Effective Restoration of Gastric and Esophageal Tissues in an In Vitro Model of GERD: Mucoadhesive and Protective Properties of Xyloglucan, Pea Proteins, and Polyacrylic Acid
Abstract
1. Introduction
2. Results
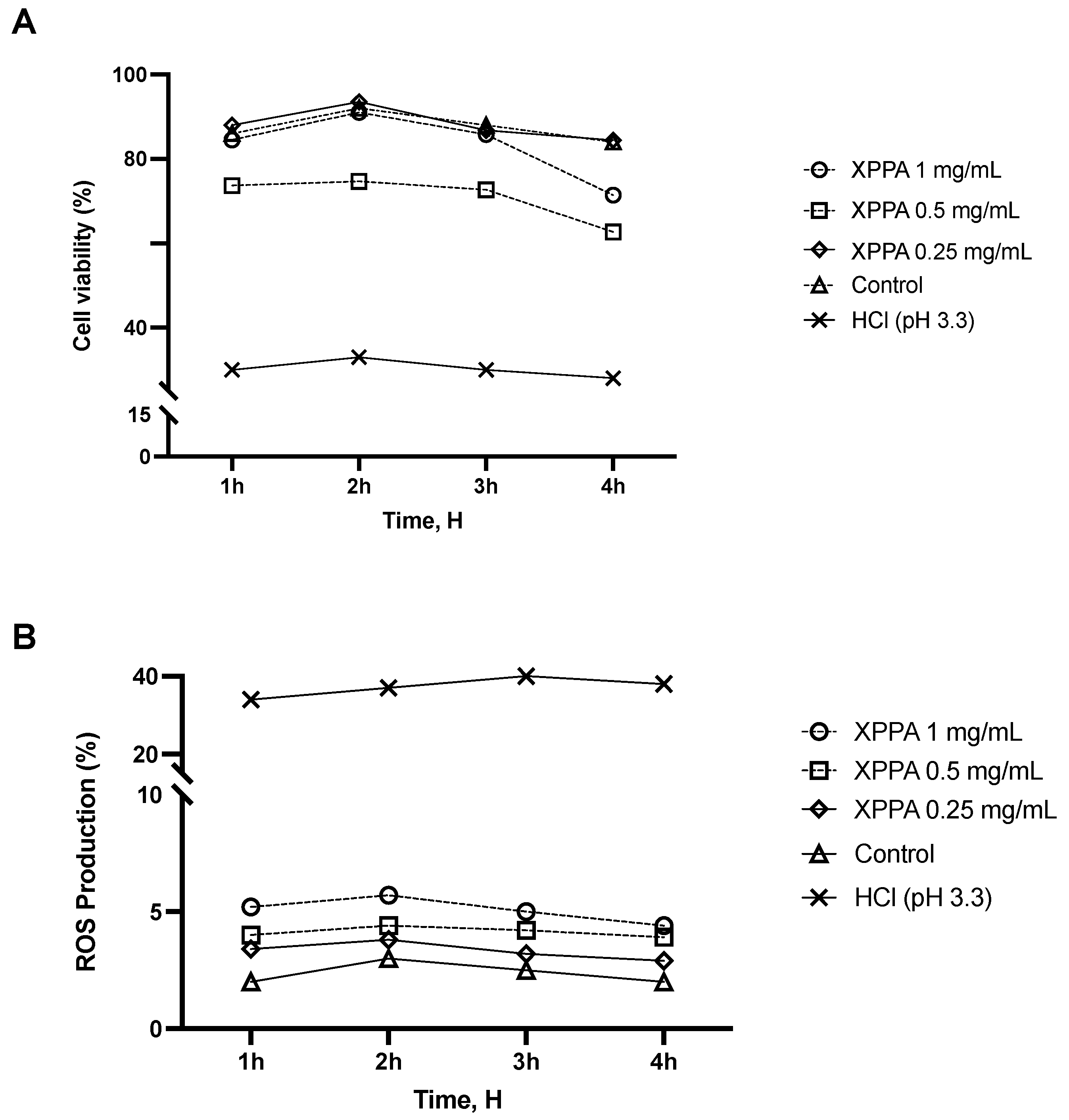
2.1. Cell Viability and ROS Production of Gastric Epithelial Cells
2.2. Effects of XPPA on Gastric Barrier Integrity
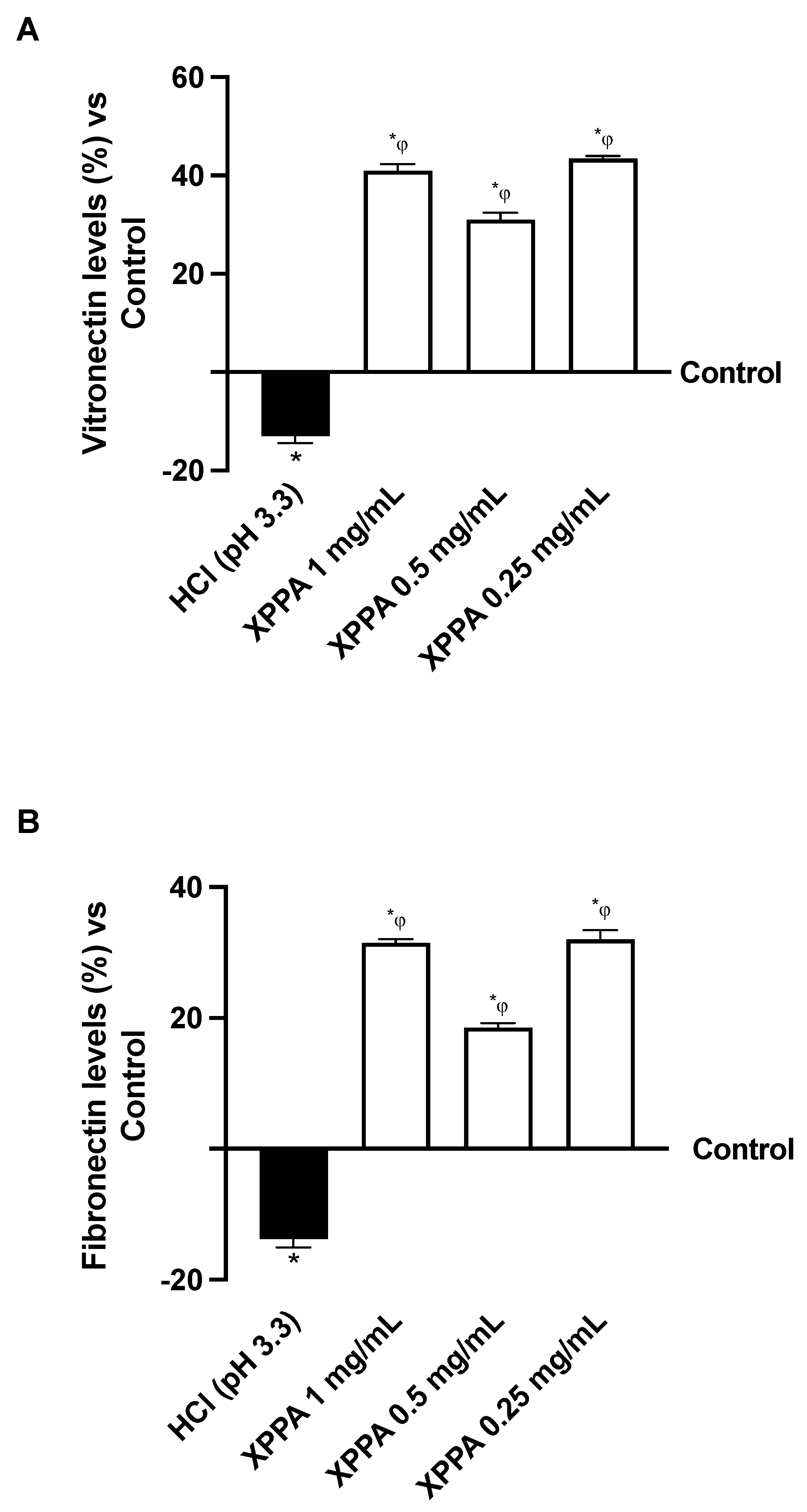
2.3. Influence of XPPA on Vitronectin and Fibronectin Quantification
2.4. Mucoadhesive Potential of XPPA
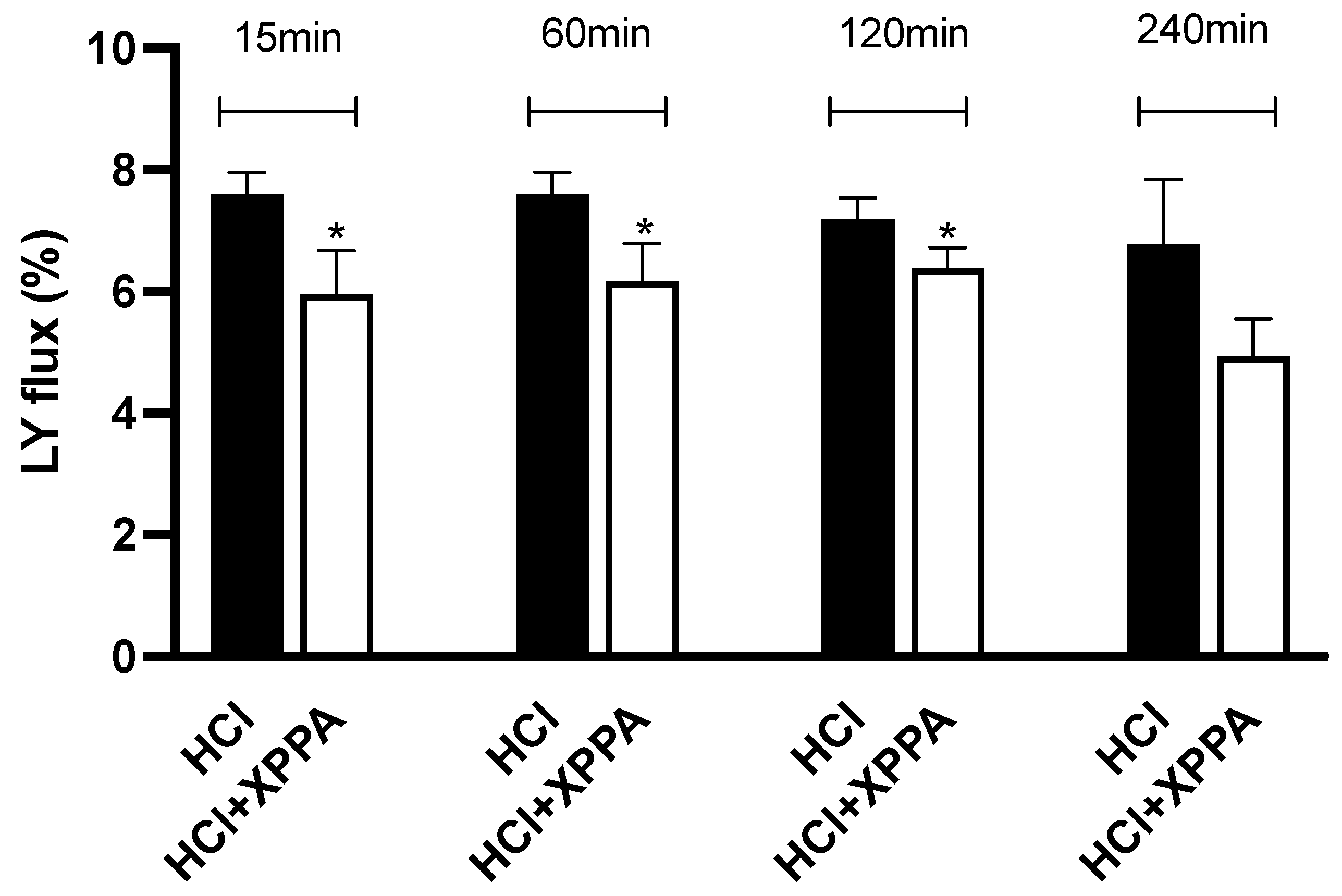
2.5. XPPA Effects on Mucosal Permeability
3. Discussion
4. Materials and Methods
4.1. Agent Preparation
4.2. Cell Culture
4.3. Experimental Protocol
4.4. Cell Viability Through MTT Test
4.5. ROS Production and Measurement
4.6. TEER Measurement for Barrier Integrity
4.7. Occludin Quantification Assay
4.8. Claudin-1 Quantification Assay
4.9. ZO-1 Quantification Assay
4.10. Vitronectin ELISA Kit
4.11. Fibronectin ELISA Kit
4.12. Mucoadhesion Test
4.13. Lucifer Yellow Assay
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, K.H.; Babic, B.; Breithaupt, W.; Dallemagne, B.; Fingerhut, A.; Furnee, E.; Granderath, F.; Horvath, P.; Kardos, P.; Pointner, R.; et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg. Endosc. 2014, 28, 1753–1773. [Google Scholar] [CrossRef]
- Shaqran, T.M.; Ismaeel, M.M.; Alnuaman, A.A.; Al Ahmad, F.A.; Albalawi, G.A.; Almubarak, J.N.; AlHarbi, R.S.; Alaqidi, R.S.; AlAli, Y.A.; Alfawaz, K.S.; et al. Epidemiology, Causes, and Management of Gastro-esophageal Reflux Disease: A Systematic Review. Cureus 2023, 15, e47420. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Group, G.C. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Paterson, W.G. Extraesophageal complications of gastroesophageal reflux disease. Can. J. Gastroenterol 1997, 11, 45B–50B. [Google Scholar]
- Chang, L. Review article: Epidemiology and quality of life in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. S7), 31–39. [Google Scholar] [CrossRef] [PubMed]
- Orlando, R.C. Overview of the mechanisms of gastroesophageal reflux. Am. J. Med. 2001, 111, 174–177. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Orlando, R.C. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 873–882. [Google Scholar] [CrossRef]
- Maev, I.V.; Livzan, M.A.; Mozgovoi, S.I.; Gaus, O.V.; Bordin, D.S. Esophageal Mucosal Resistance in Reflux Esophagitis: What We Have Learned So Far and What Remains to Be Learned. Diagnostics 2023, 13, 2664. [Google Scholar] [CrossRef] [PubMed]
- Prichard, D.O.; Byrne, A.M.; Murphy, J.O.; Reynolds, J.V.; O’Sullivan, J.; Feighery, R.; Doyle, B.; Eldin, O.S.; Finn, S.P.; Maguire, A.; et al. Deoxycholic acid promotes development of gastroesophageal reflux disease and Barrett’s oesophagus by modulating integrin-αv trafficking. J. Cell. Mol. Med. 2017, 21, 3612–3625. [Google Scholar] [CrossRef]
- Oshima, T.; Koseki, J.; Chen, X.; Matsumoto, T.; Miwa, H. Acid modulates the squamous epithelial barrier function by modulating the localization of claudins in the superficial layers. Lab. Investig. 2012, 92, 22–31. [Google Scholar] [CrossRef]
- Björkman, E.V.; Edebo, A.; Oltean, M.; Casselbrant, A. Esophageal barrier function and tight junction expression in healthy subjects and patients with gastroesophageal reflux disease: Functionality of esophageal mucosa exposed to bile salt and trypsin in vitro. Scand. J. Gastroenterol. 2013, 48, 1118–1126. [Google Scholar] [CrossRef]
- Barlow, W.J.; Orlando, R.C. The pathogenesis of heartburn in nonerosive reflux disease: A unifying hypothesis. Gastroenterology 2005, 128, 771–778. [Google Scholar] [CrossRef]
- Farré, R.; De Vos, R.; Geboes, K.; Verbecke, K.; Vanden Berghe, P.; Depoortere, I.; Blondeau, K.; Tack, J.; Sifrim, D. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut 2007, 56, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, R.; Ribolsi, M.; Maggiano, N.; Gabbrielli, A.M.; Emerenziani, S.; Guarino, M.P.; Carotti, S.; Habib, F.I.; Rabitti, C.; Cicala, M. Dilated intercellular spaces of esophageal epithelium in nonerosive reflux disease patients with physiological esophageal acid exposure. Am. J. Gastroenterol. 2005, 100, 543–548. [Google Scholar] [CrossRef]
- Tobey, N.A.; Hosseini, S.S.; Argote, C.M.; Dobrucali, A.M.; Awayda, M.S.; Orlando, R.C. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am. J. Gastroenterol. 2004, 99, 13–22. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, B.D.; Weijenborg, P.W.; Verheij, J.; van den Bergh Weerman, M.A.; Verseijden, C.; van den Wijngaard, R.M.; de Jonge, W.J.; Smout, A.J.; Bredenoord, A.J. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1815–1823.e2. [Google Scholar] [CrossRef]
- Savarino, E.; Zentilin, P.; Marabotto, E.; Pellegatta, G.; Coppo, C.; Brunacci, M.; Dulbecco, P.; Savarino, V. Drugs for improving esophageal mucosa defense: Where are we now and where are we going? Ann. Gastroenterol. 2017, 30, 585–591. [Google Scholar] [CrossRef]
- Kothadia, J.P.; Howden, C.W. Potassium-Competitive Acid Blockers for the Treatment of Gastroesophageal Reflux Disease. Foregut 2023, 4, 7–19. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Verma, P.; Samajdar, S.S.; Das, S. Vonoprazan causes symptomatic improvement in non-erosive gastroesophageal reflux disease: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102373. [Google Scholar] [CrossRef]
- Pecora, T.M.G.; Parisi, O.I.; Bertin, W.; Ragazzo, B.; Dattilo, M.; Scigliano, N.; Malivindi, R.; Amone, F.; Puoci, F. Barrier effect and wound healing activity of the medical device REF-FTP78 in the treatment of gastroesophageal reflux disease. Sci. Rep. 2022, 12, 6136. [Google Scholar] [CrossRef]
- Mahboubi, M. Aloe Vera (Aloe barbadensis) gel for the management of gastroesophageal reflux disease (GERD). Nat. Prod. J. 2021, 11, 13–20. [Google Scholar] [CrossRef]
- Sangil-Monroy, M.; Serra-Majem, L.; Monroy, J.M.M.; Andrellucchi, A.O.; Sánchez-Villegas, A.; Doreste, J.; Knipschild, P. Effects of Intake of Milk Enriched with Aloe vera on Patients with Gastrointestinal Reflux Disease. Food Nutr. Sci. 2014, 5, 936–942. [Google Scholar]
- Dutta, P.; Giri, S.; Giri, T.K. Xyloglucan as green renewable biopolymer used in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2020, 160, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Tyagi, V.K.; Patil, R.R.; Dusunge, S.B. Thiolated xyloglucan: Synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Carbohydr. Polym. 2013, 91, 618–625. [Google Scholar] [CrossRef]
- de Servi, B.; Ranzini, F.; Piqué, N. Effect of Utipro(®) (containing gelatin-xyloglucan) against Escherichia coli invasion of intestinal epithelial cells: Results of an in vitro study. Future Microbiol. 2016, 11, 651–658. [Google Scholar] [CrossRef]
- Fraile, B.; Alcover, J.; Royuela, M.; Rodríguez, D.; Chaves, C.; Palacios, R.; Piqué, N. Xyloglucan, hibiscus and propolis for the prevention of urinary tract infections: Results of in vitro studies. Future Microbiol. 2017, 12, 721–731. [Google Scholar] [CrossRef]
- Gnessi, L.; Bacarea, V.; Marusteri, M.; Piqué, N. Xyloglucan for the treatment of acute diarrhea: Results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. BMC Gastroenterol. 2015, 15, 153. [Google Scholar] [CrossRef]
- Periasamy, S.; Lin, C.H.; Nagarajan, B.; Sankaranarayanan, N.V.; Desai, U.R.; Liu, M.Y. Mucoadhesive role of tamarind xyloglucan on inflammation attenuates ulcerative colitis. J. Funct. Foods 2018, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Beaufrand, C.; Harkat, C.; Theodorou, V. The role of mucoprotectants in the management of gastrointestinal disorders. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bueno, L.; Theodorou, V.; Sekkal, S. Xyloglucan: A new agent to protect the intestinal mucosa and to prevent bacterially mediated alteration of tight junction permeability. In Proceedings of the 22nd United European Gastroenterology (UEG) Week, Vienna, Austria, 18–22 October 2014; Abstract P1675. [Google Scholar]
- Piqué, N.; Gómez-Guillén, M.D.C.; Montero, M.P. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef]
- Djemaoune, Y.; Cases, E.; Saurel, R. The Effect of High-Pressure Microfluidization Treatment on the Foaming Properties of Pea Albumin Aggregates. J. Food Sci. 2019, 84, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Ardizzone, A.; Bova, V.; Lanza, M.; Casili, G.; Cuzzocrea, S.; Esposito, E.; Campolo, M.; Paterniti, I. A Combination of Xyloglucan, Pea Protein and Chia Seed Ameliorates Intestinal Barrier Integrity and Mucosa Functionality in a Rat Model of Constipation-Predominant Irritable Bowel Syndrome. J. Clin. Med. 2022, 11, 7073. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Lanza, M.; Ardizzone, A.; Pantaleo, L.; Campolo, M.; Paterniti, I.; Cucinotta, L.; Cuzzocrea, S.; Esposito, E. Efficacy of a Product Containing Xyloglucan and Pea Protein on Intestinal Barrier Function in a Partial Restraint Stress Animal Model. Int. J. Mol. Sci. 2022, 23, 2269. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M. Mucoadhesive polymers in substance-based medical devices: Functional ingredients or what else? Front. Drug Saf. Regul. 2023, 3, 1227763. [Google Scholar] [CrossRef]
- Ardizzone, A.; Mannino, D.; Casili, G.; Campolo, M.; Paterniti, I.; Lanza, M.; Filippone, A.; Repici, A.; Bova, V.; Capra, A.P.; et al. Efficacy of an oral suspension containing xyloglucan and pea proteins on a murine model of gastroesophageal reflux disease. Phytother. Res. 2024, 38, 1610–1622. [Google Scholar] [CrossRef]
- Farhadi, A.; Fields, J.; Banan, A.; Keshavarzian, A. Reactive oxygen species: Are they involved in the pathogenesis of GERD, Barrett’s esophagus, and the latter’s progression toward esophageal cancer? Am. J. Gastroenterol. 2002, 97, 22–26. [Google Scholar] [CrossRef]
- Chen, J.; Brady, P. Gastroesophageal reflux disease: Pathophysiology, diagnosis, and treatment. Gastroenterol. Nurs. 2019, 42, 20–28. [Google Scholar] [CrossRef]
- Jaynes, M.; Kumar, A.B. The risks of long-term use of proton pump inhibitors: A critical review. Ther. Adv. Drug Saf. 2018, 10, 2042098618809927. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Yadlapati, R. Pathophysiology and treatment options for gastroesophageal reflux disease: Looking beyond acid. Ann. N. Y. Acad. Sci. 2021, 1486, 3–14. [Google Scholar] [CrossRef]
- Hartman, K.G.; Bortner, J.D.; Falk, G.W.; Yu, J.; Martín, M.G.; Rustgi, A.K.; Lynch, J.P. Modeling inflammation and oxidative stress in gastrointestinal disease development using novel organotypic culture systems. Stem Cell Res. Ther. 2013, 4 (Suppl. S1), S5. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Nagano, Y.; Shimokawa, O.; Kaneko, T.; Rai, K.; Udo, J.; Hirayama, A.; Nakamura, Y.; Indo, H.P.; Majima, H.J.; et al. Gastric acid induces mitochondrial superoxide production and lipid peroxidation in gastric epithelial cells. J. Gastroenterol. 2011, 46, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.M.; Robinson, J.R.; Leung, S.H. Binding of acrylic polymers to mu-cin/epithelial surfaces: Structure-property relationships. Crit. Rev. Ther. Drug Carr. Syst. 1988, 5, 21–67. [Google Scholar]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Gordon, S.; Daneshian, M.; Bouwstra, J.; Caloni, F.; Constant, S.; Davies, D.E.; Dandekar, G.; Guzman, C.A.; Fabian, E.; Haltner, E.; et al. Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. ALTEX-Altern. Anim. Exp. 2015, 32, 327–378. [Google Scholar] [CrossRef]
- Zuang, V.; Schäffer, M.; Tuomainen, A.M.; Amcoff, P.; Bernasconi, C.; Bremer, S.; Casati, S.; Castello, P.; Coecke, S.; Corvi, R.; et al. EURL ECVAM Progress Report on the Development, Validation and Regulatory Acceptance of Alternative Methods (2010–2013); Joint Research Centre, Institute for Health and Consumer Protection, European Commission: Ispra, Italy, 2013; Available online: http://ihcp.jrc.ec.europa.eu/ (accessed on 13 May 2024).
- Pecora, T.M.G.; Ragazzo, B.; Bertin, W.; Ragonese, A.; Mascagni, M.; Maffei, P.; Pignatello, R. Rheological Behavior of a New Mucoadhesive Oral Formulation Based on Sodium Chondroitin Sulfate, Xyloglucan and Glycerol. J. Funct. Biomater. 2021, 12, 28. [Google Scholar] [CrossRef]
- Giordano, S.; Di Renzo, M.F.; Ferracini, R.; Chiadò-Piat, L.; Comoglio, P.M. p145, a protein with associated tyrosine kinase activity in a human gastric carcinoma cell line. Mol. Cell. Biol. 1988, 8, 3510–3517. [Google Scholar] [CrossRef]
- Uberti, F.; Bardelli, C.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Role of vitamin D3 combined to alginates in preventing acid and oxidative injury in cultured gastric epithelial cells. BMC Gastroenterol. 2016, 16, 127. [Google Scholar] [CrossRef]
- Ceriotti, L.; Meloni, M. La valutazione dell’assorbimento intestinale in vitro. L’integratore Nutr. 2014, 17, 62–65. [Google Scholar]
- Atcc.org. Available online: https://www.atcc.org/products/crl-1739 (accessed on 13 May 2024).
- Khin, P.P.; Po, W.W.; Thein, W.; Sohn, U.D. Apoptotic effect of fluoxetine through the endoplasmic reticulum stress pathway in the human gastric cancer cell line AGS. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Bcrj.org.br. Available online: https://bcrj.org.br/celula/kyse-30 (accessed on 14 May 2024).
- Ceriotti, L.; Buratti, P.; Corazziari, E.S.; Meloni, M. Protective Mechanisms of Liquid Formulations for Gastro-Oesophageal Reflux Disease in a Human Reconstructed Oesophageal Epithelium Model. Med. Devices Evid. Res. 2022, 15, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, B.N.; Dos Santos, T.; Oliveira, C.; Barrias, C.C.; Granja, P.L. Bioengineering a novel 3D in vitro model of gastric mucosa for stomach permeability studies. Acta. Biomater. 2018, 82, 68–78. [Google Scholar] [CrossRef]
- Patel, D.; Smith, A.W.; Grist, N.; Barnett, P.; Smart, J.D. An in vitro mucosal model predictive of bioadhesive agents in the oral cavity. J. Control. Release 1999, 61, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, G.; Spadaccini, M.; Lamonaca, L.; Craviotto, V.; D’Amico, F.; Ceriotti, L.; Meloni, M.; Repici, A. Evaluation of Human Esophageal Epithelium Permeability in Presence of Different Formulations Containing Hyaluronic Acid and Chondroitin Sulphate. Med. Devices Evid. Res. 2020, 13, 57–66. [Google Scholar] [CrossRef]
- Ruga, S.; Galla, R.; Ferrari, S.; Invernizzi, M.; Uberti, F. Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and. Int. J. Mol. Sci. 2023, 24, 5503. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef]
- Zeng, R.; Qiu, M.; Wan, Q.; Huang, Z.; Liu, X.; Tang, D.; Knopp, D. Smartphone-Based Electrochemical Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 15155–15161. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Prados, L.; Ferruelo, A.; Puig, F.; Pandolfi, R.; Guillamat-Prats, R.; Moreno, L.; Matute-Bello, G.; Artigas, A.; Esteban, A.; et al. Fas activation alters tight junction proteins in acute lung injury. Thorax 2019, 74, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, X.; Chang, Y.; Zhang, N.; Sun, Y. Analysis of alterations of serum inflammatory cytokines and fibrosis makers in patients with essential hypertension and left ventricular hypertrophy and the risk factors. Am. J. Transl. Res. 2022, 14, 4097. [Google Scholar] [PubMed]
- Sigmaaldrich.com. Available online: https://www.sigmaaldrich.com/IT/it/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/cell-based-assays/barrier-formation-permeability-assays#yellow (accessed on 13 May 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, S.; Ferulli, F.; Galla, R.; Vicini, R.; Cattaneo, V.; Mulè, S.; Uberti, F. Effective Restoration of Gastric and Esophageal Tissues in an In Vitro Model of GERD: Mucoadhesive and Protective Properties of Xyloglucan, Pea Proteins, and Polyacrylic Acid. Int. J. Mol. Sci. 2025, 26, 4409. https://doi.org/10.3390/ijms26094409
Ferrari S, Ferulli F, Galla R, Vicini R, Cattaneo V, Mulè S, Uberti F. Effective Restoration of Gastric and Esophageal Tissues in an In Vitro Model of GERD: Mucoadhesive and Protective Properties of Xyloglucan, Pea Proteins, and Polyacrylic Acid. International Journal of Molecular Sciences. 2025; 26(9):4409. https://doi.org/10.3390/ijms26094409
Chicago/Turabian StyleFerrari, Sara, Federica Ferulli, Rebecca Galla, Riccardo Vicini, Veronica Cattaneo, Simone Mulè, and Francesca Uberti. 2025. "Effective Restoration of Gastric and Esophageal Tissues in an In Vitro Model of GERD: Mucoadhesive and Protective Properties of Xyloglucan, Pea Proteins, and Polyacrylic Acid" International Journal of Molecular Sciences 26, no. 9: 4409. https://doi.org/10.3390/ijms26094409
APA StyleFerrari, S., Ferulli, F., Galla, R., Vicini, R., Cattaneo, V., Mulè, S., & Uberti, F. (2025). Effective Restoration of Gastric and Esophageal Tissues in an In Vitro Model of GERD: Mucoadhesive and Protective Properties of Xyloglucan, Pea Proteins, and Polyacrylic Acid. International Journal of Molecular Sciences, 26(9), 4409. https://doi.org/10.3390/ijms26094409






