Natural Variation of StNADC Regulates Plant Senescence in Tetraploid Potatoes (Solanum tuberosum L.)
Abstract
1. Introduction
2. Results
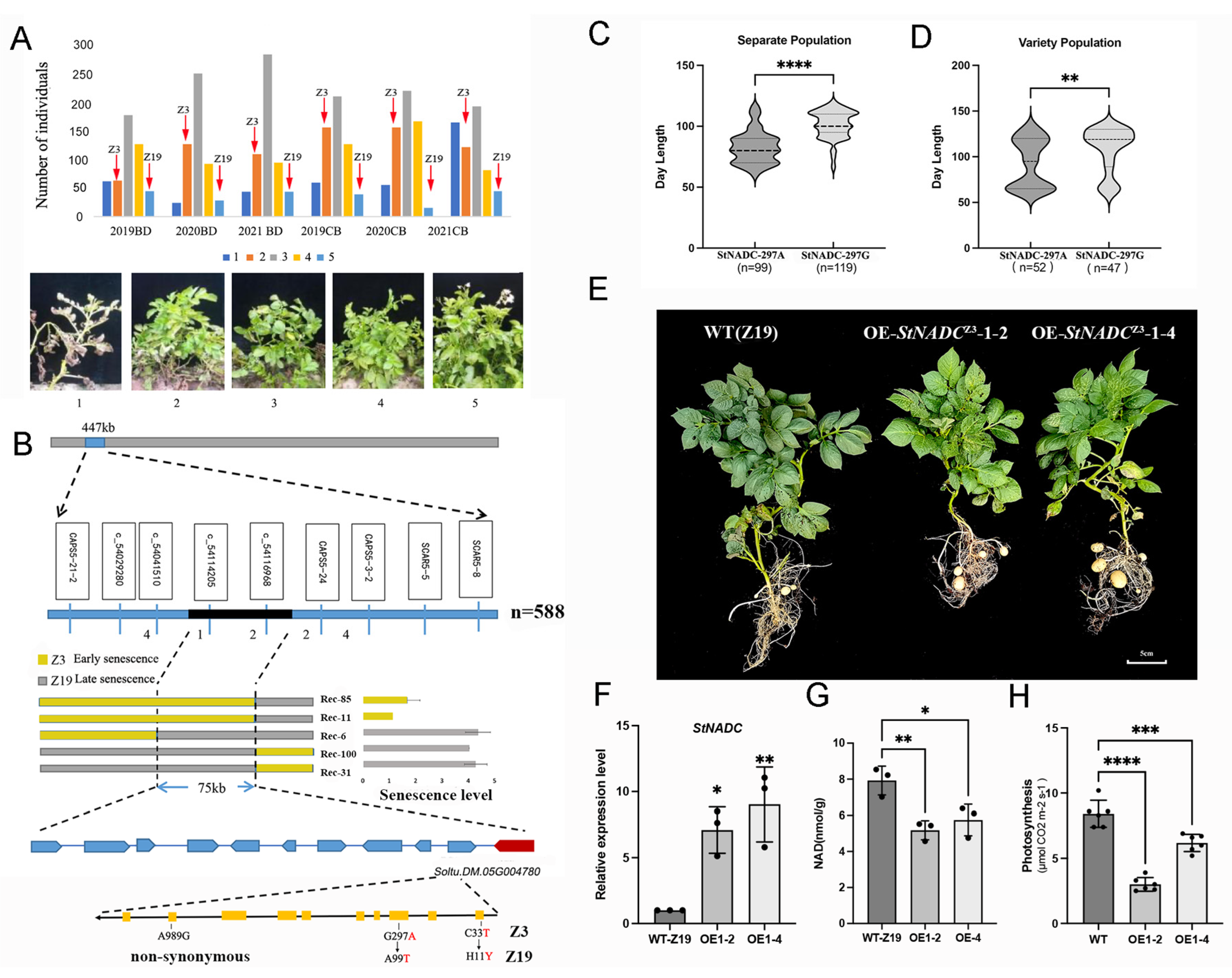
2.1. Phenotypic Analysis of Plant Senescence in the Segregating Population
2.2. Fine Mapping of the Senescence-Related QTL
2.3. Soltu.DM.05G004780 Is the Candidate Gene for the Plant Senescence Trait
2.4. StNADCZ3 Promotes Plant Senescence in Tetraploid Potatoes
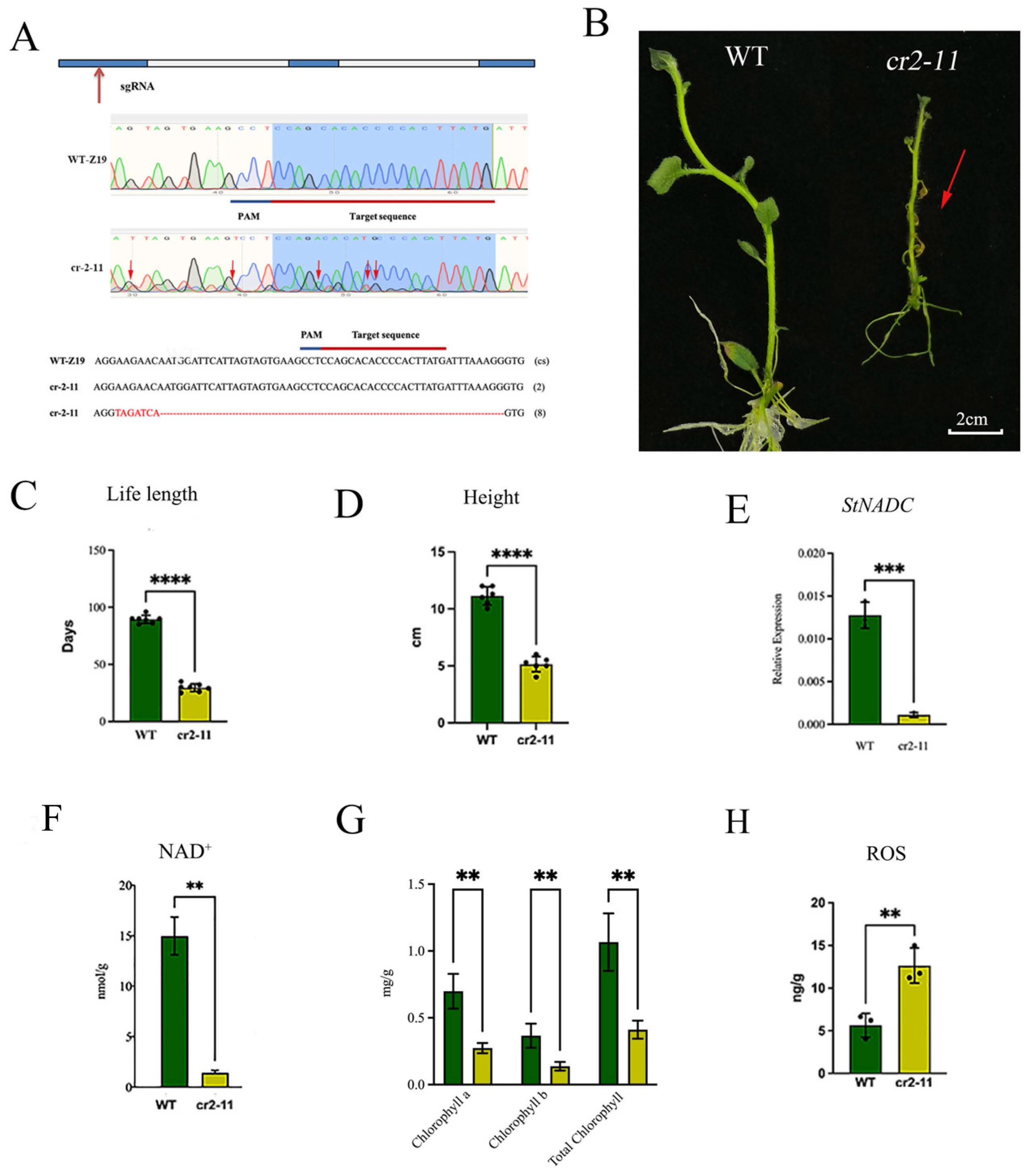
2.5. CRISPR/Cas9-Induced Mutation of StNADC Triggers Early Senescence
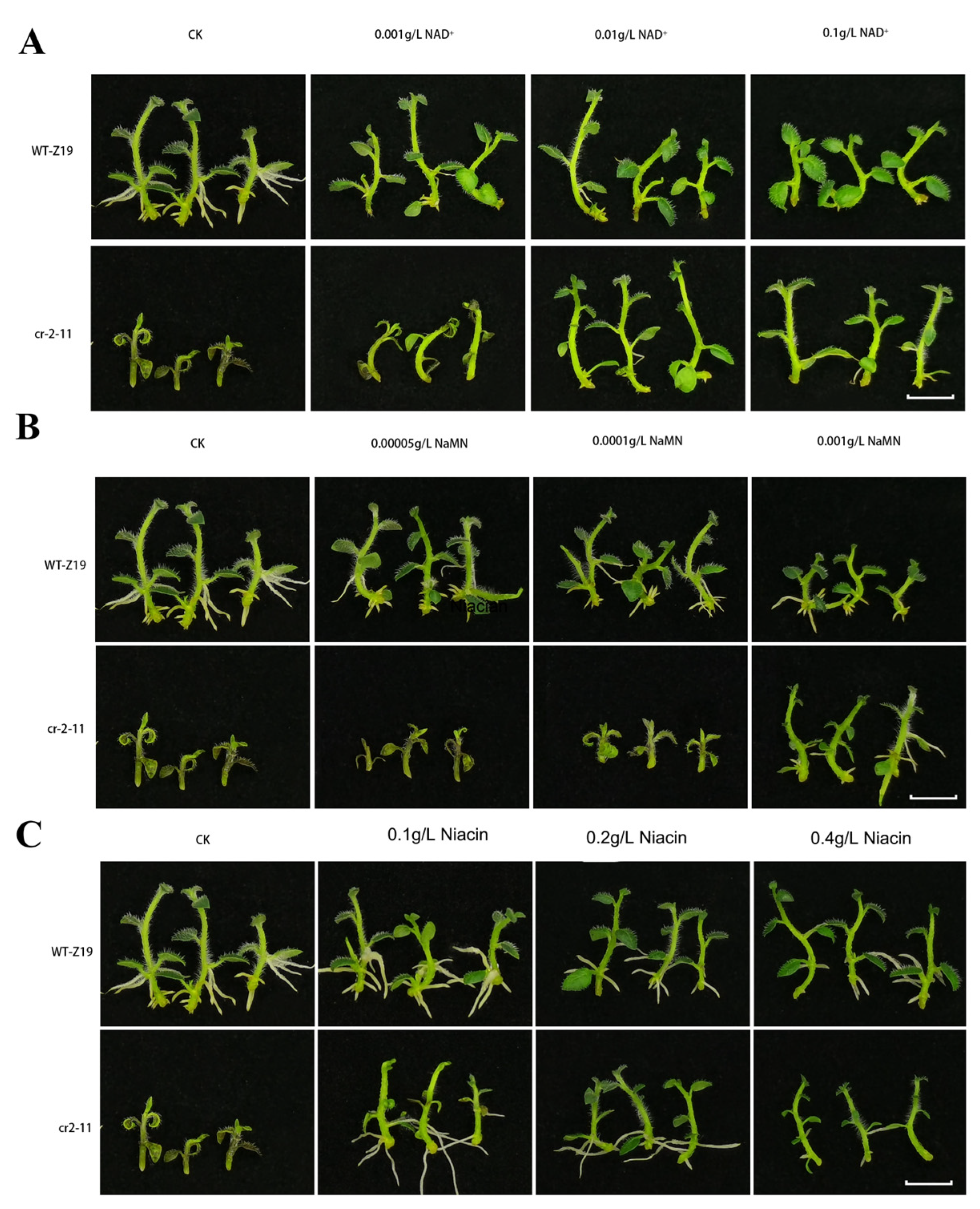
2.6. NAD and Its Intermediates Rescue the cr2–11 Mutant Phenotype
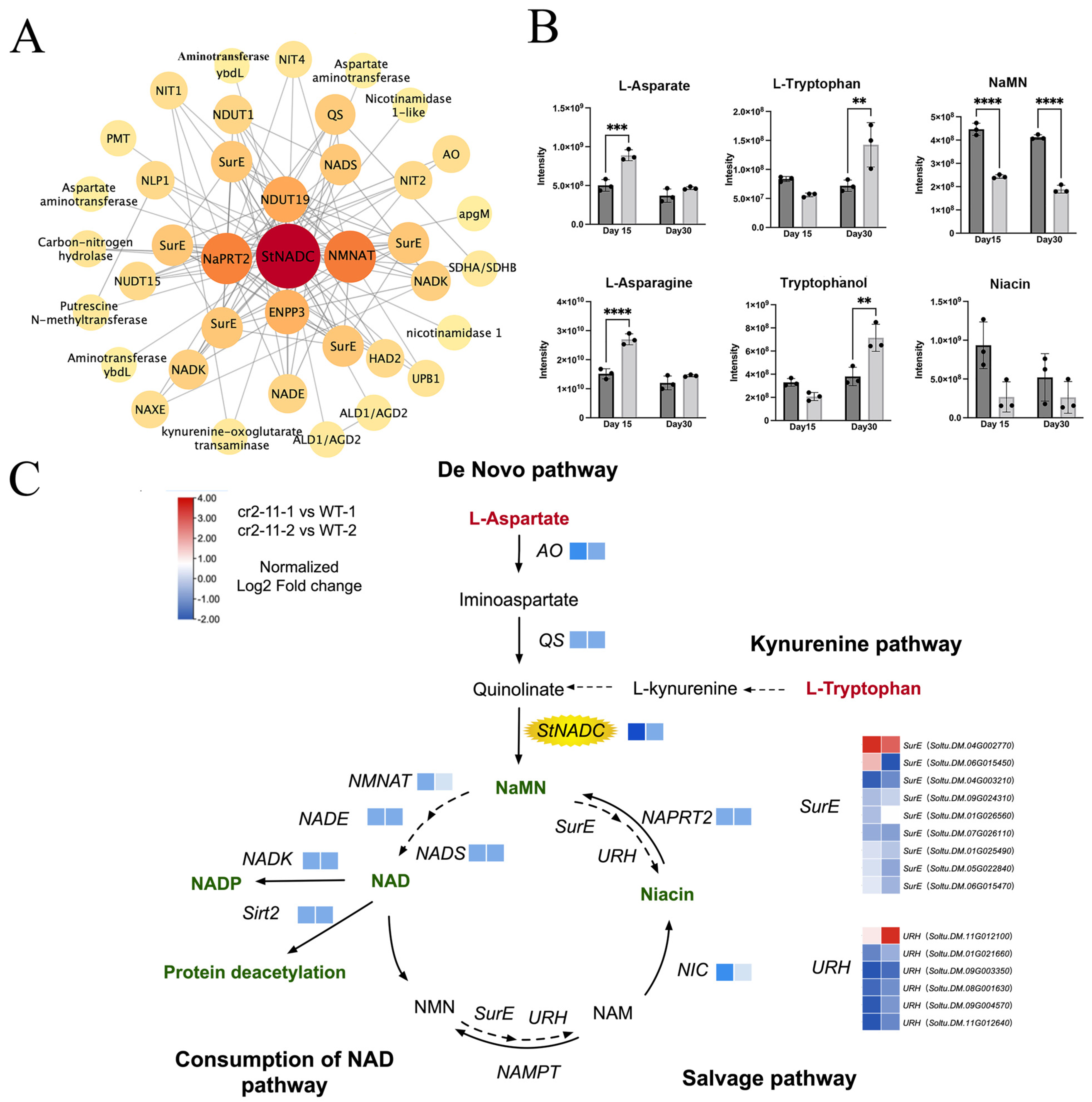
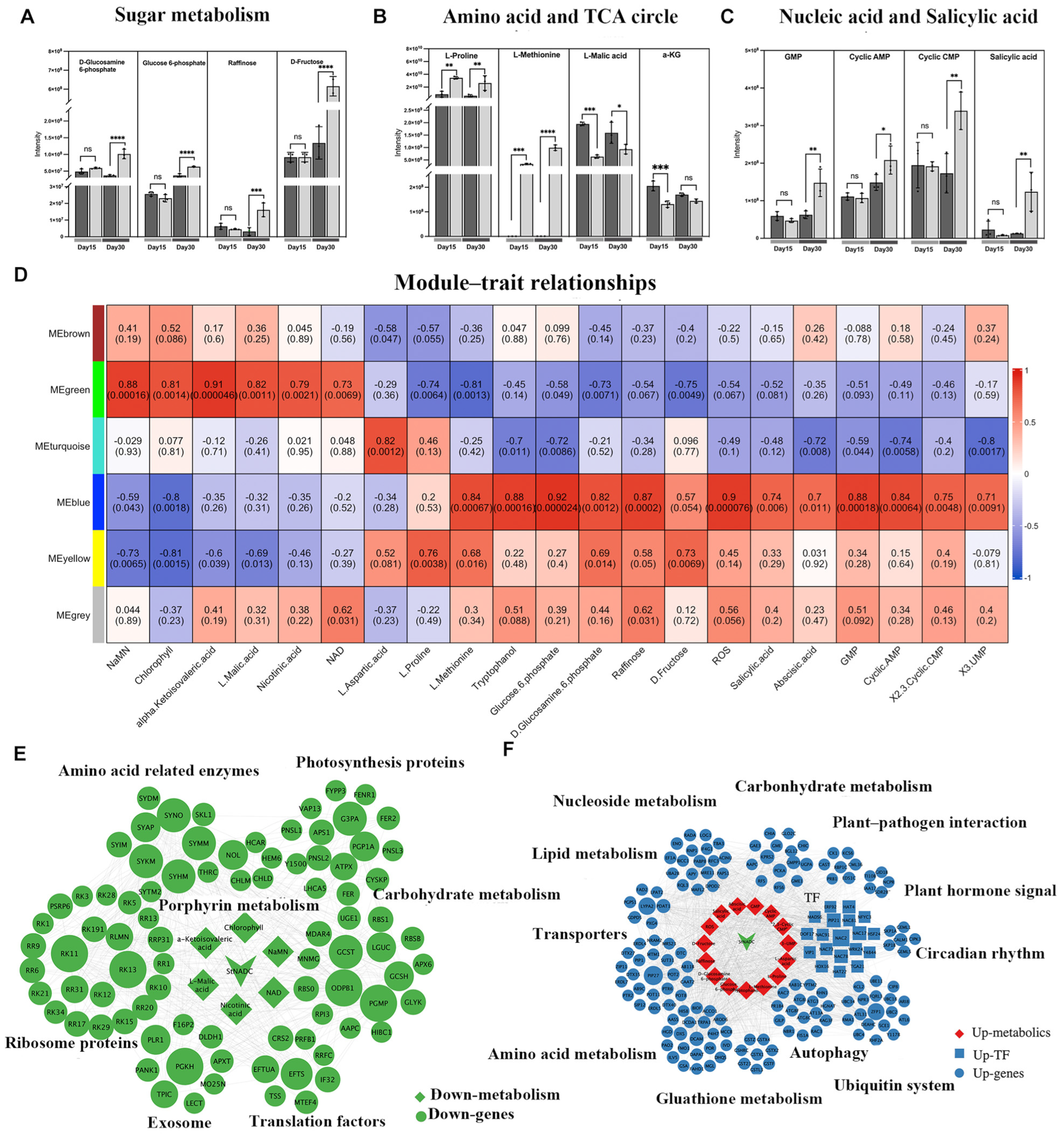
2.7. Analysis of NAD Metabolic Networks in cr2–11 Mutants
2.8. The StNADC Mutation Alters Metabolite Profiles Associated with Early Senescence
2.9. StNADC Regulates Plant Senescence Through Multiple Layers
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Design of Field Experiments and Senescence Assessment
4.3. Development of KASP Markers for Fine Mapping of Plant Senescence
4.4. Plasmid Constructs
4.5. Generation of Transgenic Lines
4.6. Maintenance of Transformed and Non-Transformed Controls
4.7. Restoration of the cr2–11 Mutant Phenotype
4.8. RNA Isolation and Quantitative PCR
4.9. Determination of Chlorophyll and ROS Contents
4.10. Measurement of NAD
4.11. RNA Sequencing
4.12. Determination of Metabolites
4.13. Co-Expression Network Analysis
4.14. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic Acid |
| AO | L-Aspartate Oxidase |
| BD | Baoding |
| CB | ChaBei |
| CDS | Coding Sequence |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| DEGs | Differentially Expressed Genes |
| GMP | Guanosine Monophosphate |
| KASP | Kompetitive Allele-Specific PCR |
| MS | Murashige and Skoog |
| NA | Niacin |
| NAD | Nicotinamide Adenine Dinucleotide |
| NaMN | Nicotinate Mononucleotide |
| NBR1 | Next to BRCA1 Gene 1 |
| QPTase | Quinolinate Phosphoribosyltransferase |
| QS | Quinolinate Synthase |
| QTL | Quantitative Trait Locus |
| ROS | Reactive Oxygen Species |
| SA | Salicylic Acid |
| SAG | Senescence-Associated Gene |
| SNP | Single Nucleotide Polymorphism |
| StABI5 | Solanum tuberosum Abscisic Acid Insensitive 5 |
| StCDF1 | Solanum tuberosum Cycling Dof Factor 1 |
| StNADC | Solanum tuberosum Nicotinate-Nucleotide Pyrophosphorylase |
| TCA | Tricarboxylic Acid Cycle |
| WT | Wild Type |
| Z3 | Zhongshu 3 |
| Z19 | Zhongshu 19 |
References
- Ye, M.; Peng, Z.; Tang, D.; Yang, Z.; Li, D.; Xu, Y.; Zhang, C.; Huang, S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 2018, 4, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Bäurle, I.; Dean, C. The timing of developmental transitions in plants. Cell 2006, 125, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC Gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Nam, H.G.; Lim, P.O. Plant leaf senescence and death - regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 2013, 126, 4823–4833. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.H.; Kim, J.; Kim, J.J.; Hong, S.; Kim, J.; Kim, J.H.; Woo, H.R.; Hyeon, C.; Lim, P.O.; et al. Time-evolving genetic networks reveal a NAC troika that negatively regulates leaf senescence in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E4930–E4939. [Google Scholar] [CrossRef]
- Kloosterman, B.; Abelenda, J.A.; Gomez, M.d.M.C.; Oortwijn, M.; de Boer, J.M.; Kowitwanich, K.; Horvath, B.M.; van Eck, H.J.; Smaczniak, C.; Prat, S.; et al. Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature 2013, 495, 246–250. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Zimmermann, P.; Zentgraf, U. The correlation between oxidative stress and leaf senescence during plant development. Cell. Mol. Biol. Lett. 2005, 10, 515–534. [Google Scholar]
- Li, Z.; Kim, J.H.; Kim, J.; Lyu, J.I.; Zhang, Y.; Guo, H.; Nam, H.G.; Woo, H.R. ATM suppresses leaf senescence triggered by DNA double-strand break through epigenetic control of senescence-associated genes in Arabidopsis. New Phytol. 2020, 227, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zou, D.; Zhao, Y.; Wang, H.L.; Zhang, Y.; Xia, X.; Luo, J.; Guo, H.; Zhang, Z. LSD 3.0: A comprehensive resource for the leaf senescence research community. Nucleic Acids Res. 2020, 48, D1069–D1075. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Noctor, G. Metabolic signalling in defence and stress: The central roles of soluble redox couples. Plant Cell Environ. 2006, 29, 409–425. [Google Scholar] [CrossRef]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD(+)-H(2)S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2019, 176, 944–945. [Google Scholar] [CrossRef]
- Poljsak, B. NAMPT-Mediated NAD Biosynthesis as the Internal Timing Mechanism: In NAD+ World, Time Is Running in Its Own Way. Rejuvenation Res. 2018, 21, 210–224. [Google Scholar] [CrossRef]
- Shi, H.; Enriquez, A.; Rapadas, M.; Martin, E.; Wang, R.; Moreau, J.; Lim, C.K.; Szot, J.O.; Ip, E.; Hughes, J.N.; et al. NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N. Engl. J. Med. 2017, 377, 544–552. [Google Scholar] [CrossRef]
- Wang, G.; Pichersky, E. Nicotinamidase participates in the salvage pathway of NAD biosynthesis in Arabidopsis. Plant J. 2007, 49, 1020–1029. [Google Scholar] [CrossRef]
- Pétriacq, P.; de Bont, L.; Tcherkez, G.; Gakière, B. NAD: Not just a pawn on the board of plant-pathogen interactions. Plant Signal Behav. 2013, 8, e22477. [Google Scholar] [CrossRef]
- Katoh, A.; Uenohara, K.; Akita, M.; Hashimoto, T. Early steps in the biosynthesis of NAD in Arabidopsis start with aspartate and occur in the plastid. Plant Physiol. 2006, 141, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ren, D.; Hu, S.; Li, G.; Dong, G.; Jiang, L.; Hu, X.; Ye, W.; Cui, Y.; Zhu, L.; et al. Down-Regulation of a Nicotinate Phosphoribosyltransferase Gene, OsNaPRT1, Leads to Withered Leaf Tips. Plant Physiol. 2016, 171, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Sedlackova, L.; Korolchuk, V.I. The crosstalk of NAD, ROS and autophagy in cellular health and ageing. Biogerontology 2020, 21, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.F.; Srikannathasan, V.; Huang, J.; Cui, H.; Fosberry, A.P.; Gu, M.; Hann, M.M.; Hibbs, M.; Homes, P.; Ingraham, K.; et al. Structural basis of DNA gyrase inhibition by antibacterial QPT-1, anticancer drug etoposide and moxifloxacin. Nat. Commun. 2015, 6, 10048. [Google Scholar] [CrossRef]
- Pétriacq, P.; de Bont, L.; Hager, J.; Didierlaurent, L.; Mauve, C.; Guérard, F.; Noctor, G.; Pelletier, S.; Renou, J.P.; Tcherkez, G.; et al. Inducible NAD overproduction in Arabidopsis alters metabolic pools and gene expression correlated with increased salicylate content and resistance to Pst-AvrRpm1. Plant J. 2012, 70, 650–665. [Google Scholar] [CrossRef]
- Xingcui, L.; Jianfei, X.; Shaoguang, D.; Jiaojiao, Z.; Chunsong, B.; Jun, H.; Guangcun, L.; Liping, J. Mapping and QTL Analysis of Early-Maturity Traits in Tetraploid Potato (Solanum tuberosum L.). Intl. J. Mol. Sci. 2018, 19, 3065. [Google Scholar] [CrossRef]
- Duan, Y.; Duan, S.; Xu, J.; Zheng, J.; Hu, J.; Li, X.; Li, B.; Li, G.; Jin, L. Late Blight Resistance Evaluation and Genome-Wide Assessment of Genetic Diversity in Wild and Cultivated Potato Species. Front. Plant Sci. 2021, 12, 710468. [Google Scholar] [CrossRef]
- Mendoza, H.A.; Haynes, F.L. Inheritance of tuber initiation in tuber bearing Solanum as influenced by photoperiod. Am. Potato J. 1977, 54, 243–252. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wen, G.; Li, G.; Li, Z.; Zhang, R.; Ma, S.; Zhou, J.; Xie, C. Mapping QTL underlying tuber starch content and plant maturity in tetraploid potato. Crop J. 2019, 7, 261–272. [Google Scholar] [CrossRef]
- Massa, A.N.; Manrique-Carpintero, N.C.; Coombs, J.J.; Zarka, D.G.; Boone, A.E.; Kirk, W.W.; Hackett, C.A.; Bryan, G.J.; Douches, D.S. Genetic Linkage Mapping of Economically Important Traits in Cultivated Tetraploid Potato (Solanum tuberosum L.). G3 Genes 2015, 5, 2357–2364. [Google Scholar] [CrossRef]
- Bradshaw, J.E.; Pande, B.; Bryan, G.J.; Hackett, C.A.; McLean, K.; Stewart, H.E.; Waugh, R. Interval mapping of quantitative trait loci for resistance to late blight [Phytophthora infestans (Mont.) de Bary], height and maturity in a tetraploid population of potato (Solanum tuberosum subsp. tuberosum). Genetics 2004, 168, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Sun, X.; Yu, L.; Wang, E.; Cheng, Z.; Liu, H.; Jiang, P.; Qin, J.; Begum, S.; Song, B. Transcription factor StABI5-like 1 binding to the FLOWERING LOCUS T homologs promotes early maturity in potato. Plant Physiol. 2022, 189, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell. Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Han, B.; Liu, X.; Dai, W.; Wang, Y.; Luo, H.; Lu, X.; Nie, J.; Xian, C.; Zhang, Z. An enzyme-mimic single Fe-N(3) atom catalyst for the oxidative synthesis of nitriles via C─C bond cleavage strategy. Sci. Adv. 2022, 8, eadd1267. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Yan, Q.; Ji, Y.; Zhang, R.; Yuan, D.; Yang, F.; Wang, J.; Wu, M.; Zhou, J. Design and Synthesis of Dual-Target Inhibitors Targeting Androgen Receptors and Glucocorticoid Receptors to Overcome Antiandrogen Resistance in Castration-Resistant Prostate Cancer. J. Med. Chem. 2024, 67, 3419–3436. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell. Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Sun, J.; Feng, Z.; Leakey, A.D.; Zhu, X.; Bernacchi, C.J.; Ort, D.R. Inconsistency of mesophyll conductance estimate causes the inconsistency for the estimates of maximum rate of Rubisco carboxylation among the linear, rectangular and non-rectangular hyperbola biochemical models of leaf photosynthesis--a case study of CO2 enrichment and leaf aging effects in soybean. Plant Sci. 2014, 226, 49–60. [Google Scholar] [CrossRef]
- Diaz, C.; Purdy, S.; Christ, A.; Morot-Gaudry, J.F.; Wingler, A.; Masclaux-Daubresse, C. Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis. A metabolic profiling approach. Plant Physiol. 2005, 138, 898–908. [Google Scholar] [CrossRef]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Duan, S.; Zhang, J.; Bian, C.; Hu, J.; Li, G.; Jin, L. Mapping and QTL Analysis of Early-Maturity Traits in Tetraploid Potato (Solanum tuberosum L.). Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.L.; Dong, L.; Wang, Z.P.; Zhang, H.Y.; Han, C.Y.; Liu, B.; Wang, X.C.; Chen, Q.J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xu, J.; Jian, Y.; Duan, S.; Hu, J.; Jin, L.; Li, G. Overexpression of galactinol synthase 1 from Solanum commersonii (ScGolS1) confers freezing tolerance in transgenic potato. Hortic. Plant J. 2022, 9, 541–552. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pham, G.M.; Hamilton, J.P.; Wood, J.C.; Burke, J.T.; Zhao, H.; Vaillancourt, B.; Ou, S.; Jiang, J.; Buell, C.R. Construction of a chromosome-scale long-read reference genome assembly for potato. Gigascience 2020, 9, giaa100. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Guo, X.; Xu, J.; Zhang, K.; Yang, Y.; Yang, Y.; Jian, Y.; Dong, D.; Huang, S.; et al. The gap-free potato genome assembly reveals large tandem gene clusters of agronomical importance in highly repeated genomic regions. Mol. Plant 2023, 16, 314–317. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Domingo-Almenara, X.; Siuzdak, G. Metabolomics Data Processing Using XCMS. Methods Mol. Biol. 2020, 2104, 11–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xu, J.; Bian, C.; Duan, S.; Hu, J.; Qin, J.; Wu, H.; He, M.; Jian, Y.; Duan, Y.; et al. Natural Variation of StNADC Regulates Plant Senescence in Tetraploid Potatoes (Solanum tuberosum L.). Int. J. Mol. Sci. 2025, 26, 4389. https://doi.org/10.3390/ijms26094389
Zhang J, Xu J, Bian C, Duan S, Hu J, Qin J, Wu H, He M, Jian Y, Duan Y, et al. Natural Variation of StNADC Regulates Plant Senescence in Tetraploid Potatoes (Solanum tuberosum L.). International Journal of Molecular Sciences. 2025; 26(9):4389. https://doi.org/10.3390/ijms26094389
Chicago/Turabian StyleZhang, Jiaojiao, Jianfei Xu, Chunsong Bian, Shaoguang Duan, Jun Hu, Junhong Qin, Huan Wu, Ming He, Yinqiao Jian, Yanfeng Duan, and et al. 2025. "Natural Variation of StNADC Regulates Plant Senescence in Tetraploid Potatoes (Solanum tuberosum L.)" International Journal of Molecular Sciences 26, no. 9: 4389. https://doi.org/10.3390/ijms26094389
APA StyleZhang, J., Xu, J., Bian, C., Duan, S., Hu, J., Qin, J., Wu, H., He, M., Jian, Y., Duan, Y., Liu, J., Wang, W., Li, G., & Jin, L. (2025). Natural Variation of StNADC Regulates Plant Senescence in Tetraploid Potatoes (Solanum tuberosum L.). International Journal of Molecular Sciences, 26(9), 4389. https://doi.org/10.3390/ijms26094389







