Topological Distribution of the Sex Hormone Receptor Expressions Highlights the Importance of Stromal ERα and Epithelial PR in Malignant Transformation of the Uterine Cervix
Abstract
1. Introduction
2. Results
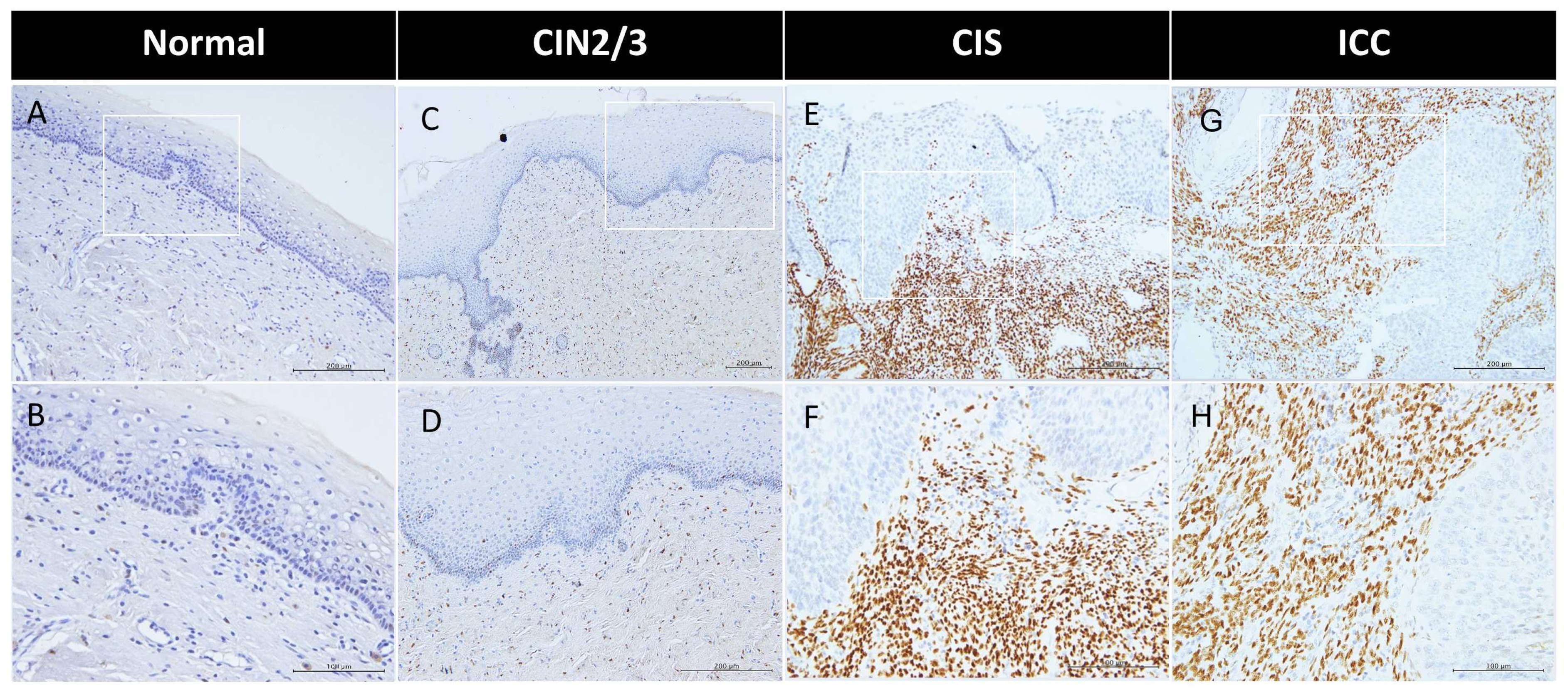
2.1. Predominant Expression of Sex Hormone Receptors in the Stroma of the Cervix with Progressive ERα Increase During Malignant Transformation
2.2. Downregulation of PRs in the Epithelium During CIS to ICC Transition
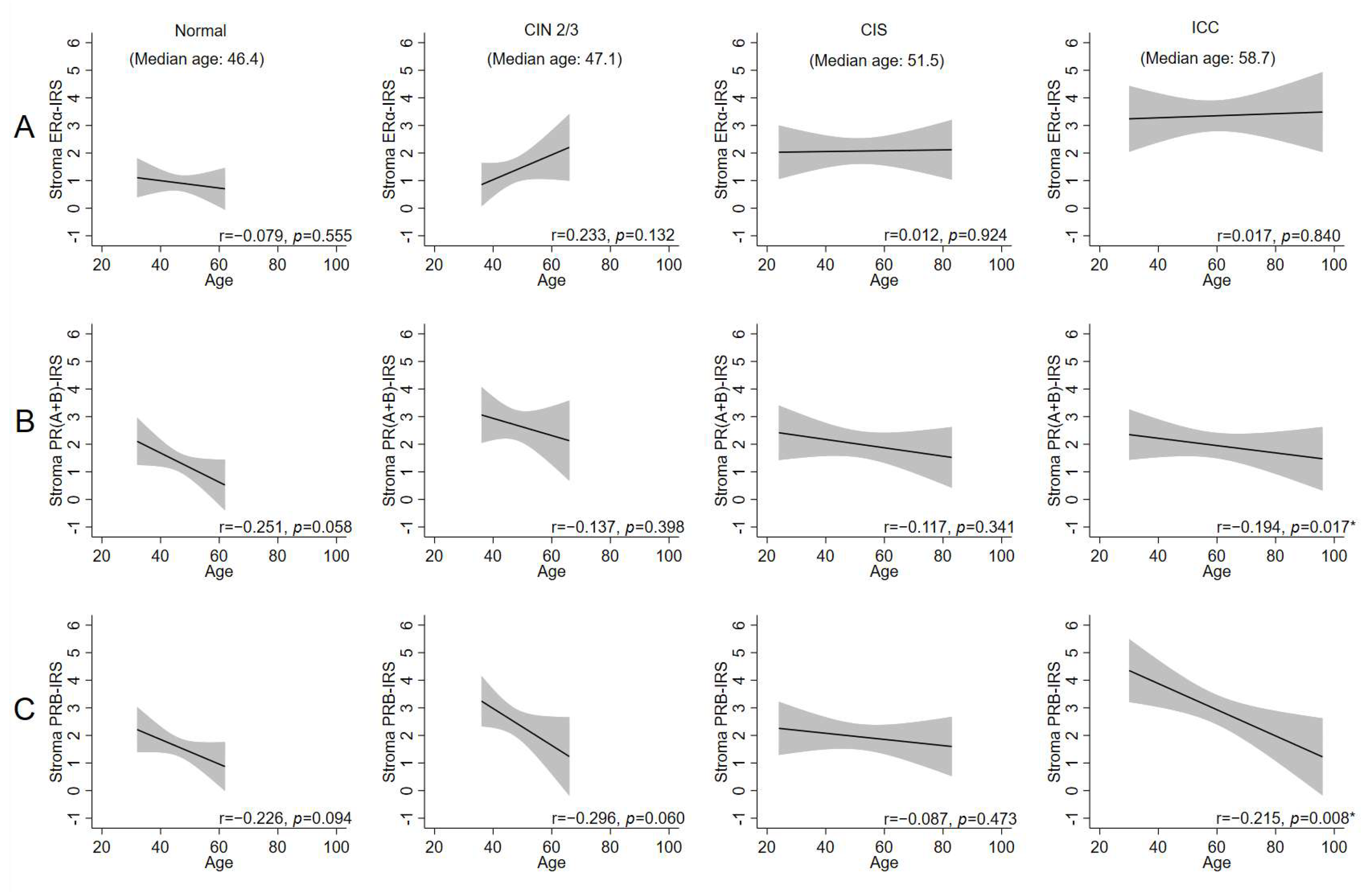
2.3. Age-Related Decrease in Stromal PRs Across Disease States, Except in CIS
2.4. Disruption of ERα–PR Expressional Correlation During CIN2/3 of Cervical Carcinogenesis
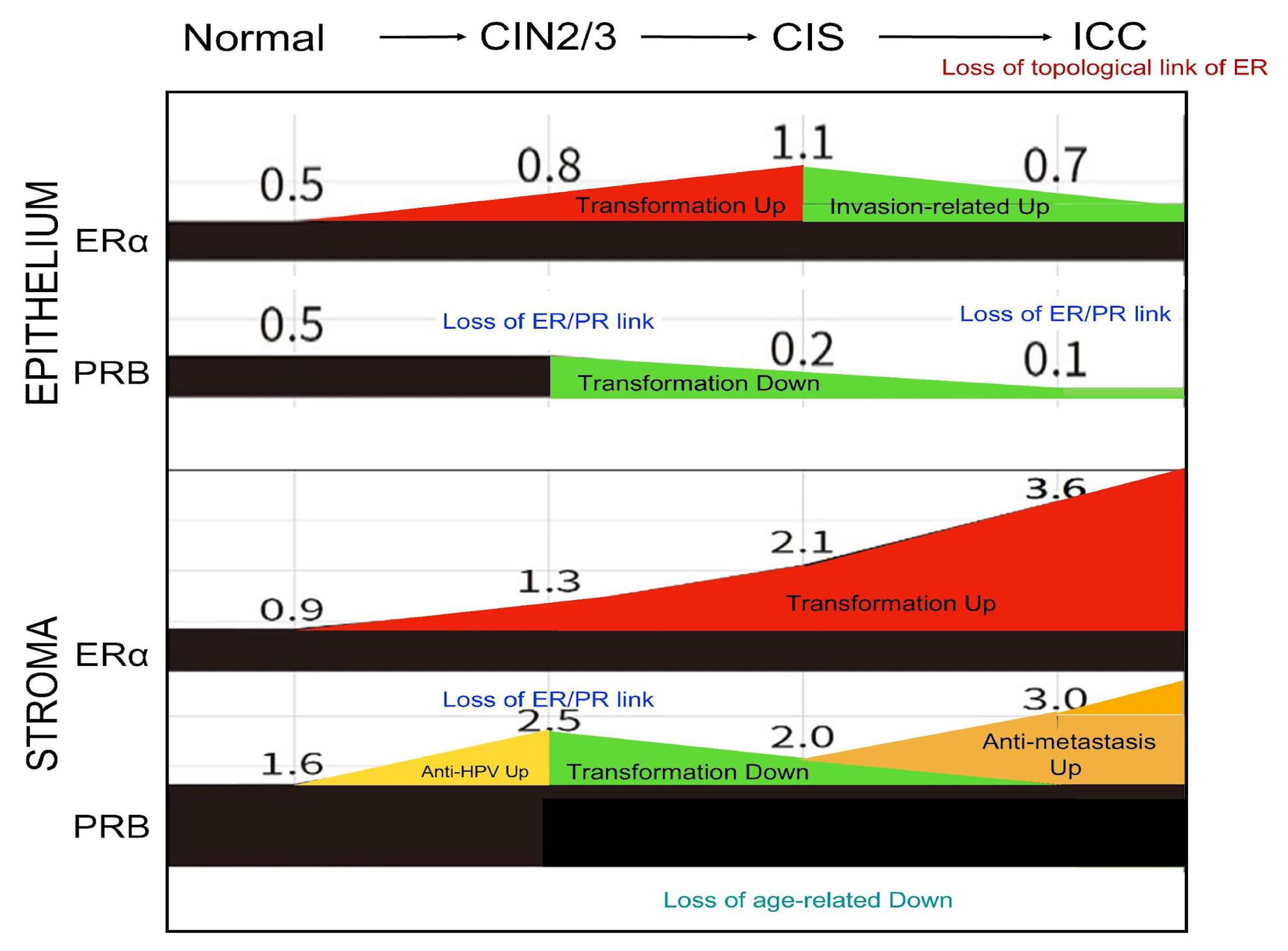
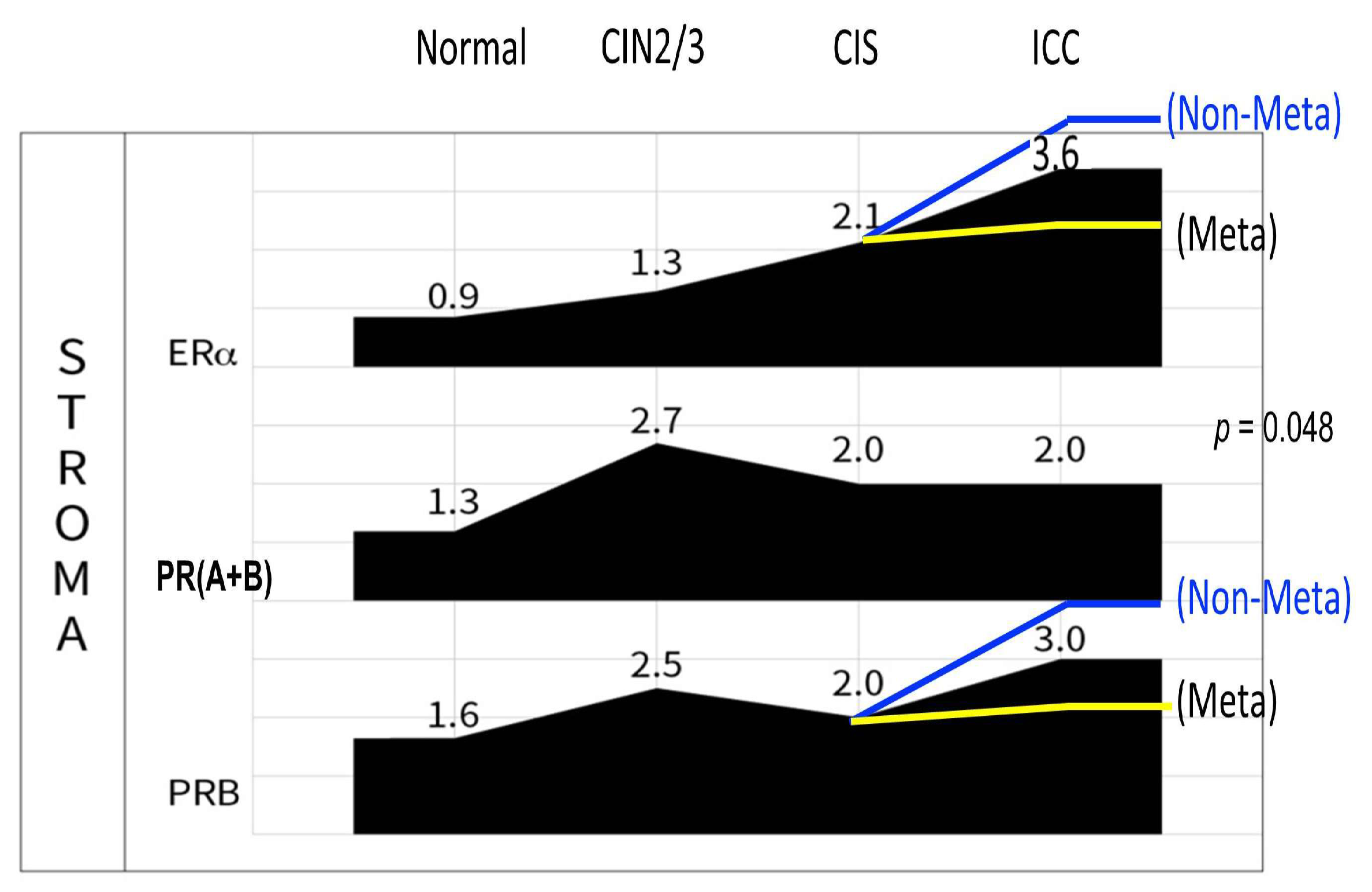
2.5. Disruption of the Topological Epithelium–Stroma Association of Sex Hormone Receptor Expression During Malignant Transformation
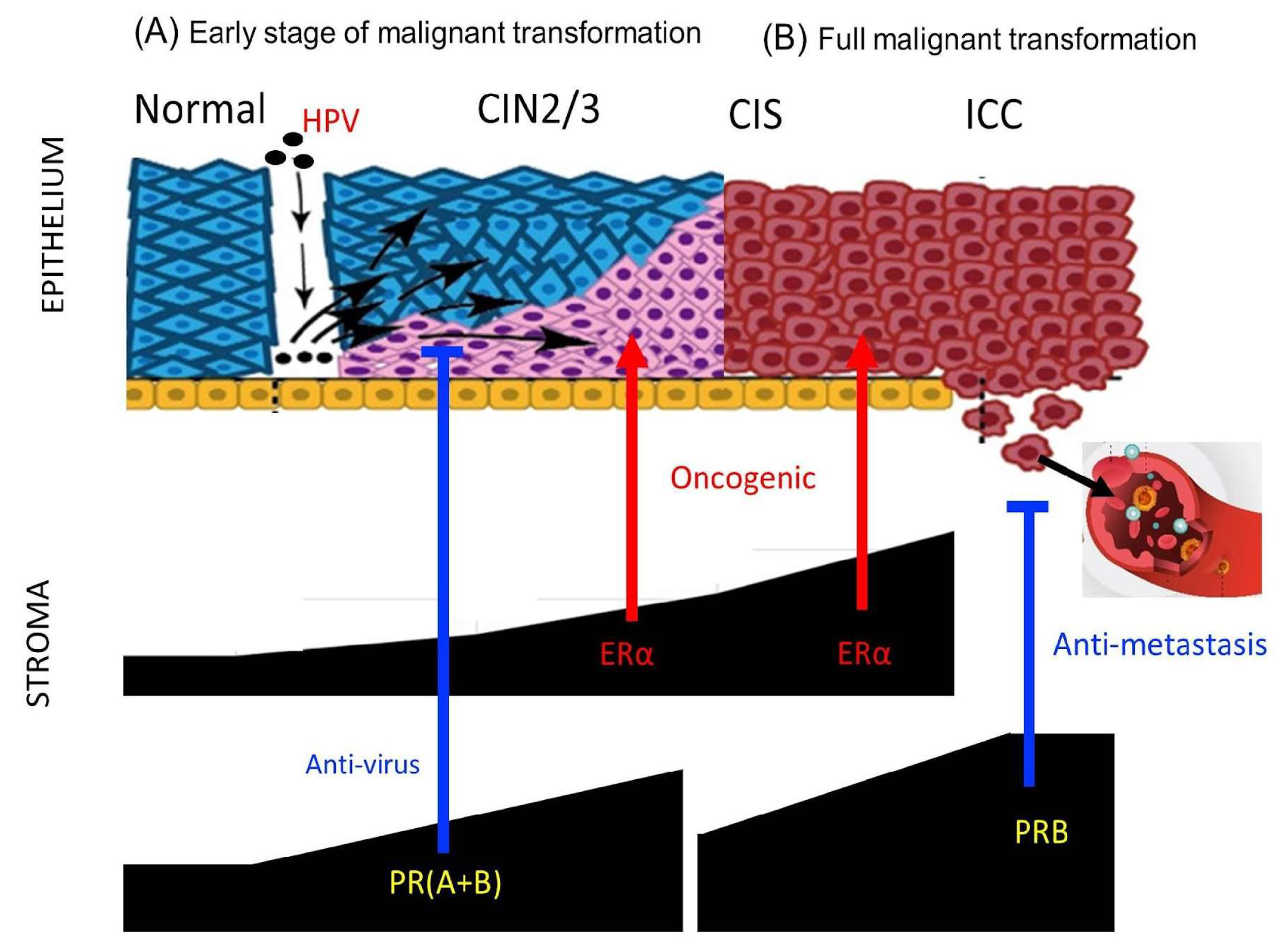
3. Discussion
4. Materials and Methods
4.1. The Patient Samples
4.2. Immunohistochemistry (IHC)
4.3. Staining Evaluation
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERα | estrogen receptor alpha |
| PR | progesterone receptor |
| PRB | progesterone receptor isoform B |
| PR(A+B) | progesterone receptor isoform A and B |
| CIS | carcinoma in situ |
| CIN2/3 | cervical intraepithelial neoplasia grade 2 and 3 |
| FFPE | formalin-fixed paraffin-embedded |
| IHC | immunohistochemistry |
| IRS | immunoreactive score |
| ICC | invasive cervical carcinoma |
References
- Plummer, M.; Peto, J.; Franceschi, S.; on behalf of the International Collaboration of Epidemiological Studies of Cervical Cancer. Time since first sexual intercourse and the risk of cervical cancer. Int. J. Cancer 2012, 130, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: Collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int. J. Cancer 2006, 119, 1108–1124. [Google Scholar] [CrossRef] [PubMed]
- Mosny, D.S.; Herholz, J.; Degen, W.; Bender, H.G. Immunohistochemical investigations of steroid receptors in normal and neoplastic squamous epithelium of the uterine cervix. Gynecol. Oncol. 1989, 35, 373–377. [Google Scholar] [CrossRef]
- Scharl, A.; Vierbuchen, M.; Graupner, J.; Fischer, R.; Bolte, A. Immunohistochemical study of distribution of estrogen receptors in corpus and cervix uteri. Arch. Gynecol. Obstet. 1988, 241, 221–233. [Google Scholar] [CrossRef]
- Nonogaki, H.; Fujii, S.; Konishi, I.; Nanbu, Y.; Ozaki, S.; Ishikawa, Y.; Mori, T. Estrogen receptor localization in normal and neoplastic epithelium of the uterine cervix. Cancer 1990, 66, 2620–2627. [Google Scholar] [CrossRef]
- Bodner, K.; Laubichler, P.; Kimberger, O.; Czerwenka, K.; Zeillinger, R.; Bodner-Adler, B. Oestrogen and progesterone receptor expression in patients with adenocarcinoma of the uterine cervix and correlation with various clinicopathological parameters. Anticancer Res. 2010, 30, 1341–1345. [Google Scholar]
- Kwasniewska, A.; Postawski, K.; Gozdzicka-Jozefiak, A.; Kwasniewski, W.; Grywalska, E.; Zdunek, M.; Korobowicz, E. Estrogen and progesterone receptor expression in HPV-positive and HPV-negative cervical carcinomas. Oncol. Rep. 2011, 26, 153–160. [Google Scholar] [CrossRef][Green Version]
- Hong, M.-K.; Wang, J.-H.; Su, C.-C.; Li, M.-H.; Hsu, Y.-H.; Chu, T.-Y. Expression of Estrogen and Progesterone Receptor in Tumor Stroma Predicts Favorable Prognosis of Cervical Squamous Cell Carcinoma. Int. J. Gynecol. Cancer 2017, 27, 1247–1255. [Google Scholar] [CrossRef]
- Hong, M.-K.; Wang, J.-H.; Li, M.-H.; Su, C.-C.; Chu, T.-Y. Progesterone receptor isoform B in the stroma of squamous cervical carcinoma: An independent favorable prognostic marker correlating with hematogenous metastasis. Taiwan J. Obstet. Gynecol. 2024, 63, 853–860. [Google Scholar] [CrossRef]
- Chung, S.-H.; Wiedmeyer, K.; Shai, A.; Korach, K.S.; Lambert, P.F. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008, 68, 9928–9934. [Google Scholar] [CrossRef]
- Chung, S.-H.; Lambert, P.F. Prevention and treatment of cervical cancer in mice using estrogen receptor antagonists. Proc. Natl. Acad. Sci. USA 2009, 106, 19467–19472. [Google Scholar] [CrossRef] [PubMed]
- Flötotto, T.; Niederacher, D.; Hohmann, D.; Heimerzheim, T.; Dall, P.; Djahansouzi, S.; Bender, H.; Hanstein, B. Molecular mechanism of estrogen receptor (ER)alpha-specific, estradiol-dependent expression of the progesterone receptor (PR) B-isoform. J. Steroid Biochem. Mol. Biol. 2004, 88, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.; Mehta, F.F.; Chung, S.-H. Medroxyprogesterone Acetate Prevention of Cervical Cancer through Progesterone Receptor in a Human Papillomavirus Transgenic Mouse Model. Am. J. Pathol. 2019, 189, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Shabani, N.; Kuhn, C.; Kunze, S.; Schulze, S.; Mayr, D.; Dian, D.; Gingelmaier, A.; Schindlbeck, C.; Willgeroth, F.; Sommer, H. Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur. J. Cancer 2007, 43, 2434–2444. [Google Scholar] [CrossRef]
- Lenhard, M.; Tereza, L.; Heublein, S.; Ditsch, N.; Himsl, I.; Mayr, D.; Friese, K.; Jeschke, U. Steroid hormone receptor expression in ovarian cancer: Progesterone receptor B as prognostic marker for patient survival. BMC Cancer 2012, 12, 553. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]
- Chung, S.-H.; Shin, M.K.; Korach, K.S.; Lambert, P.F. Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm. Cancer 2013, 4, 50–59. [Google Scholar] [CrossRef]
- Su, S.; Hua, D.; Li, J.-P.; Zhang, X.-N.; Bai, L.; Cao, L.-B.; Guo, Y.; Zhang, M.; Dong, J.-Z.; Liang, X.-W.; et al. Modulation of innate immune response to viruses including SARS-CoV-2 by progesterone. Signal Transduct. Target. Ther. 2022, 7, 137. [Google Scholar] [CrossRef]
- Harris, T.G.; Miller, L.; Kulasingam, S.L.; Feng, Q.; Kiviat, N.B.; Schwartz, S.M.; Koutsky, L.A. Depot-medroxyprogesterone acetate and combined oral contraceptive use and cervical neoplasia among women with oncogenic human papillomavirus infection. Am. J. Obstet. Gynecol. 2009, 200, 489.e1–489.e8. [Google Scholar] [CrossRef]
- Cunha, G.R. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J. Exp. Zool. 1976, 196, 361–370. [Google Scholar] [CrossRef]
- Kurita, T.; Cooke, P.S.; Cunha, G.R. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev. Biol. 2001, 240, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Baik, S.; Ho, C.; Lin, C.-Y.; Chung, S.-H. Progesterone Receptor Is a Haploinsufficient Tumor-Suppressor Gene in Cervical Cancer. Mol. Cancer Res. 2021, 19, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Bommer, G.T.; Feng, Y.; Wiese, A.B.; Fearon, E.R.; Cho, K.R. Loss of estrogen receptor 1 enhances cervical cancer invasion. Am. J. Pathol. 2010, 177, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.H.; Ahrendt, H.; Lange, C.A. Progesterone induces progesterone receptor gene (PGR) expression via rapid activation of protein kinase pathways required for cooperative estrogen receptor alpha (ER) and progesterone receptor (PR) genomic action at ER/PR target genes. Steroids 2016, 114, 48–58. [Google Scholar] [CrossRef]
- Mote, P.A.; Johnston, J.F.; Manninen, T.; Tuohimaa, P.; Clarke, C.L. Detection of progesterone receptor forms A and B by immunohistochemical analysis. J. Clin. Pathol. 2001, 54, 624–630. [Google Scholar] [CrossRef]
- Fabris, V.; Abascal, M.F.; Giulianelli, S.; May, M.; Sequeira, G.R.; Jacobsen, B.; Lombès, M.; Han, J.; Tran, L.; Molinolo, A.; et al. Isoform specificity of progesterone receptor antibodies. J. Pathol. Clin. Res. 2017, 3, 227–233. [Google Scholar] [CrossRef]
- Tone, A.A.; Virtanen, C.; Shaw, P.; Brown, T.J. Decreased progesterone receptor isoform expression in luteal phase fallopian tube epithelium and high-grade serous carcinoma. Endocr. Relat. Cancer 2011, 18, 221–234. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sänger, J.; Baum, R.P.; Prasad, V.; Hommann, M. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 2012, 5, 187–194. [Google Scholar]


| Characteristic | Normal | CIN2/3 | CIS | ICC | p Value | p for Trend |
|---|---|---|---|---|---|---|
| Number | 58 | 44 | 70 | 159 | ||
| Age (n = 330) | 46.4 ± 6.4 | 47.1 ± 7.6 | 51.5 ± 15.1 | 58.7 ± 15.4 | <0.001 * | |
| Age Group (n = 330) | <0.001 * | <0.001 ** | ||||
| <50 y/o | 43 (74.1%) | 30 (68.2%) | 36 (51.4%) | 56 (35.4%) | ||
| ≥50 y/o | 15 (25.9%) | 14 (31.8%) | 34 (48.6%) | 102 (64.6%) | ||
| Parity Group (n = 256) | <0.001 * | <0.001 ** | ||||
| 0 | 8 (14.0%) | 4 (16.0%) | 0 (0.0%) | 5 (3.7%) | ||
| 1~2 | 29 (50.9%) | 14 (56.0%) | 8 (20.0%) | 26 (19.4%) | ||
| ≥3 | 20 (35.1%) | 7 (28.0%) | 32 (80.0%) | 103 (76.9%) | ||
| Epithelium | ||||||
| ERα expression, IRS (n = 313) | 0.5 ± 0.7 | 0.8 ± 1.2 | 1.1 ± 1.4 | 0.8 ± 1.7 | 0.132 | |
| positive rate (%) | 17/55 (30.9%) | 12/34 (35.3%) | 43/70 (61.4%) | 44/154 (28.6%) | <0.001 * | 0.574 ** |
| PR(A+B) expression, IRS (n = 307) | 0.3 ± 0.6 | 0.4 ± 0.7 | 0.1 ± 0.4 | 0.1 ± 0.6 | 0.012 * | |
| positive rate (%) | 11/54 (20.4%) | 11/37 (29.7%) | 6/68 (8.8%) | 6/148 (4.1%) | <0.001 * | <0.001 ** |
| PRB expression, IRS (n = 316) | 0.5 ± 0.9 | 0.5 ± 0.8 | 0.2 ± 0.6 | 0.1 ± 0.4 | <0.001 * | |
| positive rate (%) | 14/53 (26.4%) | 11/37 (29.7%) | 11/70 (15.7%) | 12/156 (7.7%) | <0.001 * | <0.001 ** |
| Stroma | ||||||
| ERα expression, IRS (n = 322) | 0.9 ± 1.1 | 1.3 ± 1.5 | 2.1 ± 1.9 | 3.6 ± 3.3 | <0.001 * | |
| positive rate (%) | 30/58 (51.7%) | 28/43 (65.1%) | 57/70 (81.4%) | 125/151 (82.8%) | <0.001 * | <0.001 ** |
| PR(A+B)expression, IRS (n = 317) | 1.3 ± 1.3 | 2.7 ± 1.7 | 2.0 ± 2.0 | 2.1 ± 2.3 | 0.009 * | |
| positive rate (%) | 38/58 (65.5%) | 36/40 (90.0%) | 51/68 (75.0%) | 98/151 (64.9%) | 0.012 * | 0.288 ** |
| PRB expression, IRS (n = 320) | 1.6 ± 1.3 | 2.5 ± 1.6 | 2.0 ± 1.9 | 3.2 ± 3.2 | <0.001 * | |
| positive rate (%) | 45/56 (80.4%) | 38/41 (92.7%) | 55/70 (78.6%) | 118/153 (77.1%) | 0.171 * | 0.247 ** |
| Group | Stroma ERα vs. PR(A+B) | Stroma ERα vs. PRB | Epithelium ERα vs. PR(A+B) | Epithelium ERα vs. PRB | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PCC | p Value | N | PCC | p Value | N | PCC | p Value | N | PCC | p Value | ||||
| Normal | 58 | 0.528 | <0.001 * | 56 | 0.589 | <0.001 * | 54 | 0.616 | <0.001 * | 53 | 0.555 | <0.001 * | |||
| CIN2/3 | 39 | 0.292 | 0.072 | 41 | 0.253 | 0.111 | 34 | 0.534 | 0.001 * | 33 | 0.335 | 0.057 | |||
| CIS | 68 | 0.607 | <0.001 * | 70 | 0.624 | <0.001 * | 68 | 0.288 | 0.017 * | 70 | 0.389 | 0.001 * | |||
| ICC | 142 | 0.467 | <0.001 * | 148 | 0.672 | <0.001 * | 145 | 0.047 | 0.572 | 151 | 0.101 | 0.219 | |||
| Overall | 307 | 0.458 | <0.001 * | 315 | 0.661 | <0.001 * | 301 | 0.177 | 0.002 * | 307 | 0.206 | <0.001 * | |||
| Group | Stroma vs. Epithelium ERα | Stroma vs. Epithelium PR(A+B) | Stroma vs. Epithelium PRB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PCC | p Value | N | PCC | p Value | N | PCC | p Value | |||
| Normal | 55 | 0.326 | 0.015 * | 54 | 0.478 | <0.001 * | 53 | 0.424 | 0.002 * | ||
| CIN2/3 | 34 | 0.373 | 0.030 * | 35 | 0.046 | 0.794 | 37 | 0.285 | 0.087 | ||
| CIS | 70 | 0.316 | 0.008 * | 68 | -0.056 | 0.652 | 70 | 0.337 | 0.004 * | ||
| ICC | 145 | 0.293 | <0.001 * | 146 | 0.056 | 0.499 | 147 | 0.024 | 0.773 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.-K.; Wang, J.-H.; Li, M.-H.; Su, C.-C.; Cheng, C.-H.; Chu, T.-Y. Topological Distribution of the Sex Hormone Receptor Expressions Highlights the Importance of Stromal ERα and Epithelial PR in Malignant Transformation of the Uterine Cervix. Int. J. Mol. Sci. 2025, 26, 4418. https://doi.org/10.3390/ijms26094418
Hong M-K, Wang J-H, Li M-H, Su C-C, Cheng C-H, Chu T-Y. Topological Distribution of the Sex Hormone Receptor Expressions Highlights the Importance of Stromal ERα and Epithelial PR in Malignant Transformation of the Uterine Cervix. International Journal of Molecular Sciences. 2025; 26(9):4418. https://doi.org/10.3390/ijms26094418
Chicago/Turabian StyleHong, Mun-Kun, Jen-Hung Wang, Ming-Hsun Li, Cheng-Chuan Su, Chiu-Hsuan Cheng, and Tang-Yuan Chu. 2025. "Topological Distribution of the Sex Hormone Receptor Expressions Highlights the Importance of Stromal ERα and Epithelial PR in Malignant Transformation of the Uterine Cervix" International Journal of Molecular Sciences 26, no. 9: 4418. https://doi.org/10.3390/ijms26094418
APA StyleHong, M.-K., Wang, J.-H., Li, M.-H., Su, C.-C., Cheng, C.-H., & Chu, T.-Y. (2025). Topological Distribution of the Sex Hormone Receptor Expressions Highlights the Importance of Stromal ERα and Epithelial PR in Malignant Transformation of the Uterine Cervix. International Journal of Molecular Sciences, 26(9), 4418. https://doi.org/10.3390/ijms26094418






