Soft-Tissue Sarcomas—A Correlation Among Tumor Margin Infiltration, Immunological Markers, and Survival Rate
Abstract
1. Introduction
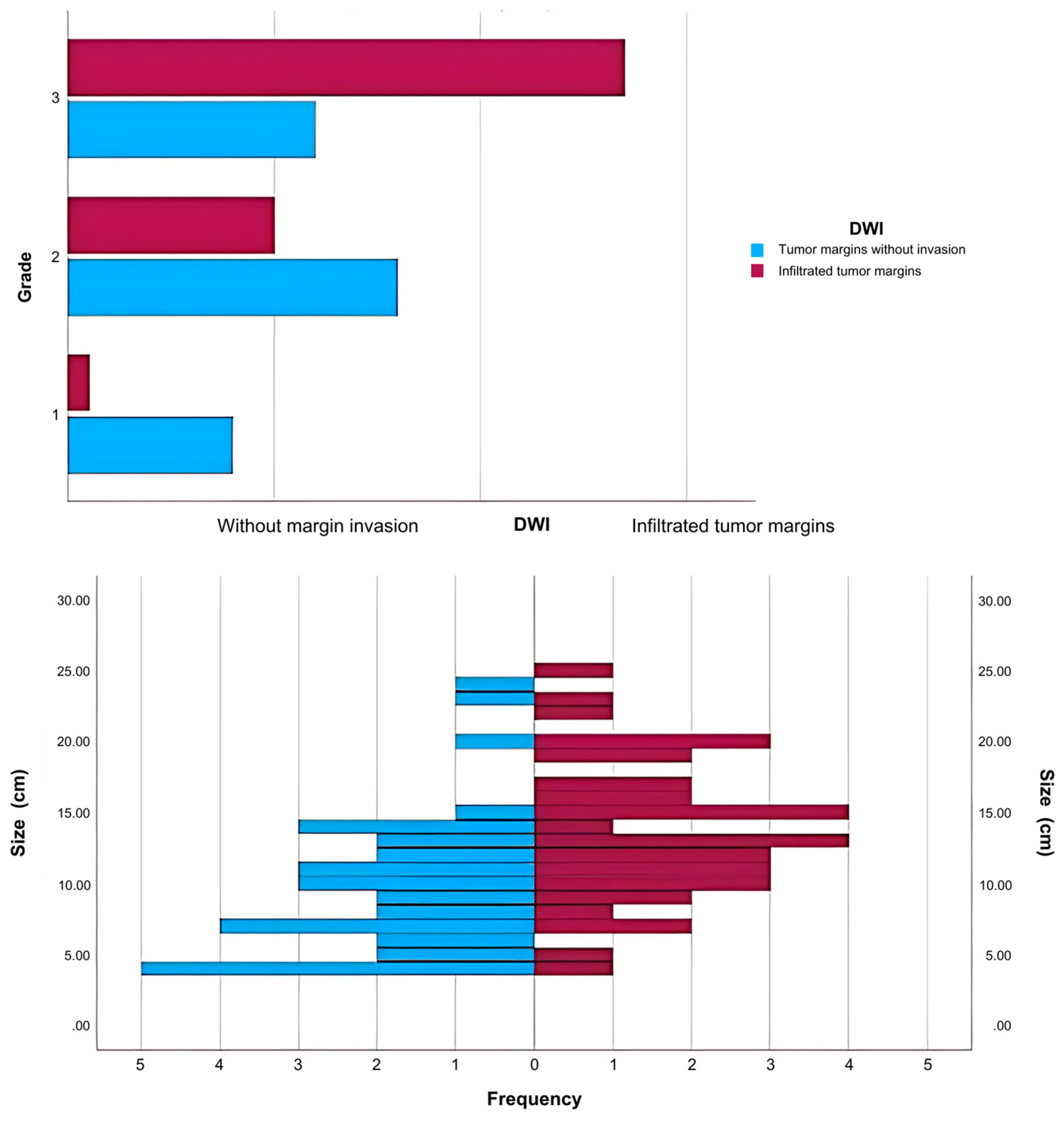
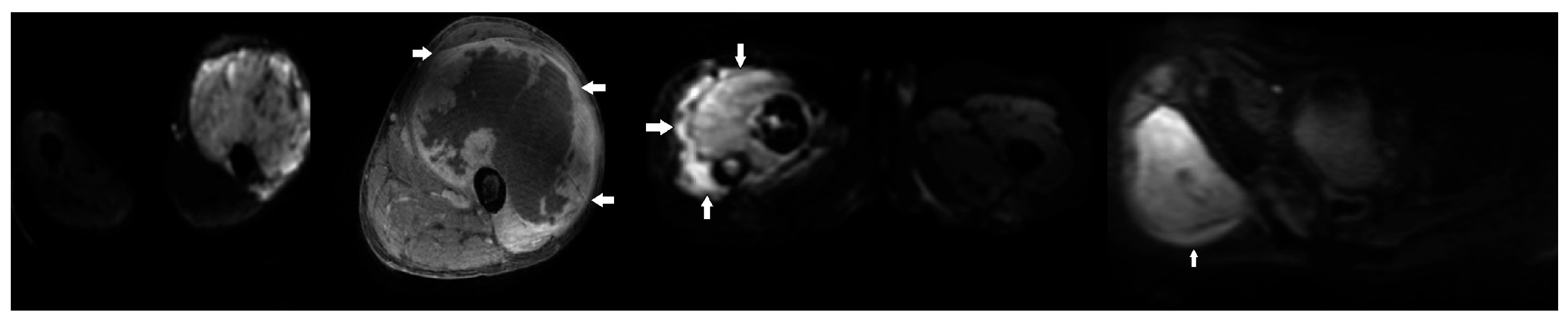
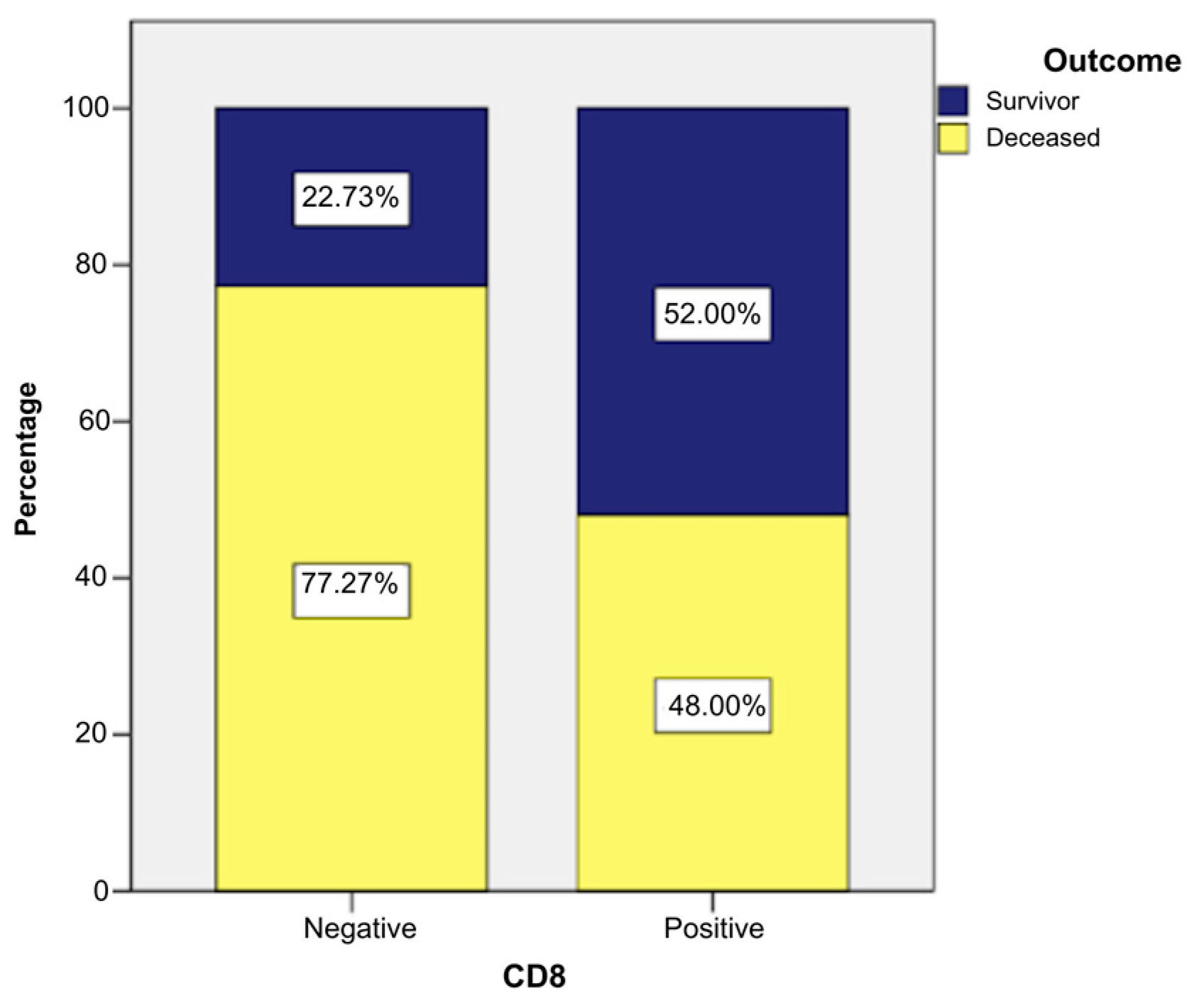
2. Results
- Grade I: 26.09% of CD4-positive patients (n = 6) vs. 4.35% CD4-negative patients (n = 2).
- Grade II: 52.17% of CD4-positive patients (n = 12) vs. 28.26% CD4-negative patients (n = 13).
- Grade III: 21.74% of CD4-positive patients (n = 5) vs. 67.39% CD4-negative patients (n = 31).
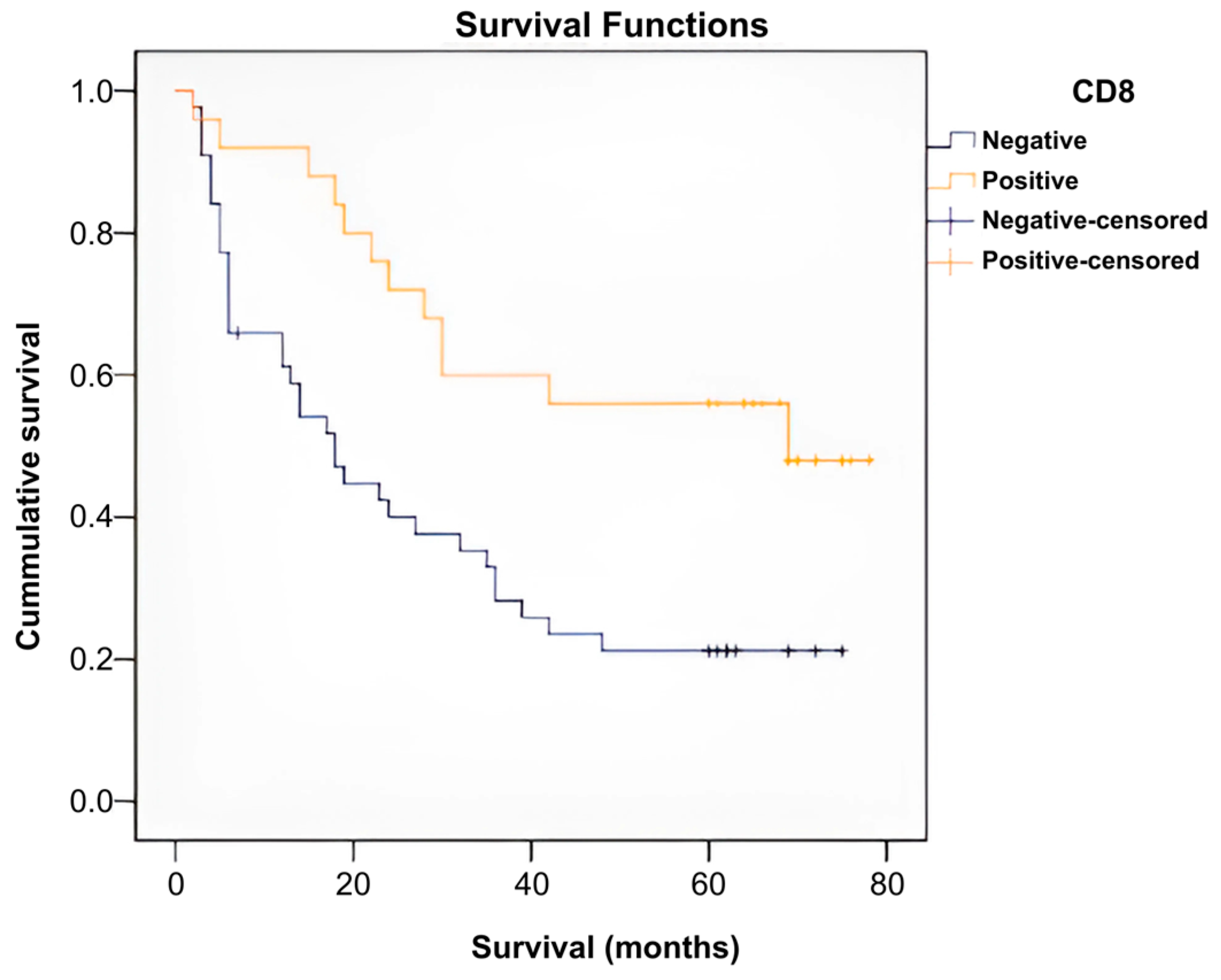
- Grade I: 28% of CD8-positive patients (n = 7) vs. 2.27% CD8-negative patients (n = 1).
- Grade II: 44% of CD8-positive patients (n = 11) vs. 31.82% CD8-negative patients (n = 14).
- Grade III: 28% of CD8-positive patients (n = 7) vs. 65.91% CD8-negative patients (n = 29).
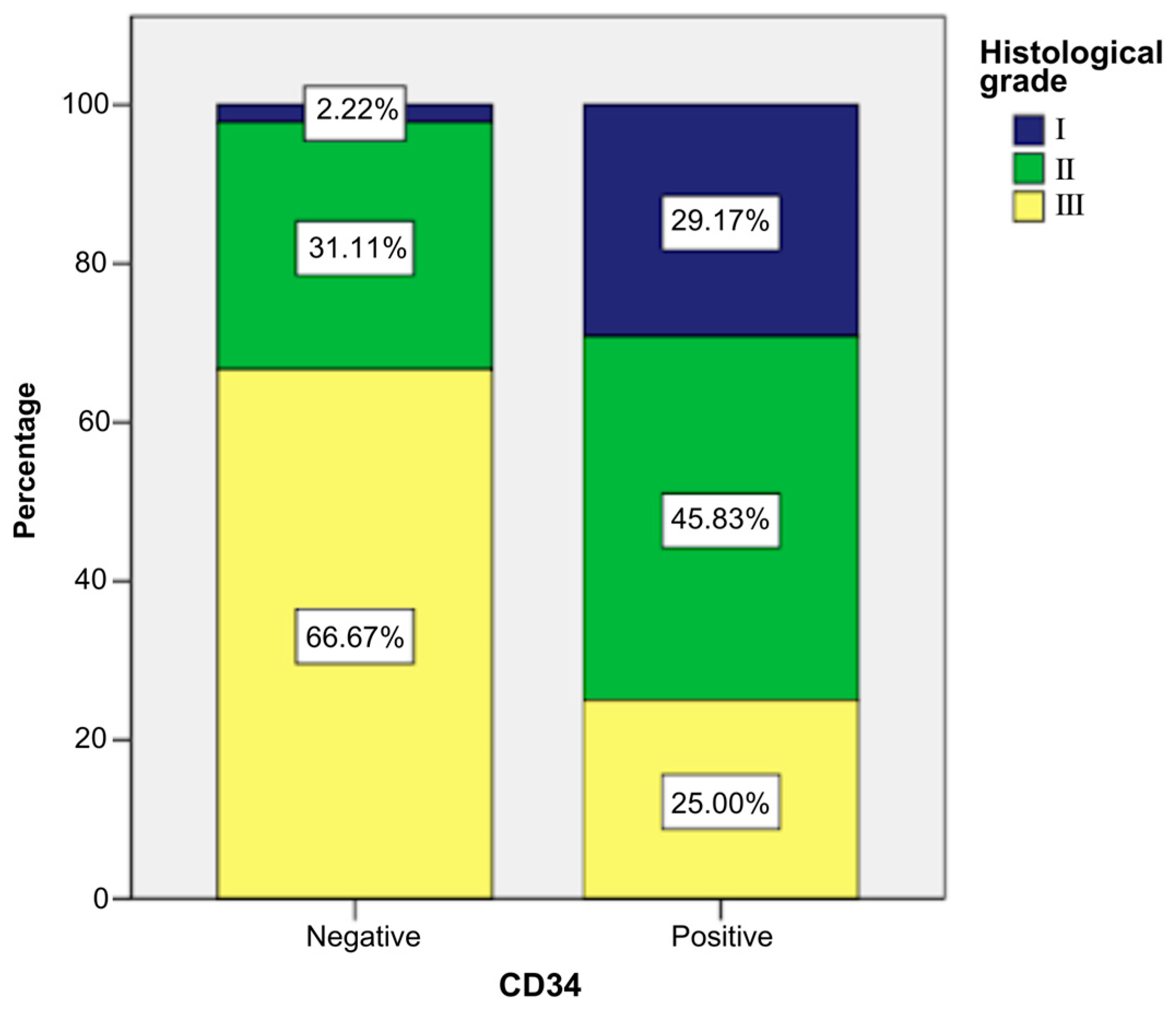
- Grade I: 29.17% of CD34-positive patients (n = 7) vs. 2.22% CD34-negative patients (n = 1).
- Grade II: 45.83% of CD34-positive patients (n = 11) vs. 31.11% CD34-negative patients (n = 14).
- Grade III: 25% of CD34-positive patients (n = 6) vs. 66.67% CD34-negative patients (n = 30).
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPECT-CT | single photon emission tomography |
| STS | soft-tissue sarcomas |
| DWI | diffusion-weighted imaging |
| MRI | magnetic resonance imaging |
| CT | computer-tomography |
| IHC | Immunohistochemistry |
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.cancer.org/cancer/sarcoma-adultsofttissuecancer/detailedguide/sarcoma-adult-soft-tissue-cancer-key-statistics (accessed on 20 December 2024).
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Li, X.Q.; Parkekh, S.G.; Rosenberg, A.E.; Mankin, H.J. Assessing prognosis for high-grade soft-tissue sarcomas: Search for a marker. Ann. Surg. Oncol. 1996, 3, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Zahm, S.H.; Fraumeni, J.F., Jr. The epidemiology of soft tissue sarcoma. Semin. Oncol. 1997, 24, 504–514. [Google Scholar] [PubMed]
- Mastrangelo, G.; Fadda, E.; Cegolon, L.; Montesco, M.C.; Ray-Coquard, I.; Buja, A.; Fedeli, U.; Frasson, A.; Spolaore, P.; Rossi, C.R. A European project on incidence, treatment, and outcome of sarcoma. BMC Public Health 2010, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Kransdorf, M.J.; Murphey, M.D.; Wessell, D.E.; Cassidy, R.C.; Czuczman, G.J.; Demertzis, J.L.; Lenchik, L.; Motamedi, K.; Pierce, J.L.; Sharma, A.; et al. ACR appropriateness criteria® soft-tissue masses. J. Am. Coll. Radiol. 2018, 15, S189–S197. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Lopes Dias, J.; Cunha, T.M. Added value of diffusion-weighted MRI in detection of cervical cancer recurrence: Comparison with morphologic and dynamic contrast-enhanced MRI sequences. Diagn. Interv. Radiol. 2015, 21, 368–375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
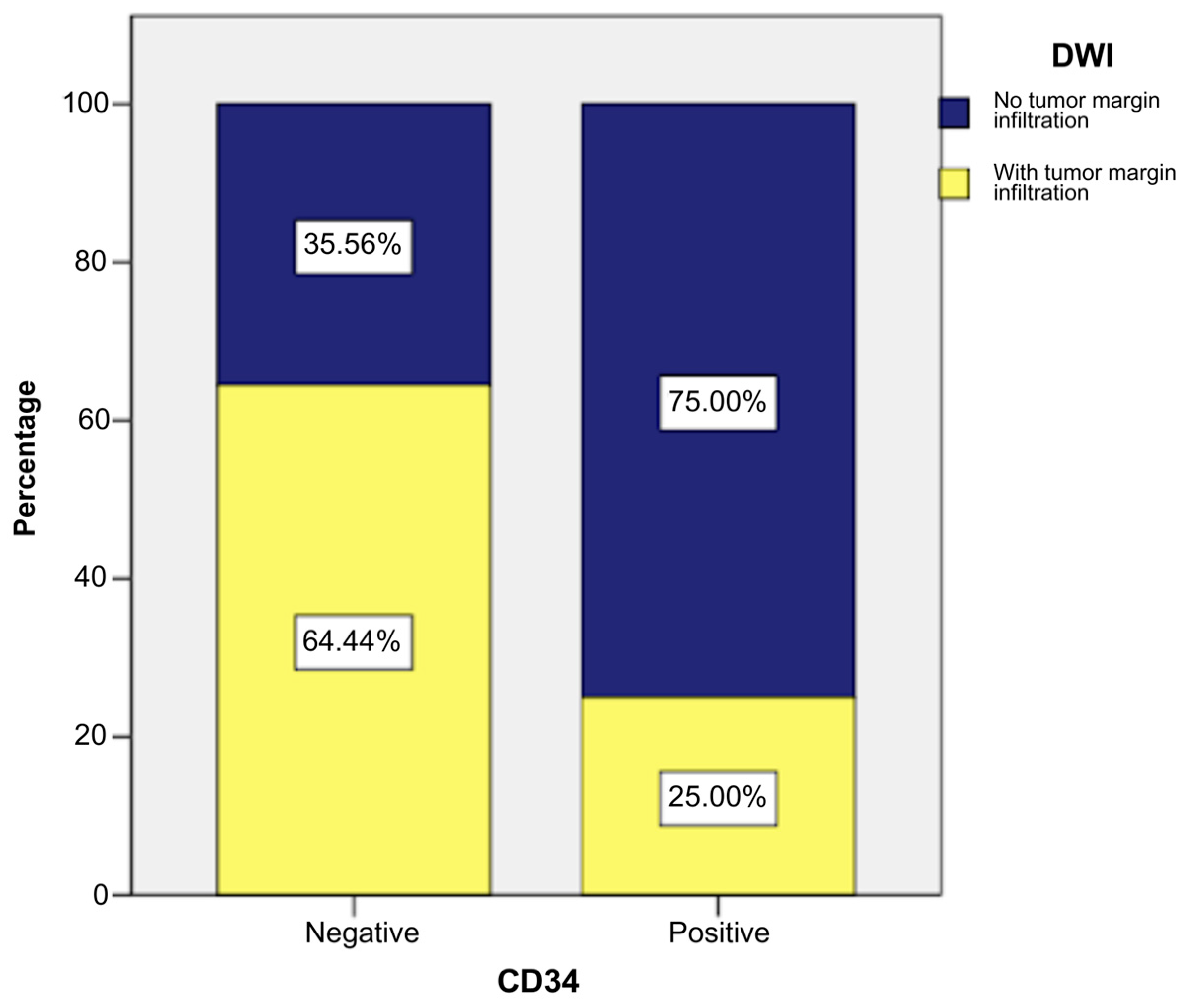
- Hong, J.H.; Jee, W.H.; Jung, C.K.; Jung, J.Y.; Shin, S.H.; Chung, Y.G. Soft tissue sarcoma: Adding diffusion-weighted imaging improves MR imaging evaluation of tumor margin infiltration. Eur. Radiol. 2019, 29, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, J.; Ge, H.; Hu, X.; Yang, K.; Liu, Y.; Hu, G.; Luo, B.; Yan, Z.; Song, K.; et al. Differentiation of malignant brain tumor types using intratumoral and peritumoral radiomic features. Front. Oncol. 2022, 12, 848846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serban, B.; Popa, M.I.G.; Cursaru, A.; Cretu, B.; Iacobescu, G.L.; Cirstoiu, C.; Iordache, S. Enhancing Diagnosis and Prognosis by Assessing the Clinical, Morphological, and Behavior Aspects in Soft Tissue Sarcomas. Cureus 2024, 16, e64025. [Google Scholar] [CrossRef] [PubMed]
- Serban, B.; Radu, E.; Cursaru, A.; Cretu, B.S.; Iordache, S.A.; Cirnu, M.; Niculae, C.F.; Cîrstoiu, C.F. Survival prognostic factors and molecular aspects in extremity soft tissue sarcoma. J. Mind Med. Sci. 2024, 11, 388–395. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.; Smădeanu, R.E.; Simionescu, A.A.; Nedelea, F.; Vlad, A.M.; Becheanu, C. Menke-Hennekam Syndrome: A Literature Review and a New Case Report. Children 2022, 9, 759. [Google Scholar] [CrossRef] [PubMed]
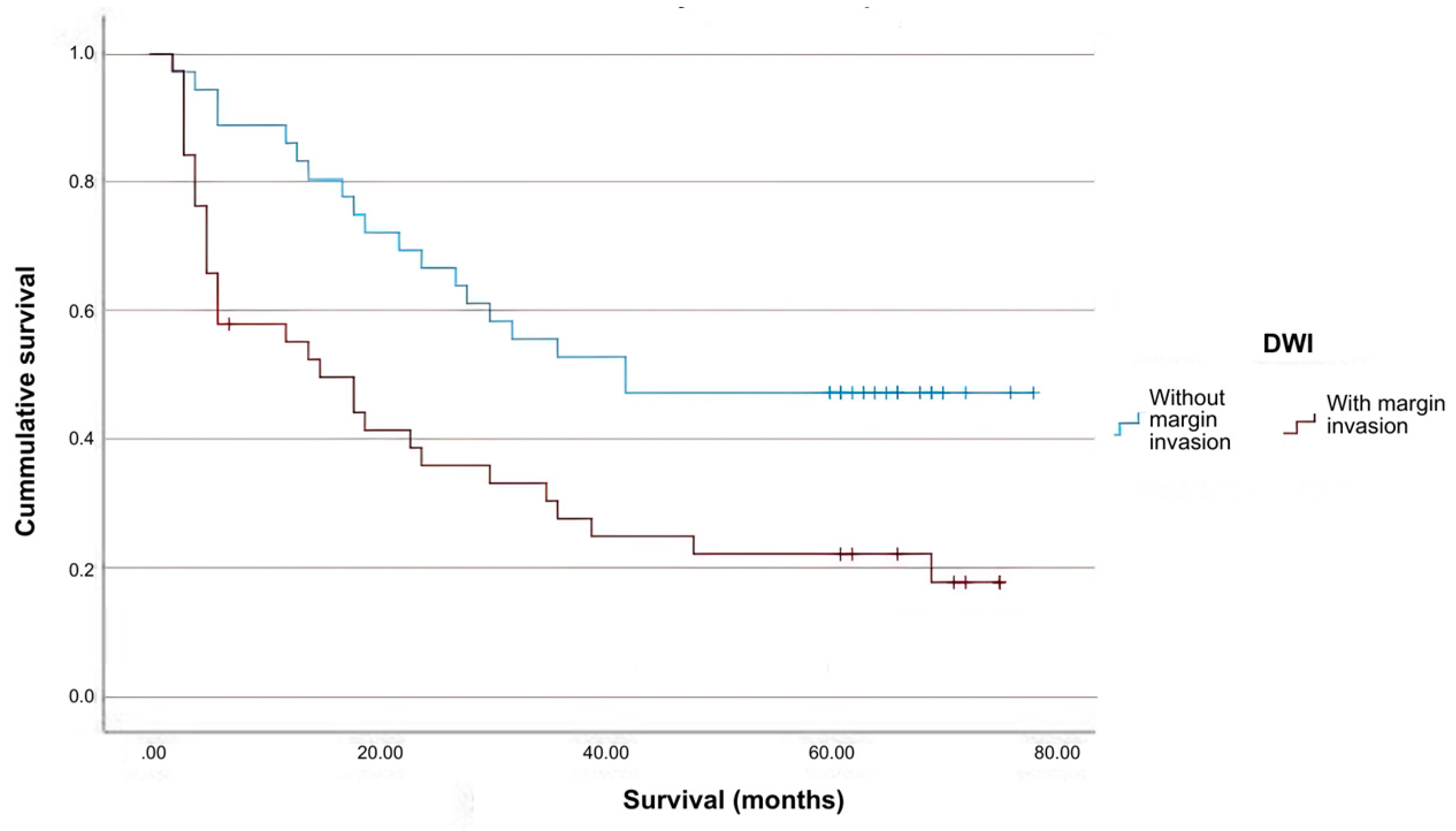




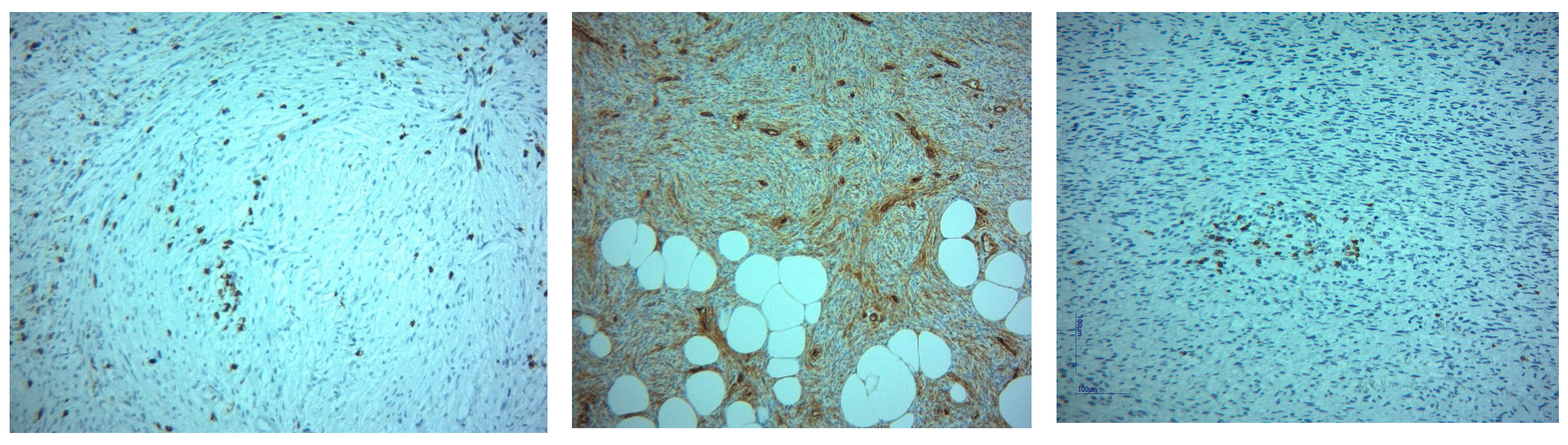
| Means and Medians for Survival Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD4 | Mean a | Median | ||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Negative | 26.325 | 3.593 | 19.283 | 33.366 | 18.000 | 3.302 | 11.527 | 24.473 |
| Positive | 58.652 | 5.693 | 47.494 | 69.811 | . | . | . | . |
| Overall | 37.738 | 3.660 | 30.565 | 44.911 | 28.000 | 5.125 | 17.956 | 38.044 |
| Means and Medians for Survival Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD8 | Mean a | Median | ||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Negativ | 28.709 | 4.070 | 20.732 | 36.687 | 18.000 | 3.227 | 11.674 | 24.326 |
| Positive | 52.360 | 5.724 | 41.141 | 63.579 | 69.000 | . | . | . |
| Overall | 37.738 | 3.660 | 30.565 | 44.911 | 28.000 | 5.125 | 17.956 | 38.044 |
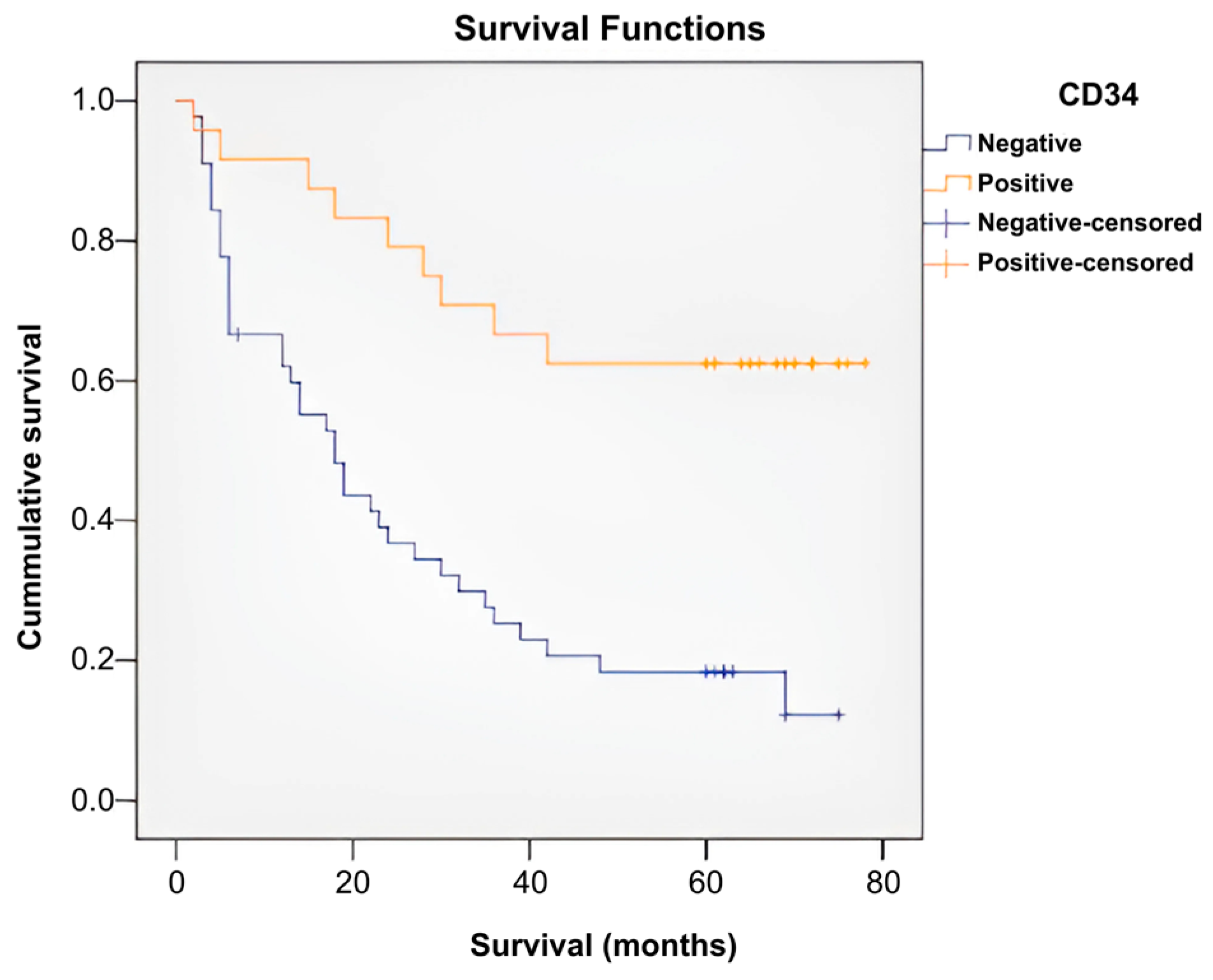
| Means and Medians for Survival Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD34 | Mean a | Median | ||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Negativ | 26.752 | 3.742 | 19.418 | 34.086 | 18.000 | 3.270 | 11.591 | 24.409 |
| Pozitiv | 57.083 | 5.736 | 45.840 | 68.327 | . | . | . | . |
| Overall | 37.738 | 3.660 | 30.565 | 44.911 | 28.000 | 5.125 | 17.956 | 38.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, B.; Cursaru, A.; Iordache, S.; Costache, M.; Cretu, B.; Dumitru, A.; Cirstoiu, C. Soft-Tissue Sarcomas—A Correlation Among Tumor Margin Infiltration, Immunological Markers, and Survival Rate. Int. J. Mol. Sci. 2025, 26, 4363. https://doi.org/10.3390/ijms26094363
Șerban B, Cursaru A, Iordache S, Costache M, Cretu B, Dumitru A, Cirstoiu C. Soft-Tissue Sarcomas—A Correlation Among Tumor Margin Infiltration, Immunological Markers, and Survival Rate. International Journal of Molecular Sciences. 2025; 26(9):4363. https://doi.org/10.3390/ijms26094363
Chicago/Turabian StyleȘerban, Bogdan, Adrian Cursaru, Sergiu Iordache, Mihai Costache, Bogdan Cretu, Adrian Dumitru, and Catalin Cirstoiu. 2025. "Soft-Tissue Sarcomas—A Correlation Among Tumor Margin Infiltration, Immunological Markers, and Survival Rate" International Journal of Molecular Sciences 26, no. 9: 4363. https://doi.org/10.3390/ijms26094363
APA StyleȘerban, B., Cursaru, A., Iordache, S., Costache, M., Cretu, B., Dumitru, A., & Cirstoiu, C. (2025). Soft-Tissue Sarcomas—A Correlation Among Tumor Margin Infiltration, Immunological Markers, and Survival Rate. International Journal of Molecular Sciences, 26(9), 4363. https://doi.org/10.3390/ijms26094363






