Extracellular Vesicles in Sport Horses: Potential Biomarkers and Modulators of Exercise Adaptation and Therapeutics
Abstract
1. Introduction
2. Biological Basis of EVs
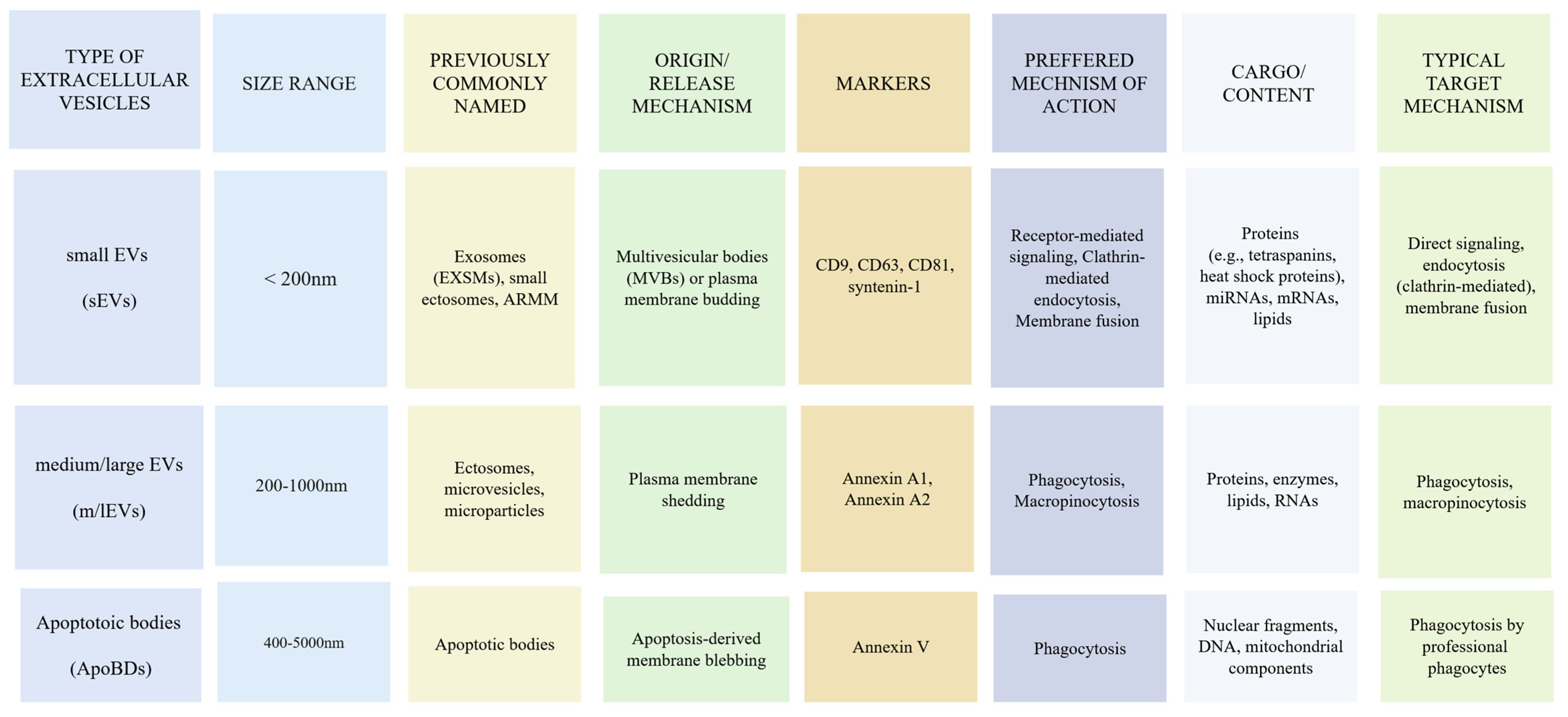
2.1. Biogenesis, Release, and Classification of EVs
2.2. Mechanisms of Extracellular Vesicle-Mediated Cargo Delivery and Cellular Modulation
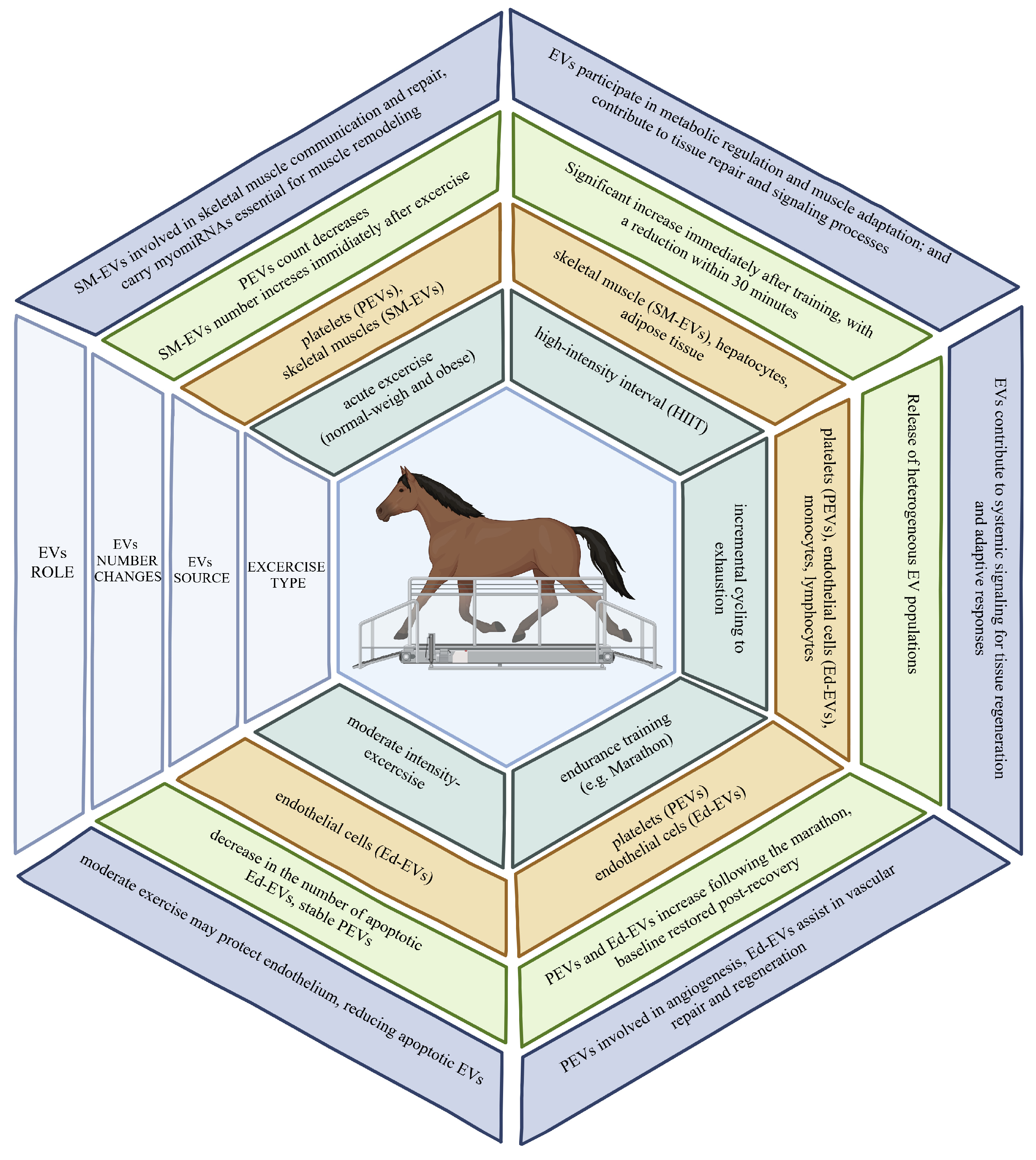
2.3. Time Course of Circulating EV Release in Relation to Exercise Type
3. Role of EVs in Athletic Performance Enhancement
| EV Source | Molecular Cargo | Role in Exercise Adaptation | Potential Use as Biomarkers | Reference |
|---|---|---|---|---|
| Skeletal Muscle EVs (SkM-EVs) | miRNAs (miR-1, miR-133a, miR-206), proteins | These miRNAs regulate muscle adaptation, myogenesis, and tissue repair during and after exercise | Can indicate muscle damage, repair, and remodeling post-exercise | [66,69] |
| Platelet EVs (PEVs) | VEGF, HGF, PDGF | Supports angiogenesis and enhances vascular response to exercise, helping oxygen and nutrient delivery | Can be used to monitor recovery, vascular health, and response to endurance training | [70,71] |
| Endothelial EVs (Ed-EVs) | MMP-2, MMP-9, miR-126 | Promotes vascular remodeling, essential for sustaining increased blood flow during prolonged exercise | Useful in detecting vascular health and adaptation to endurance exercises | [72,73] |
| Exercise-Derived EVs (Ex-EVs) | Proteins (PI3K, ERK), miRNAs, lipids | Mediates systemic metabolic adaptations and intercellular communication for energy utilization and tissue repair | Potential non-invasive biomarkers for fitness level, oxidative stress, and early detection of overtraining | [53,58,74] |
| Adipose Tissue EVs | Exerkines, miRNAs | Plays a role in fat metabolism and energy homeostasis during exercise | Can be used to monitor metabolic adaptations to exercise | [75,76] |
3.1. Antioxidant Potential of EVs
3.2. EVs as Metabolic Regulators
3.3. Angiogenic Potential of EVs
| EV Source | Key Factors Transported by EVs | Role in Angiogenesis | Role in Muscle Regeneration | References |
|---|---|---|---|---|
| Platelets (PEVs) | VEGF, HGF, PDGF, FGF-2 | Facilitates new blood vessel formation (angiogenesis) by promoting endothelial cell proliferation and migration | Enhances tissue regeneration by improving oxygen and nutrient supply to muscles | [50,87,88,89,97,98,99] |
| Endothelial Cells (Ed-EVs) | MMP-2, MMP-9, Integrins | Promotes endothelial cell adhesion and tubule formation, vital for new capillary networks | Supports tissue repair by increasing vascularization in damaged areas | [71,93,97,98] |
| Skeletal Muscle EVs (SkM-EVs) | miR-206, miR-133b, miR-146a | Does not promote angiogenesis directly, but involved in muscle communication and repair | Delivers myomiRNAs to support muscle cell proliferation and differentiation, crucial for muscle repair | [89,90,97,100,101] |
| Exercise-Derived EVs (Ex-EVs) | PI3k, ERK, VEGF, ANG1, TIE2 | Enhances endothelial proliferation and migration, leading to better blood flow and oxygen supply to exercising muscles | Plays a significant role in long-term muscle adaptation and injury recovery | [72,73,96,97,98] |
3.4. Immunomodulatory Role of Exercise-Released EVs
4. EVs as Biomarkers for Monitoring and Assessing Fitness Adaptation
5. Therapeutic Potential of EVs in Sports Medicine
| Context | Role of EVs | Specific Actions and Outcomes | Reference |
|---|---|---|---|
| Muscle Injury and Regeneration | Alteration of miRNA content in EVs after exercise-induced injury | Potential marker for muscle damage and diseases like Duchenne and myotonic dystrophy | [97,100,101] |
| Skeletal Muscle Regeneration | EVs carrying muscle-specific miRNAs (myo-miRNAs) support muscle repair | Protection of sensitive miRNAs, involvement of satellite muscle cells (SMSCs) in repair and inflammation reduction | [98,99] |
| SMSC-Derived EVs | Transfer miRNAs that regulate extracellular matrix genes like MMP-9 | Promotes muscle growth and supports the expression of regeneration-related proteins during tetanic contractions | [112,113] |
| Healing and Functional Recovery | EVs contribute to recovery post-injury | Increases muscle hypertrophy and regeneration-related proteins | [114] |
| Tendon and Ligament Repair | EV-based treatments modulate inflammation and support remodeling | Promotes macrophage polarization, enhances angiogenesis, and supports extracellular matrix remodeling | [114] |
| Cartilage Repair (OA Models) | BMSC-EXSMs promote cartilage repair by modulating inflammation and gene expression | Induces M2 macrophage polarization, reduces inflammation, upregulates miR-135b, supporting cartilage repair | [115] |
| Tendon-Bone Healing (ACLR Model) | BMSC-EXSMs support repair site by reducing inflammation | Enhances tendon-bone healing through inflammation reduction at the repair site | [116] |
| Therapeutic Potential in Sports Medicine | EVs as therapeutic agents in musculoskeletal repair | Potential for targeted therapies, especially in enhancing muscle regeneration and musculoskeletal tissue healing | [116] |
6. Methodology Limitations in Extracellular Vesicle Studies
| Parameter | Method (Widely Used) | Recommendations | Major Limitations | Most Suitable For | References |
|---|---|---|---|---|---|
| EV Quantification (Number/Concentration) | Flow cytometry (FCM), nanoparticle tracking analysis (NTA) | Report method limit of detection; describe instrument settings; present diameter distribution. | Lack specificity for EVs; low sensitivity for small EVs. | EVs down to ~100 nm (FCM), few 100 nm (NTA). | [118,119,120] |
| Particle Size (Diameter) | FCM, NTA | Due to asymmetric size distribution, detailed diameter data should be presented. | Inaccurate size measurement across full EV size range. | Small EVs (~30–200 nm). | [121,122] |
| Morphology | SEM, TEM, cryo-TEM | Report all experimental conditions; use cryo-TEM to avoid dehydration artifacts. | Underrepresentation of larger EVs; technical limitations in conventional EM. | EVs down to ~200 nm diameter. | [118,123] |
| Protein Composition | Western blotting (WB), mass spectrometry | Analyze at least one marker each from categories 1, 2 (EV markers), and 3 (purity controls). | Lack of universal molecular markers for specific EV subtypes. | Applicable to all EV types. | [119,120] |
| Nucleic Acids | Low-input RNA sequencing, quantitative PCR (qPCR) | For qPCR, report primer/adaptor sequences; for RNA-Seq, describe RNA fragmentation and RT procedures. | No standardized methods to protect surface DNA from degradation during isolation. | Applicable to all EV types. | [122,123] |
6.1. Blood Collection
6.2. Isolation and Storage
6.3. Characterization
7. Mechanistic and Clinical Challenges in Extracellular Vesicle Research: Barriers to Therapeutic and Diagnostic Applications
8. Extracellular Vesicles in Equine Science and Sport Horses
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACLR | Anterior cruciate ligament reconstruction |
| ANG1 | Angiopoietin-1 |
| ApoBDs | Apoptotic bodies |
| ARMMs | Arrestin domain-containing protein 1-mediated microvesicles |
| BMSCs-EXSMs | Bone marrow mesenchymal stem cell-derived exosomes |
| CAT | Catalase |
| DC/UC | Differential centrifugation/ultracentrifugation |
| DDFT | Deep digital flexor tendons |
| EGF | Endothelial Growth Factor |
| EMS | Equine metabolic syndrome |
| EM | Electron microscopy |
| EdEVs | Endothelial-derived EVs |
| EXSMs | Exosomes |
| ESCRT | Endosomal sorting complex required for transport |
| EVs | Extracellular vesicles |
| Ex-EVs | Exercise-derived EVs |
| FBS | Fetal bovine serum |
| FCM | Flow cytometry |
| FGF | Fibroblast growth factor |
| FR | Free radical |
| HIIT | High-intensity interval training |
| HLRE | High-load resistance exercise |
| HS | Heat shock proteins |
| HSF1 | Heat shock factor 1 |
| IL-3 | Interleukin-3 |
| ILVs | Intraluminal vesicles |
| ISEV | International Society for Extracellular Vesicles |
| ISS | Intraluminal shear stress |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-9 | Matrix metalloproteinase-9 |
| MPs | Microparticles |
| MSC-EVs | Mesenchymal stem cell-derived EVs |
| MVB | Multivesicular body |
| MVs | Microvesicles |
| Mcp1 | Monocyte chemoattractant protein 1 |
| m/lEVs | Medium/large EVs |
| NBF | Navicular bone fibrocartilage |
| NGS | Next-generation sequencing |
| NTA | Nanoparticle tracking analysis |
| OA | Osteoarthritis |
| OTS | Overtraining syndrome |
| PBS-HAT | PBS with human albumin and trehalose |
| PDGF | Platelet-derived growth factor |
| PEVs | Platelet-derived EVs |
| PGC-1a | Peroxisome proliferator-activated receptor gamma co-activator 1-alpha |
| ROS | Reactive oxygen species |
| SEM | Scanning Electron Microscopy |
| sEVs | Small EVs |
| SIRT1 | Sirtuin-1 |
| SM | Skeletal muscle |
| SM-EVs | Skeletal muscle-derived EVs |
| SMSCs | Satellite muscle cells |
| SOD2 | Superoxide dismutase 2 |
| TEM | Transmission electron microscopy |
| USADA | USA. Anti-Doping Agency |
| VEGF | Vascular endothelial growth factor |
| WB | Western blotting |
| WADA | World Anti-Doping Agency |
References
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 604274. [Google Scholar] [CrossRef] [PubMed]
- Miko, H.-C.; Zillmann, N.; Ring-Dimitriou, S.; Dorner, T.E.; Titze, S.; Bauer, R. Effects of Physical Activity on Health. Gesundheitswesen Bundesverb. Arzte Offentlichen Gesundheitsdienstes Ger. 2020, 82, S184–S195. [Google Scholar] [CrossRef]
- Burns, S.F.; Hardman, A.E.; Stensel, D.J. Brisk Walking Offsets the Increase in Postprandial TAG Concentrations Found When Changing to a Diet with Increased Carbohydrate. Br. J. Nutr. 2009, 101, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Giannetto, C.; Fazio, F.; Panzera, F.; Piccione, G. Training Program Intensity Induces an Acute Phase Response in Clinically Healthy Horses. J. Equine Vet. Sci. 2020, 88, 102986. [Google Scholar] [CrossRef]
- Ben-Zeev, T.; Okun, E. High-Intensity Functional Training: Molecular Mechanisms and Benefits. Neuromol. Med. 2021, 23, 335–338. [Google Scholar] [CrossRef]
- Barbosa, A.; Siniossoglou, S. New Kid on the Block: Lipid Droplets in the Nucleus. FEBS J. 2020, 287, 4838–4843. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.S. Illuminating the Physiology of Extracellular Vesicles. Stem Cell Res. Ther. 2016, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular Vesicles: Masters of Intercellular Communication and Potential Clinical Interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Alberro, A.; Iparraguirre, L.; Fernandes, A.; Otaegui, D. Extracellular Vesicles in Blood: Sources, Effects, and Applications. Int. J. Mol. Sci. 2021, 22, 8163. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N.S.; Dhillon, N.K. Extracellular Vesicles: Novel Communicators in Lung Diseases. Respir. Res. 2020, 21, 175. [Google Scholar] [CrossRef] [PubMed]
- Zmigrodzka, M.; Witkowska-Pilaszewicz, O.; Pingwara, R.; Pawlak, A.; Winnicka, A. Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 9831. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, C.; Chen, M.; Yang, T.; Wang, X.; Gao, Y.; Wang, L.; Wen, Y.; Chen, G.; Wang, X.; et al. Extracellular Vesicle-Mediated Delivery of miR-101 Inhibits Lung Metastasis in Osteosarcoma. Theranostics 2020, 10, 411–425. [Google Scholar] [CrossRef]
- Verweij, F.J.; Balaj, L.; Boulanger, C.M.; Carter, D.R.F.; Compeer, E.B.; D’Angelo, G.; El Andaloussi, S.; Goetz, J.G.; Gross, J.C.; Hyenne, V.; et al. The Power of Imaging to Understand Extracellular Vesicle Biology In Vivo. Nat. Methods 2021, 18, 1013–1026. [Google Scholar] [CrossRef]
- Menck, K.; Bleckmann, A.; Schulz, M.; Ries, L.; Binder, C. Isolation and Characterization of Microvesicles from Peripheral Blood. J. Vis. Exp. JoVE 2017, 119, 55057. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Winnicka, A. Platelets Extracellular Vesicles as Regulators of Cancer Progression—An Updated Perspective. Int. J. Mol. Sci. 2020, 21, 5195. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.D.; Rimmer, M.P. Extracellular Vesicles Arising from Apoptosis: Forms, Functions, and Applications. J. Pathol. 2023, 260, 592–608. [Google Scholar] [CrossRef]
- Witwer, K.W.; Théry, C. Extracellular Vesicles or Exosomes? On Primacy, Precision, and Popularity Influencing a Choice of Nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-Derived Microvesicles: Shedding Light on Novel Microenvironment Modulators and Prospective Cancer Biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- D’Souza-Schorey, C.; Schorey, J.S. Regulation and Mechanisms of Extracellular Vesicle Biogenesis and Secretion. Essays Biochem. 2018, 62, 125–133. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular Organelles Important in Intercellular Communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.J.; Speth, J.M.; Penke, L.R.; Wettlaufer, S.H.; Swanson, J.A.; Peters-Golden, M. Mechanisms and Modulation of Microvesicle Uptake in a Model of Alveolar Cell Communication. J. Biol. Chem. 2017, 292, 20897–20910. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular Microvesicles/Exosomes: Discovery, Disbelief, Acceptance, and the Future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome Maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-like Vesicles, and Apoptotic Bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Bergsmedh, A.; Szeles, A.; Henriksson, M.; Bratt, A.; Folkman, M.J.; Spetz, A.-L.; Holmgren, L. Horizontal Transfer of Oncogenes by Uptake of Apoptotic Bodies. Proc. Natl. Acad. Sci. USA 2001, 98, 6407–6411. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III–Dependent Sorting of Tetraspanins to Exosomes. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Kowal, E.J.K.; Ter-Ovanesyan, D.; Regev, A.; Church, G.M. Extracellular Vesicle Isolation and Analysis by Western blotting. Methods Mol. Biol. 2017, 1660, 143–152. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Vicario, E.; Pucci, M.; Toscani, D.; Manno, M.; Raccosta, S.; Giuliani, N.; Alessandro, R. Multiple Myeloma-Derived Exosomes Are Enriched of Amphiregulin (AREG) and Activate the Epidermal Growth Factor Pathway in the Bone Microenvironment Leading to Osteoclastogenesis. J. Hematol. Oncol. 2019, 12, 2. [Google Scholar] [CrossRef]
- Liu, A.; Pan, W.; Zhuang, S.; Tang, Y.; Zhang, H. Cancer Cell-Derived Exosomal miR-425-3p Induces White Adipocyte Atrophy. Adipocyte 2022, 11, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef]
- Chaput, N.; Taïeb, J.; Schartz, N.E.C.; André, F.; Angevin, E.; Zitvogel, L. Exosome-Based Immunotherapy. Cancer Immunol. Immunother. 2004, 53, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes Provide a Protective and Enriched Source of miRNA for Biomarker Profiling Compared to Intracellular and Cell-Free Blood. J. Extracell. Vesicles 2014, 3, 23743. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An Emerging Focus on Lipids in Extracellular Vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of Extracellular Vesicle DNA at the Single-vesicle Level by Nano-flow Cytometry. J. Extracell. Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic Roles of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.C.; Dean, T.T.; Minari, K.; Basso, L.G.M.; Vance, T.D.R.; Serrão, V.H.B. A Frame-by-Frame Glance at Membrane Fusion Mechanisms: From Viral Infections to Fertilization. Biomolecules 2023, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, D.V.; Zubarev, A.Y. Transport Phenomena in Complex Systems (Part 1). Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2021, 379, 20200301. [Google Scholar] [CrossRef]
- Guescini, M.; Canonico, B.; Lucertini, F.; Maggio, S.; Annibalini, G.; Barbieri, E.; Luchetti, F.; Papa, S.; Stocchi, V. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS ONE 2015, 10, e0125094. [Google Scholar] [CrossRef]
- Mathiesen, A.; Hamilton, T.; Carter, N.; Brown, M.; McPheat, W.; Dobrian, A. Endothelial Extracellular Vesicles: From Keepers of Health to Messengers of Disease. Int. J. Mol. Sci. 2021, 22, 4640. [Google Scholar] [CrossRef]
- Rakobowchuk, M.; Ritter, O.; Wilhelm, E.N.; Isacco, L.; Bouhaddi, M.; Degano, B.; Tordi, N.; Mourot, L. Divergent Endothelial Function but Similar Platelet Microvesicle Responses Following Eccentric and Concentric Cycling at a Similar Aerobic Power Output. J. Appl. Physiol. 2017, 122, 1031–1039. [Google Scholar] [CrossRef]
- Wilhelm, E.N.; González-Alonso, J.; Parris, C.; Rakobowchuk, M. Exercise Intensity Modulates the Appearance of Circulating Microvesicles with Proangiogenic Potential upon Endothelial Cells. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H1297–H1310. [Google Scholar] [CrossRef]
- Kirk, R.J.; Peart, D.J.; Madden, L.A.; Vince, R.V. Repeated Supra-maximal Sprint Cycling with and without Sodium Bicarbonate Supplementation Induces Endothelial Microparticle Release. Eur. J. Sport Sci. 2014, 14, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sossdorf, M.; Otto, G.P.; Claus, R.A.; Gabriel, H.H.W.; Lösche, W. Cell-Derived Microparticles Promote Coagulation after Moderate Exercise. Med. Sci. Sports Exerc. 2011, 43, 1169–1176. [Google Scholar] [CrossRef]
- Jenkins, N.T.; Padilla, J.; Boyle, L.J.; Credeur, D.P.; Laughlin, M.H.; Fadel, P.J. Disturbed Blood Flow Acutely Induces Activation and Apoptosis of the Human Vascular Endothelium. Hypertension 2013, 61, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Serviente, C.; Burnside, A.; Witkowski, S. Moderate-Intensity Exercise Reduces Activated and Apoptotic Endothelial Microparticles in Healthy Midlife Women. J. Appl. Physiol. 2019, 126, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E. Physical Exercise Induces Rapid Release of Small Extracellular Vesicles into the Circulation. J. Extracell. Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Eguchi, A.; Tamai, Y.; Fukuda, S.; Tempaku, M.; Izuoka, K.; Iwasa, M.; Takei, Y.; Togashi, K. Protein Composition of Circulating Extracellular Vesicles Immediately Changed by Particular Short Time of High-Intensity Interval Training Exercise. Front. Physiol. 2021, 12, 693007. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Bollati, V.; Pergoli, L.; Iodice, S.; De Col, A.; Tamini, S.; Cicolini, S.; Tringali, G.; De Micheli, R.; Cella, S.G.; et al. Effects of an Acute Bout of Exercise on Circulating Extracellular Vesicles: Tissue-, Sex-, and BMI-Related Differences. Int. J. Obes. 2020, 44, 1108–1118. [Google Scholar] [CrossRef]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.-M. Platelets, Endothelial Cells and Leukocytes Contribute to the Exercise-Triggered Release of Extracellular Vesicles into the Circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- Kearns, C.F.; McKeever, K.H.; Abe, T. Overview of Horse Body Composition and Muscle Architecture: Implications for Performance. Vet. J. 2002, 164, 224–234. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.S.; Cox, J.; Jensen, L.J.; Meissner, F.; Mann, M. Secretome Analysis of Lipid-Induced Insulin Resistance in Skeletal Muscle Cells by a Combined Experimental and Bioinformatics Workflow. J. Proteome Res. 2015, 14, 4885–4895. [Google Scholar] [CrossRef]
- Mytidou, C.; Koutsoulidou, A.; Katsioloudi, A.; Prokopi, M.; Kapnisis, K.; Michailidou, K.; Anayiotos, A.; Phylactou, L.A. Muscle-derived Exosomes Encapsulate myomiRs and Are Involved in Local Skeletal Muscle Tissue Communication. FASEB J. 2021, 35, e21279. [Google Scholar] [CrossRef]
- Maggio, S.; Canonico, B.; Ceccaroli, P.; Polidori, E.; Cioccoloni, A.; Giacomelli, L.; Ferri Marini, C.; Annibalini, G.; Gervasi, M.; Benelli, P.; et al. Modulation of the Circulating Extracellular Vesicles in Response to Different Exercise Regimens and Study of Their Inflammatory Effects. Int. J. Mol. Sci. 2023, 24, 3039. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Sudo, Y.; Makino, T.; Kimura, S.; Tomita, K.; Noguchi, M.; Sakurai, H.; Shimizu, M.; Takahashi, Y.; Sato, R.; et al. Skeletal Muscle Releases Extracellular Vesicles with Distinct Protein and microRNA Signatures That Function in the Muscle Microenvironment. PNAS Nexus 2022, 1, pgac173. [Google Scholar] [CrossRef]
- Russell, A.P.; Lamon, S.; Boon, H.; Wada, S.; Güller, I.; Brown, E.L.; Chibalin, A.V.; Zierath, J.R.; Snow, R.J.; Stepto, N.; et al. Regulation of miRNAs in Human Skeletal Muscle Following Acute Endurance Exercise and Short-term Endurance Training. J. Physiol. 2013, 591, 4637–4653. [Google Scholar] [CrossRef]
- Nair, V.D.; Ge, Y.; Li, S.; Pincas, H.; Jain, N.; Seenarine, N.; Amper, M.A.S.; Goodpaster, B.H.; Walsh, M.J.; Coen, P.M.; et al. Sedentary and Trained Older Men Have Distinct Circulating Exosomal microRNA Profiles at Baseline and in Response to Acute Exercise. Front. Physiol. 2020, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Aswad, H.; Jalabert, A.; Rome, S. Depleting Extracellular Vesicles from Fetal Bovine Serum Alters Proliferation and Differentiation of Skeletal Muscle Cells in Vitro. BMC Biotechnol. 2016, 16, 32. [Google Scholar] [CrossRef]
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-Induced Skeletal Muscle Atrophy and Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2307–2319. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Hood, D.A. Exercise Is Mitochondrial Medicine for Muscle. Sports Med. Health Sci. 2019, 1, 11–18. [Google Scholar] [CrossRef]
- Wahl, P.; Wehmeier, U.F.; Jansen, F.J.; Kilian, Y.; Bloch, W.; Werner, N.; Mester, J.; Hilberg, T. Acute Effects of Different Exercise Protocols on the Circulating Vascular microRNAs -16, -21, and -126 in Trained Subjects. Front. Physiol. 2016, 7, 643. [Google Scholar] [CrossRef] [PubMed]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Lin, W.; Yan, X.; Chen, X.; Xu, G. Fatiguing Freestyle Swimming Modifies miRNA Profiles of Circulating Extracellular Vesicles in Athletes. Eur. J. Appl. Physiol. 2023, 123, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Heiston, E.M.; Ballantyne, A.; Stewart, N.R.; La Salvia, S.; Musante, L.; Lanningan, J.; Erdbrügger, U.; Malin, S.K. Insulin Infusion Decreases Medium-Sized Extracellular Vesicles in Adults with Metabolic Syndrome. Am. J. Physiol.-Endocrinol. Metab. 2022, 323, E378–E388. [Google Scholar] [CrossRef]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029827. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Liu, T.; Zha, L. Extracellular Vesicles Regulate the Transmission of Insulin Resistance and Redefine Noncommunicable Diseases. Front. Mol. Biosci. 2023, 9, 1024786. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxid. Med. Cell. Longev. 2022, 2022, 3553617. [Google Scholar] [CrossRef]
- Lisi, V.; Moulton, C.; Fantini, C.; Grazioli, E.; Guidotti, F.; Sgrò, P.; Dimauro, I.; Capranica, L.; Parisi, A.; Di Luigi, L.; et al. Steady-State Redox Status in Circulating Extracellular Vesicles: A Proof-of-Principle Study on the Role of Fitness Level and Short-Term Aerobic Training in Healthy Young Males. Free Radic. Biol. Med. 2023, 204, 266–275. [Google Scholar] [CrossRef]
- Paronetto, M.P.; Dimauro, I.; Grazioli, E.; Palombo, R.; Guidotti, F.; Fantini, C.; Sgrò, P.; De Francesco, D.; Di Luigi, L.; Capranica, L.; et al. Exercise-Mediated Downregulation of MALAT1 Expression and Implications in Primary and Secondary Cancer Prevention. Free Radic. Biol. Med. 2020, 160, 28–39. [Google Scholar] [CrossRef]
- Wu, S.F.; Hooten, N.N.; Freeman, D.W.; Mode, N.A.; Zonderman, A.B.; Evans, M.K. Extracellular Vesicles in Diabetes Mellitus Induce Alterations in Endothelial Cell Morphology and Migration. J. Transl. Med. 2020, 18, 230. [Google Scholar] [CrossRef]
- Iversen, M.B.; Gottfredsen, R.H.; Larsen, U.G.; Enghild, J.J.; Praetorius, J.; Borregaard, N.; Petersen, S.V. Extracellular Superoxide Dismutase Is Present in Secretory Vesicles of Human Neutrophils and Released upon Stimulation. Free Radic. Biol. Med. 2016, 97, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Abdelsaid, K.; Sudhahar, V.; Harris, R.A.; Das, A.; Youn, S.; Liu, Y.; McMenamin, M.; Hou, Y.; Fulton, D.; Hamrick, M.W.; et al. Exercise Improves Angiogenic Function of Circulating Exosomes in Type 2 Diabetes: Role of Exosomal SOD3. FASEB J. 2022, 36, e22177. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Kolodziej, F.; McDonagh, B.; Burns, N.; Goljanek-Whysall, K. MicroRNAs as the Sentinels of Redox and Hypertrophic Signalling. Int. J. Mol. Sci. 2022, 23, 14716. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.C.; Silva, A.; James, P.F.; Wu, S.S.X.; Howitt, J. Exercise Increases the Release of NAMPT in Extracellular Vesicles and Alters NAD + Activity in Recipient Cells. Aging Cell 2022, 21, e13647. [Google Scholar] [CrossRef] [PubMed]
- Kissane, R.W.P.; Wright, O.; Al’Joboori, Y.D.; Marczak, P.; Ichiyama, R.M.; Egginton, S. Effects of Treadmill Training on Microvascular Remodeling in the Rat after Spinal Cord Injury. Muscle Nerve 2019, 59, 370–379. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a Therapeutic Target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-Derived Microparticles Induce Angiogenesis and Stimulate Post-Ischemic Revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef]
- Nie, Y.; Sato, Y.; Garner, R.T.; Kargl, C.; Wang, C.; Kuang, S.; Gilpin, C.J.; Gavin, T.P. Skeletal Muscle-derived Exosomes Regulate Endothelial Cell Functions via Reactive Oxygen Species-activated Nuclear factor-κB Signalling. Exp. Physiol. 2019, 104, 1262–1273. [Google Scholar] [CrossRef]
- Janowska-Wieczorek, A.; Wysoczynski, M.; Kijowski, J.; Marquez-Curtis, L.; Machalinski, B.; Ratajczak, J.; Ratajczak, M.Z. Microvesicles Derived from Activated Platelets Induce Metastasis and Angiogenesis in Lung Cancer. Int. J. Cancer 2005, 113, 752–760. [Google Scholar] [CrossRef]
- Gorski, T.; De Bock, K. Metabolic Regulation of Exercise-Induced Angiogenesis. Vasc. Biol. 2019, 1, H1–H8. [Google Scholar] [CrossRef] [PubMed]
- Di Credico, A.; Izzicupo, P.; Gaggi, G.; Di Baldassarre, A.; Ghinassi, B. Effect of Physical Exercise on the Release of Microparticles with Angiogenic Potential. Appl. Sci. 2020, 10, 4871. [Google Scholar] [CrossRef]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the Matrix Metalloproteinases MMP-2, MMP-9, and MT1-MMP as Membrane Vesicle-Associated Components by Endothelial Cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef]
- Bittencourt, C.R.D.O.; Izar, M.C.D.O.; Schwerz, V.L.; Póvoa, R.M.D.S.; Fonseca, H.A.R.; Fonseca, M.I.H.; Bianco, H.T.; França, C.N.; Ferreira, C.E.D.S.; Fonseca, F.A.H. Effects of High-Intensity Training of Professional Runners on Myocardial Hypertrophy and Subclinical Atherosclerosis. PLoS ONE 2016, 11, e0166009. [Google Scholar] [CrossRef]
- Boyle, L.J.; Credeur, D.P.; Jenkins, N.T.; Padilla, J.; Leidy, H.J.; Thyfault, J.P.; Fadel, P.J. Impact of Reduced Daily Physical Activity on Conduit Artery Flow-Mediated Dilation and Circulating Endothelial Microparticles. J. Appl. Physiol. 2013, 115, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Bryl-Górecka, P.; Sathanoori, R.; Al-Mashat, M.; Olde, B.; Jögi, J.; Evander, M.; Laurell, T.; Erlinge, D. Effect of Exercise on the Plasma Vesicular Proteome: A Methodological Study Comparing Acoustic Trapping and Centrifugation. Lab. Chip 2018, 18, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Bittel, D.C.; Jaiswal, J.K. Contribution of Extracellular Vesicles in Rebuilding Injured Muscles. Front. Physiol. 2019, 10, 828. [Google Scholar] [CrossRef]
- Sandonà, M.; Di Pietro, L.; Esposito, F.; Ventura, A.; Silini, A.R.; Parolini, O.; Saccone, V. Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 652970. [Google Scholar] [CrossRef]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef]
- Lovett, J.A.C.; Durcan, P.J.; Myburgh, K.H. Investigation of Circulating Extracellular Vesicle MicroRNA Following Two Consecutive Bouts of Muscle-Damaging Exercise. Front. Physiol. 2018, 9, 1149. [Google Scholar] [CrossRef]
- Hudson, M.B.; Woodworth-Hobbs, M.E.; Zheng, B.; Rahnert, J.A.; Blount, M.A.; Gooch, J.L.; Searles, C.D.; Price, S.R. miR-23a Is Decreased during Muscle Atrophy by a Mechanism That Includes Calcineurin Signaling and Exosome-Mediated Export. Am. J. Physiol.-Cell Physiol. 2014, 306, C551–C558. [Google Scholar] [CrossRef] [PubMed]
- Catitti, G.; De Bellis, D.; Vespa, S.; Simeone, P.; Canonico, B.; Lanuti, P. Extracellular Vesicles as Players in the Anti-Inflammatory Inter-Cellular Crosstalk Induced by Exercise Training. Int. J. Mol. Sci. 2022, 23, 14098. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The Potential of Endurance Exercise-Derived Exosomes to Treat Metabolic Diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Hornung, S.; Taha, H.B.; Bitan, G. Biomarkers for Parkinsonian Disorders in CNS-Originating EVs: Promise and Challenges. Acta Neuropathol. 2023, 145, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Neupane, Y.R.; Lim, X.C.; Shekhani, R.; Czarny, B.; Wacker, M.G.; Pastorin, G.; Wang, J.-W. Extracellular Vesicles in Cardiovascular Disease. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 103, pp. 47–95. ISBN 978-0-12-824616-0. [Google Scholar]
- Conkright, W.R.; Beckner, M.E.; Sahu, A.; Mi, Q.; Clemens, Z.J.; Lovalekar, M.; Flanagan, S.D.; Martin, B.J.; Ferrarelli, F.; Ambrosio, F.; et al. Men and Women Display Distinct Extracellular Vesicle Biomarker Signatures in Response to Military Operational Stress. J. Appl. Physiol. 2022, 132, 1125–1136. [Google Scholar] [CrossRef]
- Beckner, M.E.; Conkright, W.R.; Sahu, A.; Mi, Q.; Clemens, Z.J.; Martin, B.J.; Flanagan, S.D.; Ferrarelli, F.; Ambrosio, F.; Nindl, B.C. Utility of Extracellular Vesicles as a Potential Biological Indicator of Physiological Resilience during Military Operational Stress. Physiol. Rep. 2022, 10, e15219. [Google Scholar] [CrossRef]
- Oliveira, G.P.; Porto, W.F.; Palu, C.C.; Pereira, L.M.; Petriz, B.; Almeida, J.A.; Viana, J.; Filho, N.N.A.; Franco, O.L.; Pereira, R.W. Effects of Acute Aerobic Exercise on Rats Serum Extracellular Vesicles Diameter, Concentration and Small RNAs Content. Front. Physiol. 2018, 9, 532. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Basal Hormones and Biochemical Markers as Predictors of Overtraining Syndrome in Male Athletes: The EROS-BASAL Study. J. Athl. Train. 2019, 54, 906–914. [Google Scholar] [CrossRef]
- Grzędzicka, J.; Dąbrowska, I.; Malin, K.; Witkowska-Piłaszewicz, O. Exercise-Related Changes in the Anabolic Index (Testosterone to Cortisol Ratio) and Serum Amyloid A Concentration in Endurance and Racehorses at Different Fitness Levels. Front. Vet. Sci. 2023, 10, 1148990. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Murach, K.A.; Vechetti, I.J.; Van Pelt, D.W.; Crow, S.E.; Dungan, C.M.; Figueiredo, V.C.; Kosmac, K.; Fu, X.; Richards, C.I.; Fry, C.S.; et al. Fusion-Independent Satellite Cell Communication to Muscle Fibers During Load-Induced Hypertrophy. Function 2020, 1, zqaa009. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Akiyama, H.; Honda, H.; Shimizu, K. Electrical Pulse Stimulation-Induced Tetanic Exercise Simulation Increases the Secretion of Extracellular Vesicles from C2C12 Myotubes. Biochem. Biophys. Res. Commun. 2023, 672, 177–184. [Google Scholar] [CrossRef]
- Shojaee, A. Equine Tendon Mechanical Behaviour: Prospects for Repair and Regeneration Applications. Vet. Med. Sci. 2023, 9, 2053–2069. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, B. TGF-Β1-Modified MSC-Derived Exosomal miR-135b Attenuates Cartilage Injury via Promoting M2 Synovial Macrophage Polarization by Targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Tong, K.; Zhu, J.; Wang, H.; Chen, B.; Chen, L. BMSC-Derived Exosomes Promote Tendon-Bone Healing After Anterior Cruciate Ligament Reconstruction by Regulating M1/M2 Macrophage Polarization in Rats. Stem Cell Res. Ther. 2022, 13, 295. [Google Scholar] [CrossRef]
- Voss, S.C.; Jaganjac, M.; Al-Thani, A.M.; Grivel, J.-C.; Raynaud, C.M.; Al-Jaber, H.; Al-Menhali, A.S.; Merenkov, Z.A.; Alsayrafi, M.; Latiff, A.; et al. Analysis of RBC-Microparticles in Stored Whole Blood Bags—A Promising Marker to Detect Blood Doping in Sports? Drug Test. Anal. 2017, 9, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, C.; Lin, X.; Zhou, J.; Zhang, J.; Zheng, W.; Wang, T.; Cui, Y. Establishment of a Simplified Dichotomic Size-exclusion Chromatography for Isolating Extracellular Vesicles toward Clinical Applications. J. Extracell. Vesicles 2021, 10, e12145. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of Storage Conditions Stabilizing Extracellular Vesicles Preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.-M.; Wang, Z. Preserving Extracellular Vesicles for Biomedical Applications: Consideration of Storage Stability before and after Isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef]
- Rai, A.; Fang, H.; Fatmous, M.; Claridge, B.; Poh, Q.H.; Simpson, R.J.; Greening, D.W. A Protocol for Isolation, Purification, Characterization, and Functional Dissection of Exosomes. Methods Mol. Biol. 2021, 2261, 105–149. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and Characterization of Extracellular Vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Karimi, N.; Dalirfardouei, R.; Dias, T.; Lötvall, J.; Lässer, C. Tetraspanins Distinguish Separate Extracellular Vesicle Subpopulations in Human Serum and Plasma—Contributions of Platelet Extracellular Vesicles in Plasma Samples. J. Extracell. Vesicles 2022, 11, e12213. [Google Scholar] [CrossRef]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A Framework for Standardized Reporting of Extracellular Vesicle Flow Cytometry Experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- Welsh, J.A.; Arkesteijn, G.J.A.; Bremer, M.; Cimorelli, M.; Dignat-George, F.; Giebel, B.; Görgens, A.; Hendrix, A.; Kuiper, M.; Lacroix, R.; et al. A Compendium of Single Extracellular Vesicle Flow Cytometry. J. Extracell. Vesicles 2023, 12, e12299. [Google Scholar] [CrossRef]
- Cizmar, P.; Yuana, Y. Detection and Characterization of Extracellular Vesicles by Transmission and Cryo-Transmission Electron Microscopy. In Extracellular Vesicles; Kuo, W.P., Jia, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1660, pp. 221–232. ISBN 978-1-4939-7251-7. [Google Scholar]
- Goo, J.; Lee, Y.; Lee, J.; Kim, I.-S.; Jeong, C. Extracellular Vesicles in Therapeutics: A Comprehensive Review on Applications, Challenges, and Clinical Progress. Pharmaceutics 2024, 16, 311. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, W.; Li, Y.; Lai, R.; Qiu, X.; Chen, X.; Chen, Z.; Mi, B.; Wu, M.; et al. Translational Challenges and Prospective Solutions in the Implementation of Biomimetic Delivery Systems. Pharmaceutics 2023, 15, 2623. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, Opportunity, and Perspective on Exosome Isolation—Efforts for Efficient Exosome-Based Theranostics. Theranostics 2020, 10, 3684. [Google Scholar] [CrossRef]
- Ortiz, A. Not All Extracellular Vesicles Were Created Equal: Clinical Implications. Ann. Transl. Med. 2017, 5, 111. [Google Scholar] [CrossRef]
- Klymiuk, M.C.; Balz, N.; Elashry, M.I.; Heimann, M.; Wenisch, S.; Arnhold, S. Exosomes Isolation and Identification from Equine Mesenchymal Stem Cells. BMC Vet. Res. 2019, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Almiñana, C.; Rudolf Vegas, A.; Tekin, M.; Hassan, M.; Uzbekov, R.; Fröhlich, T.; Bollwein, H.; Bauersachs, S. Isolation and Characterization of Equine Uterine Extracellular Vesicles: A Comparative Methodological Study. Int. J. Mol. Sci. 2021, 22, 979. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Lazzari, B.; Perrini, C.; Pizzi, F.; Stella, A.; Cremonesi, F.; Capra, E. MicroRNAs of Equine Amniotic Mesenchymal Cell-Derived Microvesicles and Their Involvement in Anti-Inflammatory Processes. Cell Transplant. 2018, 27, 45–54. [Google Scholar] [CrossRef]
- Perrini, C.; Strillacci, M.G.; Bagnato, A.; Esposti, P.; Marini, M.G.; Corradetti, B.; Bizzaro, D.; Idda, A.; Ledda, S.; Capra, E.; et al. Microvesicles Secreted from Equine Amniotic-Derived Cells and Their Potential Role in Reducing Inflammation in Endometrial Cells in an in-Vitro Model. Stem Cell Res. Ther. 2016, 7, 169. [Google Scholar] [CrossRef]
- Gabryś, J.; Gurgul, A.; Szmatoła, T.; Kij-Mitka, B.; Andronowska, A.; Karnas, E.; Kucharski, M.; Wojciechowska-Puchałka, J.; Kochan, J.; Bugno-Poniewierska, M. Correction: Gabryś et al. Follicular Fluid-Derived Extracellular Vesicles Influence on In Vitro Maturation of Equine Oocyte: Impact on Cumulus Cell Viability, Expansion and Transcriptome. Int. J. Mol. Sci. 2024, 25, 6812. [Google Scholar] [CrossRef]
- Weiss, C.; Kornicka-Grabowska, K.; Mularczyk, M.; Siwinska, N.; Marycz, K. Extracellular Microvesicles (MV’s) Isolated from 5-Azacytidine-and-Resveratrol-Treated Cells Improve Viability and Ameliorate Endoplasmic Reticulum Stress in Metabolic Syndrome Derived Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2020, 16, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Finding, E.J.T.; Lawson, C.; Elliott, J.; Harris, P.A.; Menzies-Gow, N.J. Cell Specific Microvesicles Vary with Season and Disease Predisposition in Healthy and Previously Laminitic Ponies. Vet. Immunol. Immunopathol. 2018, 202, 85–92. [Google Scholar] [CrossRef]
- Arévalo-Turrubiarte, M.; Baratta, M.; Ponti, G.; Chiaradia, E.; Martignani, E. Extracellular Vesicles from Equine Mesenchymal Stem Cells Decrease Inflammation Markers in Chondrocytes in Vitro. Equine Vet. J. 2022, 54, 1133–1143. [Google Scholar] [CrossRef]
- Reynolds, D.E.; Vallapureddy, P.; Morales, R.-T.T.; Oh, D.; Pan, M.; Chintapula, U.; Linardi, R.L.; Gaesser, A.M.; Ortved, K.; Ko, J. Equine Mesenchymal Stem Cell Derived Extracellular Vesicle Immunopathology Biomarker Discovery. J. Extracell. Biol. 2023, 2, e89. [Google Scholar] [CrossRef]
- Connard, S.S.; Gaesser, A.M.; Clarke, E.J.; Linardi, R.L.; Even, K.M.; Engiles, J.B.; Koch, D.W.; Peffers, M.J.; Ortved, K.F. Plasma and Synovial Fluid Extracellular Vesicles Display Altered microRNA Profiles in Horses with Naturally Occurring Post-Traumatic Osteoarthritis: An Exploratory Study. J. Am. Vet. Med. Assoc. 2024, 262, S83–S96. [Google Scholar] [CrossRef]
- Elashry, M.I.; Speer, J.; De Marco, I.; Klymiuk, M.C.; Wenisch, S.; Arnhold, S. Extracellular Vesicles: A Novel Diagnostic Tool and Potential Therapeutic Approach for Equine Osteoarthritis. Curr. Issues Mol. Biol. 2024, 46, 13078–13104. [Google Scholar] [CrossRef] [PubMed]
- Quam, V.G.; Belacic, Z.A.; Long, S.; Rice, H.C.; Dhar, M.S.; Durgam, S. Equine Bone Marrow MSC-Derived Extracellular Vesicles Mitigate the Inflammatory Effects of Interleukin-1β on Navicular Tissues In Vitro. Equine Vet. J. 2025, 57, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Kornicka-Garbowska, K.; Pędziwiatr, R.; Woźniak, P.; Kucharczyk, K.; Marycz, K. Microvesicles Isolated from 5-Azacytidine-and-Resveratrol-Treated Mesenchymal Stem Cells for the Treatment of Suspensory Ligament Injury in Horse—A Case Report. Stem Cell Res. Ther. 2019, 10, 394. [Google Scholar] [CrossRef]
- Gaesser, A.M.; Usimaki, A.I.J.; Barot, D.A.; Linardi, R.L.; Molugu, S.; Musante, L.; Ortved, K.F. Equine Mesenchymal Stem Cell-Derived Extracellular Vesicle Productivity but Not Overall Yield Is Improved via 3-D Culture with Chemically Defined Media. J. Am. Vet. Med. Assoc. 2024, 262, S97–S108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yao, X.; Zeng, Y.; Wang, J.; Ren, W.; Wang, T.; Li, X.; Yang, L.; Yang, X.; Meng, J. The Impact of the Competition on miRNA, Proteins, and Metabolites in the Blood Exosomes of the Yili Horse. Genes 2025, 16, 224. [Google Scholar] [CrossRef]
- de Oliveira, G.P.; Porto, W.F.; Palu, C.C.; Pereira, L.M.; Reis, A.M.M.; Marçola, T.G.; Teixeira-Neto, A.R.; Franco, O.L.; Pereira, R.W. Effects of Endurance Racing on Horse Plasma Extracellular Particle miRNA. Equine Vet. J. 2021, 53, 618–627. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milczek-Haduch, D.; Żmigrodzka, M.; Witkowska-Piłaszewicz, O. Extracellular Vesicles in Sport Horses: Potential Biomarkers and Modulators of Exercise Adaptation and Therapeutics. Int. J. Mol. Sci. 2025, 26, 4359. https://doi.org/10.3390/ijms26094359
Milczek-Haduch D, Żmigrodzka M, Witkowska-Piłaszewicz O. Extracellular Vesicles in Sport Horses: Potential Biomarkers and Modulators of Exercise Adaptation and Therapeutics. International Journal of Molecular Sciences. 2025; 26(9):4359. https://doi.org/10.3390/ijms26094359
Chicago/Turabian StyleMilczek-Haduch, Dominika, Magdalena Żmigrodzka, and Olga Witkowska-Piłaszewicz. 2025. "Extracellular Vesicles in Sport Horses: Potential Biomarkers and Modulators of Exercise Adaptation and Therapeutics" International Journal of Molecular Sciences 26, no. 9: 4359. https://doi.org/10.3390/ijms26094359
APA StyleMilczek-Haduch, D., Żmigrodzka, M., & Witkowska-Piłaszewicz, O. (2025). Extracellular Vesicles in Sport Horses: Potential Biomarkers and Modulators of Exercise Adaptation and Therapeutics. International Journal of Molecular Sciences, 26(9), 4359. https://doi.org/10.3390/ijms26094359






