The Emerging Oncogenic Role of RARγ: From Stem Cell Regulation to a Potential Cancer Therapy
Abstract
1. Introduction
2. RARγ Is Expressed by Normal Stem Cells
3. A Role for RARγ During Embryogenesis and Adult Haematopoiesis
4. RARγ Plays a Role in Cancer
4.1. AML
4.2. Cholangiocarcinoma
4.3. Colorectal Cancer
4.4. Head and Neck Cancer
4.5. Hepatocellular Cancer
4.6. Ovarian Cancer
4.7. Pancreatic Cancer
4.8. Prostate Cancer
4.9. Renal Cancer
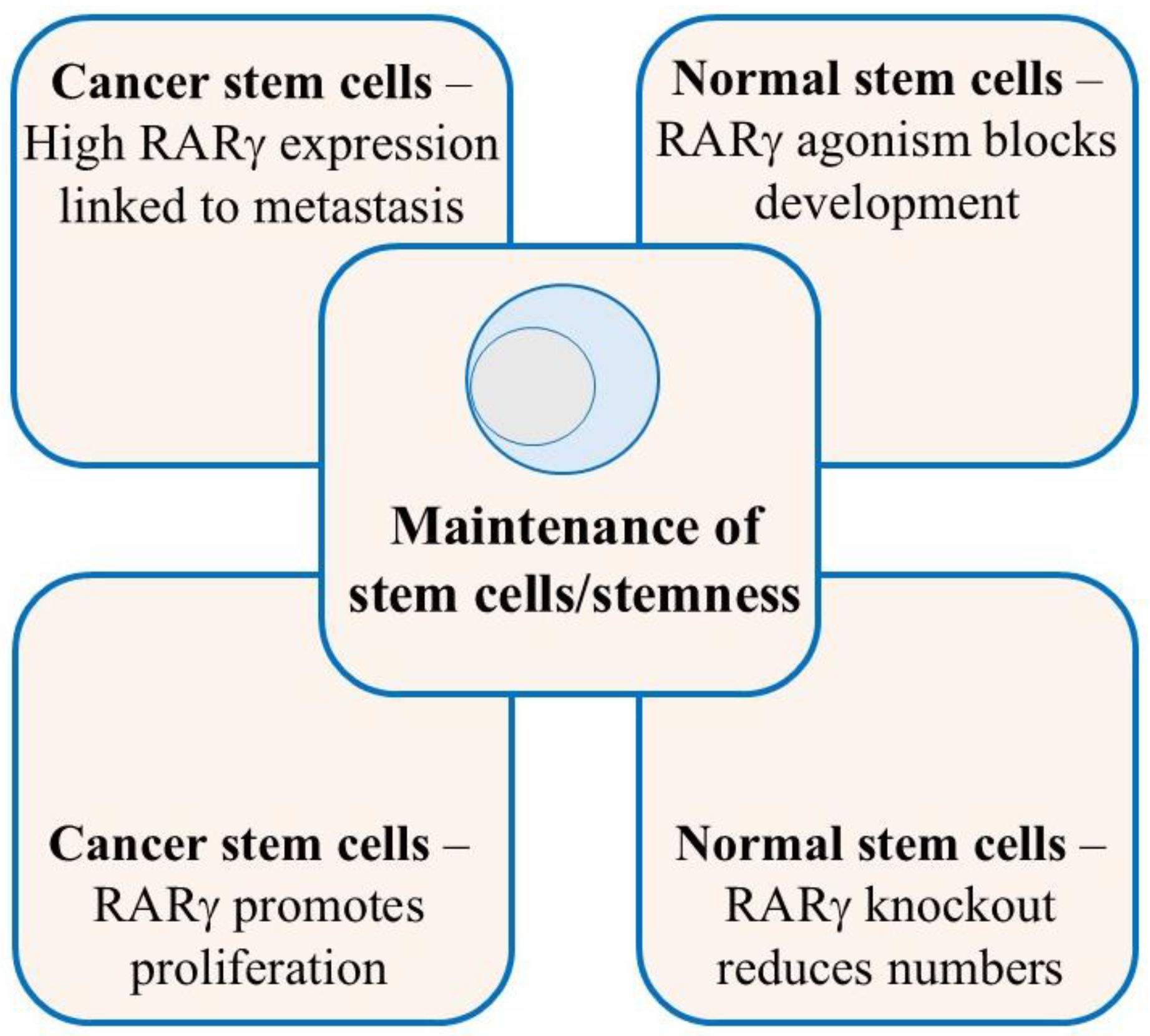
5. RARγ Ensures the Maintenance of Normal and Cancer Stem Cells
6. Targeting RARγ to Kill Cancer Stem Cells
6.1. AML
6.2. Hepatocellular Cancer
6.3. Pancreatic Cancer
6.4. Paediatric Brain
6.5. Prostate Cancer
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Bozic, I.; Antal, T.; Outsuki, H.; Carter, H.; Chen, S.; Karshin, R.; Kinzler, R.; Vogelstein, B.; Nowak, M.A. Accumulation of driver and passenger mutations during tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 18543–18550. [Google Scholar] [CrossRef] [PubMed]
- Loukas, I.; Simeoni, F.; Milan, M.; Inglese, P.; Patel, H.; Goldstone, R.; East, P.; Strohbuecker, S.; Mitter, R.; Talsania, B.; et al. Selective advantage of epigeenetically disrupted cancer cells via phenotypic inertia. Cancer Cell 2023, 41, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell theory. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Sell, S. On the stem cell origin of cancer. Am. J. Pathol. 2010, 176, 2584–2594. [Google Scholar] [CrossRef]
- Ruberte, E.; Dolle, P.; Krust, A.; Zelent, A.; Morris-Kay, G.; Chambon, P. Specific spatial and temporal distribution of retinoic acid receptor gamma transcripts during mouse embryogenesis. Development 1990, 108, 213–222. [Google Scholar] [CrossRef]
- Waxman, J.S.; Yelon, D. Comparison of the expression patterns of newly identified zebrafish retinoic acid and retinoid X receptors. Dev. Dyn. 2007, 236, 587–595. [Google Scholar] [CrossRef]
- Hale, L.A.; Tallafuss, A.; Yan, Y.-L.; Dudley, L.; Eisen, J.S.; Postlethwait, J.H. Characterization of the retinoic acid receptor genes raraa, rarab and rarg during zebrafish development. Gene Expr. Patterns 2006, 6, 546–555. [Google Scholar] [CrossRef]
- Grandel, H.; Lun, K.; Rauch, G.-J.; Rhinn, M.; Piotrowski, T.; Houart, C.; Sordino, P.; Kuchler, A.M.; Schulte-Merker, S.; Geisler, R.; et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 2002, 129, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Aloinger-Ziegelbauer, H.; Dreyer, C. The pattern of expression in normal development of Xenopus laevis and after manipulation of the main body axis. Mech. Dev. 1993, 41, 33–46. [Google Scholar] [CrossRef]
- Purton, L.E.; Dworkin, S.; Olsen, G.H.; Walkley, C.R.; Fabb, S.A.; Collins, S.J.; Chambon, P. RARγ is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 2006, 203, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Heyworth, C.M.; Glasgow, A.; Huang, Q.H.; Petrie, K.; Lanotte, M.; Benoit, G.; Gallagher, R.; Waxman, S.; Enver, T.; et al. Lineage restriction of the RARalpha gene expression in myeloid differentiation. Blood 2001, 98, 2563–2567. [Google Scholar] [CrossRef]
- Kastner, P.; Chan, S. Function of RARa during the maturation of neutrophils. Oncogene 2001, 20, 7178–7185. [Google Scholar] [CrossRef]
- Kashyap, V.; Gudes, L.J.; Brenit, F.; Funk, P.; Viale, A.; Scandura, J.M. Epigenomic reorganization of the clustered hox genes in embryonic stem cells induced by retinoic acid. J. Biol. Chem. 2011, 286, 3250–3260. [Google Scholar] [CrossRef]
- Handberg-Thorsager, M.; Gutierrez-Mazariegos, J.; Arold, S.T.; Nadenala, E.K.; Bertucci, P.Y.; German, P.; Tomancek, P.; Pierzchalski, K.; Jones, J.W.; Albalat, R.; et al. The ancestral retinoic acid receptor was a low-affinity sensor triggering neuronal differentiation. Sci. Adv. 2018, 4, eaao1261. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mao, X.; Zhou, X.; Su, Y.; Zhou, X.; Shi, K.; Zhao, S. An optimal method for neuronal differentiation of embryonic stem cells in vitro. J. Neurosci. Methods 2020, 330, 108486. [Google Scholar] [CrossRef]
- Martinez-Ceballos, E.; Gudas, L. Hoxa1 is required for the retinoic acid-induced differentiation of embrtonic stem cells into neurons. J. Neurosci. Res. 2008, 86, 2809–2819. [Google Scholar] [CrossRef]
- Al Tanoury, Z.; Gaouar, S.; Piskunov, A.; Ye, T.; Urban, S.; Jost, B.; Keime, C.; Davidson, I.; Dierich, A.; Rochette-Egly, C. Phosphorylation of the retinoic acid receptor RARγ2 is crucial for the neuronal differentiation of mouse embryonic stem cells. J. Cell Sci. 2014, 127, 2095–2105. [Google Scholar] [CrossRef]
- Chatagnon, A.; Veber, P.; Marin, V.; Bede, J.; Triqueneaux, G.; Semon, M.; Laudet, V.; d’Ache-Buc, F.; Benoit, G. RAR/RXR binding dynamics distinguish pluripotency from differentiation associated cis-regulatory elements. Nucleic Acid. Res. 2015, 43, 4833–4848. [Google Scholar] [CrossRef] [PubMed]
- Wai, H.A.; Kawakami, K.; Wada, H.; Muller, F.; Vernalis, A.B.; Brown, G.; Johnson, W.E.B. The development and growth of tissues derived from cranial neural crest and primitive mesoderm is dependent on the ligation status of retinoic acid receptor γ: Evidence that retinoic acid receptor γ functions to maintain stem/progenitors in the absence of retinoic acid. Stem Cells Dev. 2015, 24, 507–518. [Google Scholar]
- Shimono, K.; Tung, W.-e.; Maolino, C.; Chi, A.H.-T.; Didizian, J.H.; Mundy, C.; Chandraratna, R.A.; Mishina, Y.; Enomoto-Iwamoto, M.; Pacifici, M.; et al. Potent inhibition of heterotopic ossification by nuclear receptor-g agonists. Nat. Med. 2011, 17, 454–460. [Google Scholar] [CrossRef]
- Janesick, A.; Nguyen, T.T.L.; Aisaki, K.-i.; Igarashi, K.; Kitajima, S.; Chandraratna, R.A.S.; Kanno, J.; Blumberg, B. Active repression by RARγ signalling is required for vertebrate axial elongation. Development 2014, 141, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Shi, J.Y.; Shirkov, Y.; Brown, G.; Zelent, A.; Petrie, K. Expression of retinoic acid receptor gamma is regulated by miR-30a. Klin. Pediatr. 2023, 235, 002. [Google Scholar]
- Brown, G. Deregulation of all-trans retinoic acid signaling and development in cancer. Int. J. Mol. Sci. 2023, 24, 12089. [Google Scholar] [CrossRef] [PubMed]
- Conserva, M.R.; Redavid, I.; Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. RARG gene dysregulation in acute myeloid leukemia. Front. Mol. Biosci. 2019, 6, 114. [Google Scholar] [CrossRef]
- Liu, T.; Weng, T.; Qi, L.; Liu, Y.; Shen, M.; Wong, F.; Fang, Y.; Liu, S.; Wen, L.; Chen, S.; et al. CPSF-6-RARγ interacts with histone deacetylase 3 to promote myeloid transformation in RARG-fusion acute myeloid leukemia. Nat. Commun. 2025, 16, 616. [Google Scholar] [CrossRef]
- Huang, G.L.; Luo, Q.; Rui, G.; Zhang, W.; Zhang, Q.Y.; Chen, Q.X.; Shen, D.Y. Oncogenic activity of retinoic acid receptor gamma is exhibited through activation of the Akt/NF-kB and Wnt/b-catenin pathways in cholangiocarcinoma. Mol. Cell. Biol. 2013, 33, 3416–3425. [Google Scholar] [CrossRef]
- Huang, G.-L.; Song, W.; Zhou, P.; Fu, Q.-R.; Lin, C.-L.; Chen, Q.-X.; Shen, D.-Y. Oncogenic retinoic acid receptor γ knockdown reverses muti-drug resistance of human colorectal cancer via Wnt/b-catenin pathway. Cell Cycle 2017, 16, 685–691. [Google Scholar] [CrossRef]
- Irmer, B.; Wlochowitz, D.; Krekeler, C.; Richter, K.M.; Chandrabalan, S.; Bayerlova, M.; Wolff, A.; Lenz, G.; Conradi, L.-C.; Schildhaus, H.-U.; et al. Consensus molecular subtyping of colorectal brain metastases reveals a metabolism signature associated with poor patient survival. Mol. Oncol. 2025, 19, 614–634. [Google Scholar] [CrossRef]
- Su, Y.-C.; Wu, S.; Hsu, K.-F.; Jian, S.-S.; Kuo, P.-C.; Shiau, A.-L.; Wu, C.-L.; Wang, Y.-K.; Hsiao, J.-R. Oncogenic RARγ isoforms promote head and neck cancer proliferation through vinexin-b-mediated cell cycle acceleration and autocrine activation of EGFR signal. Int. J. Biol. Sci. 2025, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Wu, H.; Zhang, H.P.; Lu, N.; Ye, P.; Yu, F.-H.; Zhou, H.; Li, W.G.; Cao, X.; Lin, Y.Y.; et al. Oncogenic potential of retinoic acid receptor-gamma in hepatocellular carcinoma. Cancer Res. 2010, 70, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.-T.; Wang, J.-R.; Zhu, X.-L.; He, X.-S.; Guo, P.-D.; Zhang, S.; Li, X.-M.; Li, J.-M.; Wu, H. RARγ-induced E-cadherin downregulation promotes hepatocellular carcinoma invasion and metastasis. J. Exp. Clin. Cancer Res. 2016, 35, 164. [Google Scholar] [CrossRef]
- Xiu, L.; Zhao, Y.; Li, N.; Zeng, J.; Liu, J.; Fu, Y.; Gao, Q.; Wu, L. High expression of RARG accelerates ovarian cancer progression by regulating cell proliferation. Front. Oncol. 2022, 12, 1063031. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Dou, X.; Zhang, N.; Wen, B.; Zhang, M.; Zhang, Q.; Xu, S.; Zhou, J.; Liu, T. Retinoic acid receptor gamma is required for proliferation of pancreatic cancer cells. Cell Biol. Int. 2023, 47, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Yamakowa, K.; Koyangi-Aoi, M.; Machinaga, A.; Kakiuchi, N.; Hirano, T.; Kodama, Y.; Aoi, T. Blockage of retinoic acid signalling via RARγ suppressed the proliferation of pancreatic cancer cells by arresting the cell cycle progression in G1-S phase. Cancer Cell Int. 2023, 23, 94. [Google Scholar] [CrossRef]
- Richter, F.; Joyce, A.; Fromwitz, F.; Wang, S.; Watson, J.; Watson, R.; Irwin JR, T.J.; Huang, H.F.S. Immunohistological localisation of the retinoic acid receptors in in human prostate. J. Androl. 2002, 23, 830–838. [Google Scholar] [CrossRef]
- Petrie, K.; Urban-Wojciuk, Z.; Sbirkov, Y.; Graham, A.; Hamann, A.; Brown, G. Retinoic acid receptor γ is a therapeutically targetable driver of growth and survival in prostate cancer. Cancer Rep. 2020, 3, e1284. [Google Scholar] [CrossRef]
- De Felice, D.; Alaimo, A.; Bressan, D.; Genovesi, S.; Marmacchi, E.; Annesi, N.; Beccaceci, G.; Dalfovo, D.; Cutrupi, F.; Medaglia, S. Rarγ-Foxa1 signaling promotes luminal identity in prostate progenitors and is disrupted in prostate cancer. EMBO Rep. 2024, 26, 443–469. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Nyushko, K.M.; Zaretsky, A.R.; Shagin, D.A.; Kaprin, A.D.; Alekseev, B.Y.; Snezhkina, A.V. Upregulation of Rarb, Rarg, and Rorc Genes in Clear Cell Renal Cell. Carcinoma. Biomed. Pharmacol. J. 2016, 9, 967–975. [Google Scholar]
- Khillan, J.S. Vitamin A/retinol and the maintenance of pluripotency of stem cells. Nutrients 2014, 6, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Khillan, J.S. A novel signaling by vitamin a/retinol promotes self-renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells 2010, 28, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Marchwicka, A.; Cunningham, A.; Toellner, K.-M.; Marcinkowska, E. Antagonising retinoic acid receptors increases myeloid cell production by cultured human hematopoietic stem cells. Arch. Immunol. Ther. Exp. 2017, 65, 69–81. [Google Scholar] [CrossRef]
- Idres, N.; Marill, J.; Flexer, M.A.; Chabot, G.G. Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J. Biol. Chem. 2002, 277, 31491–31498. [Google Scholar] [CrossRef]
- Paquali, D.; Thaller, C.; Eichele, G. Abnormal level of retinoic acid in prostate cancer. J. Clin. Endocrinol. Metab. 1996, 81, 2186–2191. [Google Scholar]
- Boyd, D.L.; Chisholm, G.D.; Habib, F.K. Nuclear retinoic acid binding protein in the human prostate. J. Endocrinol. 1985, 105, 157–162. [Google Scholar] [CrossRef]
- Jutley, J.K.; Kelleher, J.; Whelan, P.; Mikel, J. Cytosolic retinoic acid-binding protein in human prostatic dysplasia and neoplasm. Prostate 1987, 11, 127–132. [Google Scholar] [CrossRef]
- Bleul, T.; Ruhl, R.; Bulashevska, S.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reduced retinoids and retinoid receptors’ expression in pancreatic cancer: A link to patient survival. Mol. Carcinog. 2015, 54, 870–879. [Google Scholar] [CrossRef]
- Guo, X.; Nanys, D.M.; Ruiz, A.; Rando, R.R.; Bok, L.J. Reduced levels of retinyl esters and vitamin A in human renal cancers. Cancer Res. 2001, 61, 2774–2781. [Google Scholar]
- Sharma, R.B.; Wang, Q.; Khillian, J.S. Amplification of tumour inducing putative cancer stem cells (CSCs) by vitamin A/retinol from mouse mammary tumours. Biochem. Biophys. Res. Comm. 2013, 436, 625–631. [Google Scholar]
- Hayden, L.J.; Satre, M.A. Alterations in cellular retinal metabolism contribute to differential retinoid responsiveness in normal human mammary epithelial cells versus breast cancer cells. Breast Cancer Res. Treat. 2002, 72, 95–105. [Google Scholar] [CrossRef]
- Mira-Y.-Lopez, R.; Zheng, W.L.; Kuppumbatti, Y.S.; Rexer, B.; Jing, Y.; Ong, D.E. Retinol conversion to retinoic acid is impaired in breast cancer cells. J. Cell Physiol. 2000, 185, 302–309. [Google Scholar] [CrossRef]
- Williams, S.J.; Cvetkovic, D.; Hamilton, T.C. Vitamin A metabolism is impaired in human ovarian cancer. Gynecol. Oncol. 2009, 112, 637–645. [Google Scholar] [CrossRef]
- Kroptova, E.S.; Zinovieva, O.L.; Zyryanova, A.F.; Dybovaya, V.I.; Praolov, V.S.; Beresten, S.F.; Operina, N.Y.; Mashkova, T.D. Altered expression of multiple genes involved in retinoic acid biosynthesis in human colorectal cancer. Path. Oncol. Res. 2014, 20, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Jette, C.; Peterson, P.W.; Sandoval, I.T.; Manos, E.J.; Hadley, E.; Ireland, C.M.; Jones, D.A. The tumor suppressor adenomatous polyposis coli and caudal related homeodomain protein regulate expression of retinal dehydrogenase L. J. Biol. Chem. 2004, 279, 34397–34405. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kwan, H.; Cho, H.; Chung, J.-Y.; Hewitt, S.M.; Kim, J.-H. ALDH1A2 is a candidate tumor suppressor gene in ovarian cancer. Cancer 2019, 11, 1553. [Google Scholar] [CrossRef]
- Kroptova, E.S.; Zinov’eva, O.L.; Zyranova, A.F.; Chinzonov, E.L.; Afanas’ev, S.G.; Cherdyntseva, N.V.; Berensten, S.F.; lu Oparina, N.; Mashkeva, T.D. Expression of genes involved in retinoic acid biosynthesis in human gastric cancer. Mol. Biol. 2013, 47, 317–330. [Google Scholar] [CrossRef]
- Kuznetsova, E.S.; Zinovieva, O.L.; Oparina, N.Y.; Prokofjeva, M.M.; Spirin, P.V.; Favorskaya, I.A.; Zborovskaya, I.B.; Lisitsyn, N.A.; Prassolov, V.S.; Mashkova, T.D. Abnormal expression of genes that regulate retinoid metabolism in non-small-cell lung cancer. Mol. Biol. 2016, 50, 220–229. [Google Scholar] [CrossRef]
- Seidensaal, K.; Nollert, A.; Fiege, A.H.; Muller, M.; Fleming, T.; Gunkel, N.; Zaoui, K.; Grabe, N.; Weichert, N.; Weber, K.-J.; et al. Impaired aldehyde dehydrogenase subfamily member 2A-dependent retinoic acid signaling is related with a mesenchymal-like phenotype and an unfavourable prognosis of head and neck squamous cell carcinoma. Mol. Cancer 2015, 14, 204. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Bauer, K.; Hackl, H.; Schlerka, A.; Keller, E.; Hladik, A.; Steiber, D.; Zuber, J.; Staber, P.B.; Hoelbl-Kovacis, A.; et al. All-trans retinoic acid enhances, and a pan-RAR antagonist counteracts, the stem cell promoting activity of EV11 in acute myeloid leukaemia. Cell Death Differ. 2019, 10, 944. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, C.; Cheng, H.; Wu, Y.-L.; Liu, J.; Chen, Z.; Huang, J.-g.; Erickson, R.E.; Chen, L.; Zhang, H.; et al. Targeting to the non-genomic activity of retinoic acid receptor-gamma by acacetin in hepatocellular carcinoma. Sci. Rep. 2017, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Petrie, K. The RARγ oncogene: An Achilles heel for some cancers. Int. J. Mol. Sci. 2021, 22, 3632. [Google Scholar] [CrossRef]
- Hammond, L.A.; Krinks, C.H.V.; Durham, J.; Tomkins, S.E.; Burnett, R.D.; Jones, E.L.; Chandraratna, R.A.S.; Brown, G. Antagonists of retinoic acid receptors (RARs) are potent growth inhibitors of prostate carcinoma cells. Br. J. Cancer 2001, 85, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Keedwell, R.G.; Zhao, Y.; Hammond, L.A.; Wen, K.; Qin, S.; Atangan, L.I.; Shurland, D.L.; Wallace, D.M.; Bird, R.; Reitmair, A.; et al. An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium. Br. J. Cancer 2004, 91, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.W.; Wang, X.; Roberts, S.S.; Griffey, S.M.; Reczek, P.R.; Wolgemuth, D.J. Oral administration of a retinoic receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 2011, 152, 2492–2502. [Google Scholar] [CrossRef]
- Schulze, G.E.; Clay, R.J.; Mezza, L.E.; Bregman, C.L.; Buroker, R.A.; Frantz, J.D. BMS-189453, a novel retinoid receptor antagonist, is a potent testicular toxin. Toxicol. Sci. 2001, 59, 297–308. [Google Scholar] [CrossRef]
- Podlesny-Drabinoik, A.; Sobska, J.; de Lera, A.R.; Golembiowska, K.; Kaminski, K.; Dolle, P.; Cebrat, M.; Krezel, W. Distinct retinoic acid receptor (RAR) control differentiation of embryonal carcinoma cells to dopaminergic or striatopallidal medium spiny neurons. Sci. Rep. 2017, 7, 13671. [Google Scholar]
- Monk, M.; Hobling, S. Human embryonic genes can be re-expressed in cancer cells. Oncogene 2001, 20, 8085–8091. [Google Scholar] [CrossRef]
- Lord, A.; Ficz, G. Corrupted devolution: How normal cells are reborn as cancer precursors. Int. J. Biochem. Cell Biol. 2022, 149, 106263. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Liu, H.; Lu, D.; Chen, X.; Zenonos, Z.; Campos, L.S.; Rad, R.; Guo, G.; Zhang, S.; et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc. Natl. Acad. Sci. USA 2011, 108, 18283–18288. [Google Scholar] [CrossRef] [PubMed]
- Holyoake, T.; Jiang, X.; Eaves, C.; Eaves, A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood 1999, 94, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Jorgensen, H.G.; Allen, E.; Pearson, C.; Akorn, M.J.; Richmond, L.; Holyoake, T.L. Primitive, quiescent, Phildelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002, 99, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Cancer stem cells and chemoresitance: The smartest survives the raid. Pharmacol. Ther. 2016, 160, 145–158. [Google Scholar] [CrossRef]
- Hantusch, B.; Kenner, L.; Stanulovic, V.S.; Hoogenkamp, M.; Brown, G. Targeting androgen, thyroid hormone, and vitamin A and D receptors to treat prostate cancer. Int. J. Mol. Sci. 2024, 25, 9245. [Google Scholar] [CrossRef]
- Larange, A.; Cheroutre, H. Retinoic acid and retinoic acid receptors as pleiotropic modulators of the immune system. Ann. Rev. Immunol. 2016, 14, 369–394. [Google Scholar] [CrossRef]
| Malignancy | Evidence That RARγ Is an Oncogene |
|---|---|
| AML | RARG gene rearrangements [27]; CPSF6-RARγ interacts with histone deacetylase 3 to promote myeloid transformation [28]. |
| Cholangiocarcinoma | Overexpression in patients’ cells; activation of the Akt/NF-κB and Wnt/β-catenin pathways [29] |
| Colorectal | Overexpression in patients’ cells and RARγ knockdown increased sensitivity to chemotherapeutics. [30]; vitamin A metabolism signature for brain metastasis [31] |
| Head and neck | Activation of the epidermal growth factor receptor and downstream Akt, Erk, Src, and YAP signalling [32] |
| Hepatocellular | Overexpression in patients’ cells; activation of the Akt/NF-κB pathways [33] and downregulation of E-cadherin for metastasis [34] |
| Ovarian | Overexpression in patients’ cells and cell line knockdown reduced xenograft growth [35] |
| Pancreatic | Overexpression in patients’ cells; chromatin epigenetic activation from histone H3 K27 acetylation [36,37] |
| Prostate | Overexpression in patients’ cells [38]; promotion of proliferation [39]; RARγ-Foxa1 promotion of progenitor cell identity/dedifferentiation [40]. |
| Renal | Overexpression in patients’ cells [41] |
| Malignancy | Agent | Outcome from Antagonism of RARs |
|---|---|---|
| AML | Pan-RAR antagonist AGN193109 | Delayed leukemogenesis in vivo and reduced stemness of Evi1high AML cells [61] |
| Hepatocellular | Acacetin Inhibits RARγ transactivation | Apoptosis of cell line cells [62] |
| Pancreatic | RARγ antagonists LY2955303 MM11225 | G1 arrest of cell line cells and reduced proliferation of pancreatic cancer organoids [37] |
| Paediatric brain | Pan-RAR antagonist AGN194310 | Growth arrest and cell death of patients’ cells and the prevention of neurosphere formation [63] |
| Prostate | Pan-RAR antagonist AGN194310 RARγ antagonist AGN205728 | G1 arrest and necroptosis of cell line and patients’ cells [39,64,65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, G. The Emerging Oncogenic Role of RARγ: From Stem Cell Regulation to a Potential Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 4357. https://doi.org/10.3390/ijms26094357
Brown G. The Emerging Oncogenic Role of RARγ: From Stem Cell Regulation to a Potential Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(9):4357. https://doi.org/10.3390/ijms26094357
Chicago/Turabian StyleBrown, Geoffrey. 2025. "The Emerging Oncogenic Role of RARγ: From Stem Cell Regulation to a Potential Cancer Therapy" International Journal of Molecular Sciences 26, no. 9: 4357. https://doi.org/10.3390/ijms26094357
APA StyleBrown, G. (2025). The Emerging Oncogenic Role of RARγ: From Stem Cell Regulation to a Potential Cancer Therapy. International Journal of Molecular Sciences, 26(9), 4357. https://doi.org/10.3390/ijms26094357






