. Transl. Med. 2024, 14, eadj6779. [Google Scholar] [CrossRef]
- Novo, M.; Pérez-González, C.; Freire, S.; Al-Soufi, W. Early Aggregation of Amyloid-β(1–42) Studied by Fluorescence Correlation Spectroscopy. In Protein Aggregation; Cieplak, A.S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2023; Volume 2551, pp. 1–14. ISBN 978-1-07-162596-5. [Google Scholar]
- Novo, M.; Freire, S.; Al-Soufi, W. Critical Aggregation Concentration for the Formation of Early Amyloid-β (1-42) Oligomers. Sci. Rep. 2018, 8, 1783. [Google Scholar] [CrossRef]
- Manzano, I.; Taylor, N.; Csordas, M.; Vezeau, G.E.; Salis, H.M.; Zydney, A.L. Purification of Cas9—RNA Complexes by Ultrafiltration. Biotechnol. Prog. 2021, 37, e3104. [Google Scholar] [CrossRef]
- Camperi, J.; Moshref, M.; Dai, L.; Lee, H.Y. Physicochemical and Functional Characterization of Differential CRISPR-Cas9 Ribonucleoprotein Complexes. Anal. Chem. 2022, 94, 1432–1440. [Google Scholar] [CrossRef]
- Ponomareva, N.I.; Brezgin, S.A.; Kostyusheva, A.P.; Slatinskaya, O.V.; Bayurova, E.O.; Gordeychuk, I.V.; Maksimov, G.V.; Sokolova, D.V.; Babaeva, G.; Khan, I.I.; et al. Stochastic Packaging of Cas Proteins into Exosomes. Mol. Biol. 2024, 58, 147–156. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Wu, X.; Zhao, Y.; Liu, Y. Cascade-Responsive Nanoparticles for Efficient CRISPR/Cas9-Based Glioblastoma Gene Therapy. ACS Appl. Mater. Interfaces 2025, 17, 4480–4489. [Google Scholar] [CrossRef]
- Konstantinidou, S.; Lindstaedt, A.; Schmidt, T.J.N.; Nocilla, F.; Maltinti, G.; Rocco, M.A.; Landi, E.; De Carli, A.; Crucitta, S.; Lai, M.; et al. A Transfection-Free Approach of Gene Editing via a Gold-Based Nanoformulation of the Cas9 Protein. bioRxiv 2024. [Google Scholar] [CrossRef]
- Im, S.H.; Jang, M.; Park, J.-H.; Chung, H.J. Finely Tuned Ionizable Lipid Nanoparticles for CRISPR/Cas9 Ribonucleoprotein Delivery and Gene Editing. J. Nanobiotechnol. 2024, 22, 175. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, S.E.; Shepelev, M.V.; Mazurov, D.V.; Kruglova, N.A. Efficient Genome Editing Using ‘NanoMEDIC’ AsCas12a-VLPs Produced with Pol II-Transcribed crRNA. Int. J. Mol. Sci. 2024, 25, 12768. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Manufacturing Changes and Comparability for Human Cellular and Gene Therapy Products; Draft Guidance for Industry; FDA: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/media/170198/download (accessed on 5 April 2025).
- Brooks, D.L.; Whittaker, M.N.; Said, H.; Dwivedi, G.; Qu, P.; Musunuru, K.; Ahrens-Nicklas, R.C.; Alameh, M.-G.; Wang, X. A Base Editing Strategy Using mRNA-LNPs for in Vivo Correction of the Most Frequent Phenylketonuria Variant. Hum. Genet. Genom. Adv. 2024, 5, 100253. [Google Scholar] [CrossRef] [PubMed]
- Miskalis, A.; Shirguppe, S.; Winter, J.; Elias, G.; Swami, D.; Nambiar, A.; Stilger, M.; Woods, W.S.; Gosstola, N.; Gapinske, M.; et al. SPLICER: A Highly Efficient Base Editing Toolbox That Enables In Vivo Therapeutic Exon Skipping. Nat. Commun. 2024, 15, 10354. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Quality, Non-Clinical and Clinical Aspects of Gene Therapy Medicinal Products; EMA/CAT/80183/2014; EMA: London, UK, 2018; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-clinical-aspects-gene-therapy-medicinal-products_en.pdf (accessed on 5 April 2025).
- European Medicines Agency. Nanotechnology-Based Medicinal Products for Human Use: EU Horizon Scanning Report; EMA: London, UK, 2025; Available online: https://www.ema.europa.eu/en/documents/report/nanotechnology-based-medicinal-products-human-use-eu-horizon-scanning-report_en.pdf (accessed on 5 April 2025).
- European Medicines Agency. Guideline on the Follow-Up of Patients Administered with Gene Therapy Medicinal Products; EMA/CHMP/GTWP/60436/2007; EMA: London, UK, 2009; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-follow-patients-administered-gene-therapy-medicinal-products_en.pdf (accessed on 5 April 2025).
- Ossandon, H.; Armijo, N.; Vargas, C.; Repetto, G.M.; Espinoza, M.A. Challenges for Gene Therapy in the Financial Sustainability of Health Systems: A Scoping Review. Orphanet J. Rare Dis. 2024, 19, 243. [Google Scholar] [CrossRef]
- Foley, R.A.; Ayoub, P.G.; Sinha, V.; Juett, C.; Sanoyca, A.; Duggan, E.C.; Lathrop, L.E.; Bhatt, P.; Coote, K.; Illek, B.; et al. Lipid Nanoparticles for the Delivery of CRISPR/Cas9 Machinery to Enable Site-Specific Integration of CFTR and Mutation-Agnostic Disease Rescue. bioRxiv 2025. [Google Scholar] [CrossRef]
- Schubert, M.S.; Thommandru, B.; Woodley, J.; Turk, R.; Yan, S.; Kurgan, G.; McNeill, M.S.; Rettig, G.R. Optimized Design Parameters for CRISPR Cas9 and Cas12a Homology-Directed Repair. Sci. Rep. 2021, 11, 19482. [Google Scholar] [CrossRef]
- Chenouard, V.; Leray, I.; Tesson, L.; Remy, S.; Allan, A.; Archer, D.; Caulder, A.; Fortun, A.; Bernardeau, K.; Cherifi, Y.; et al. Excess of Guide RNA Reduces Knockin Efficiency and Drastically Increases On-Target Large Deletions. iScience 2023, 26, 106399. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Miao, Y.; Walker, R.C.; Jinek, M.; McCammon, J.A. CRISPR-Cas9 Conformational Activation as Elucidated from Enhanced Molecular Simulations. Proc. Natl. Acad. Sci. USA 2017, 114, 7260–7265. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Nishimasu, H.; Kodera, N.; Hirano, S.; Ando, T.; Uchihashi, T.; Nureki, O. Real-Space and Real-Time Dynamics of CRISPR-Cas9 Visualized by High-Speed Atomic Force Microscopy. Nat. Commun. 2017, 8, 1430. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seijas, A.; Cora, D.; Novo, M.; Al-Soufi, W.; Sánchez, L.; Arana, Á.J. CRISPR/Cas9 Delivery Systems to Enhance Gene Editing Efficiency. Int. J. Mol. Sci. 2025, 26, 4420. https://doi.org/10.3390/ijms26094420
Seijas A, Cora D, Novo M, Al-Soufi W, Sánchez L, Arana ÁJ. CRISPR/Cas9 Delivery Systems to Enhance Gene Editing Efficiency. International Journal of Molecular Sciences. 2025; 26(9):4420. https://doi.org/10.3390/ijms26094420
Chicago/Turabian StyleSeijas, Ana, Diego Cora, Mercedes Novo, Wajih Al-Soufi, Laura Sánchez, and Álvaro J. Arana. 2025. "CRISPR/Cas9 Delivery Systems to Enhance Gene Editing Efficiency" International Journal of Molecular Sciences 26, no. 9: 4420. https://doi.org/10.3390/ijms26094420
APA StyleSeijas, A., Cora, D., Novo, M., Al-Soufi, W., Sánchez, L., & Arana, Á. J. (2025). CRISPR/Cas9 Delivery Systems to Enhance Gene Editing Efficiency. International Journal of Molecular Sciences, 26(9), 4420. https://doi.org/10.3390/ijms26094420







