Exploring Nepicastat Activity: Beyond DβH
Abstract
1. Introduction
2. Results and Discussion
2.1. NEP Exhibits a Weak Antimicrobial Activity Depending on the Strain Used
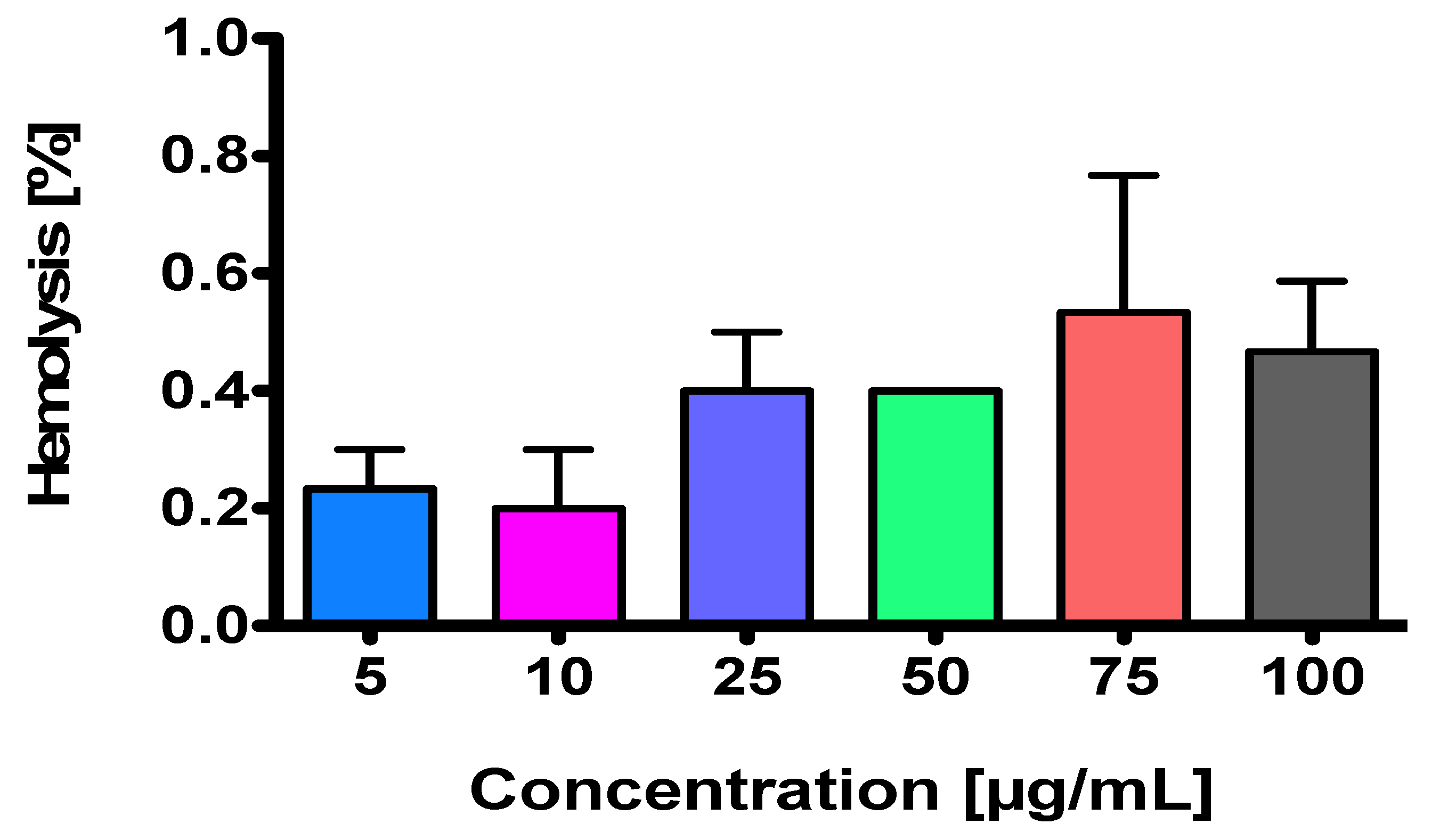
2.2. NEP Is a Non-Hemolysis-Inducing Agent
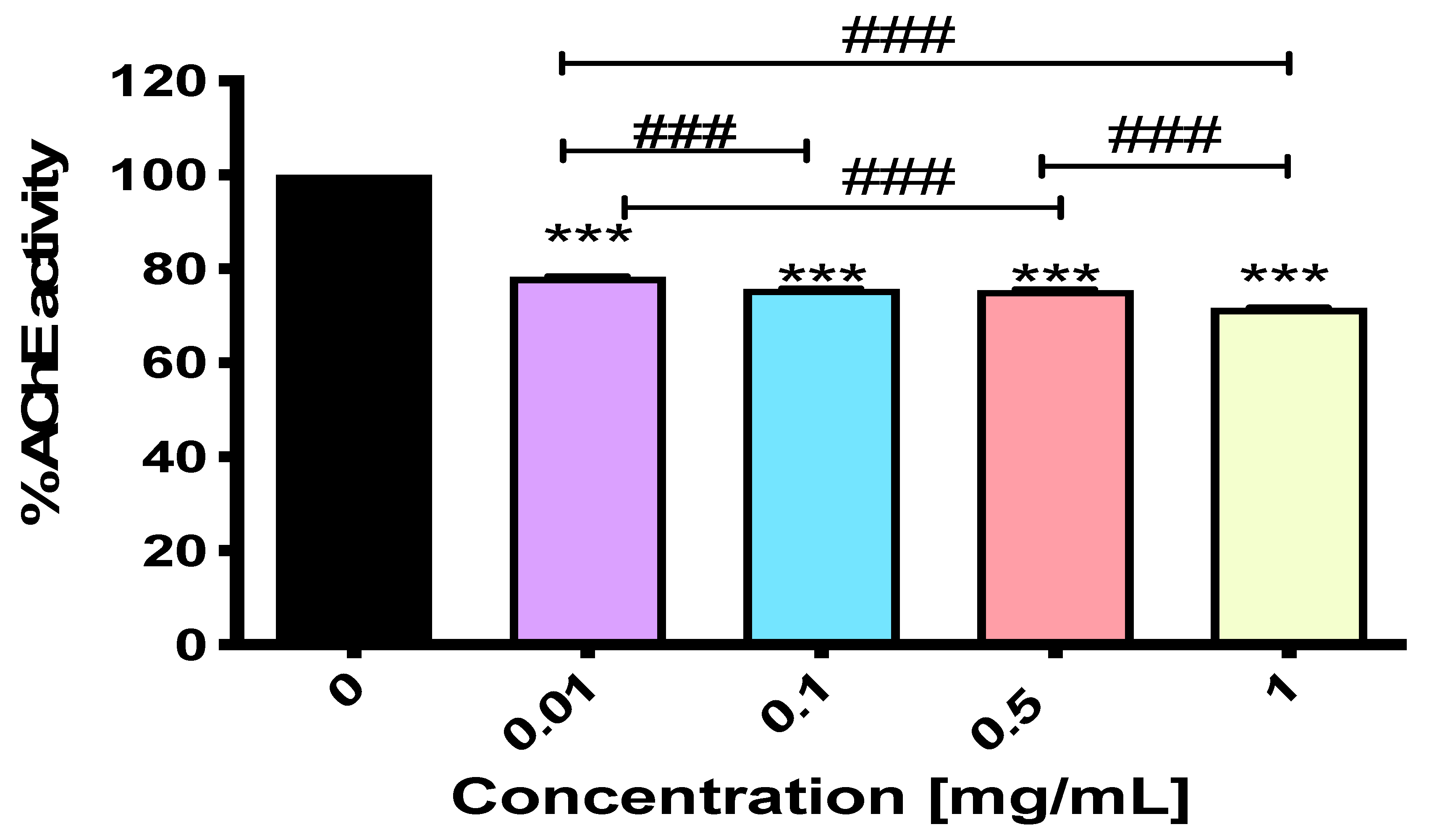
2.3. NEP Exerts Activity Against Acetylcholinesterase
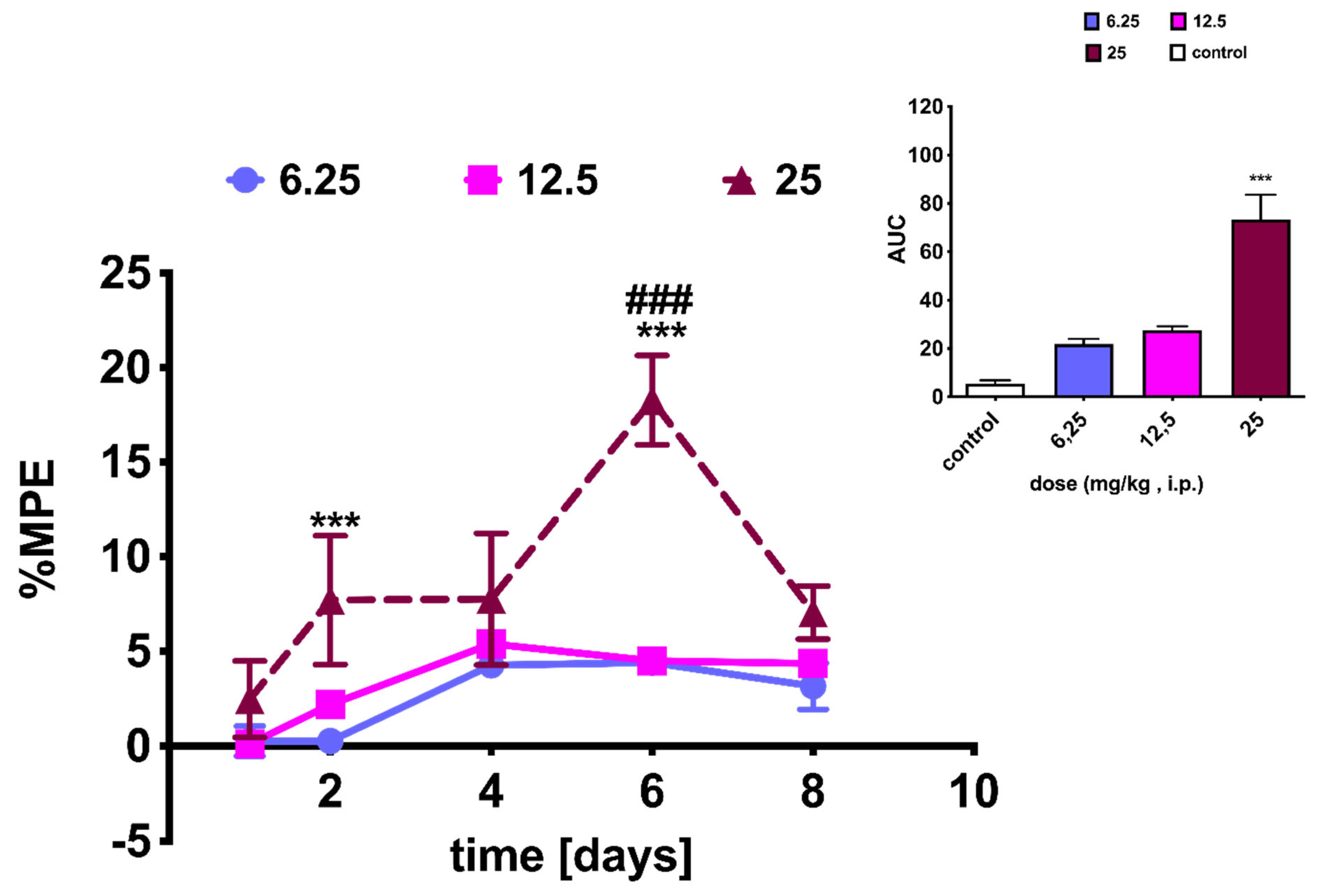
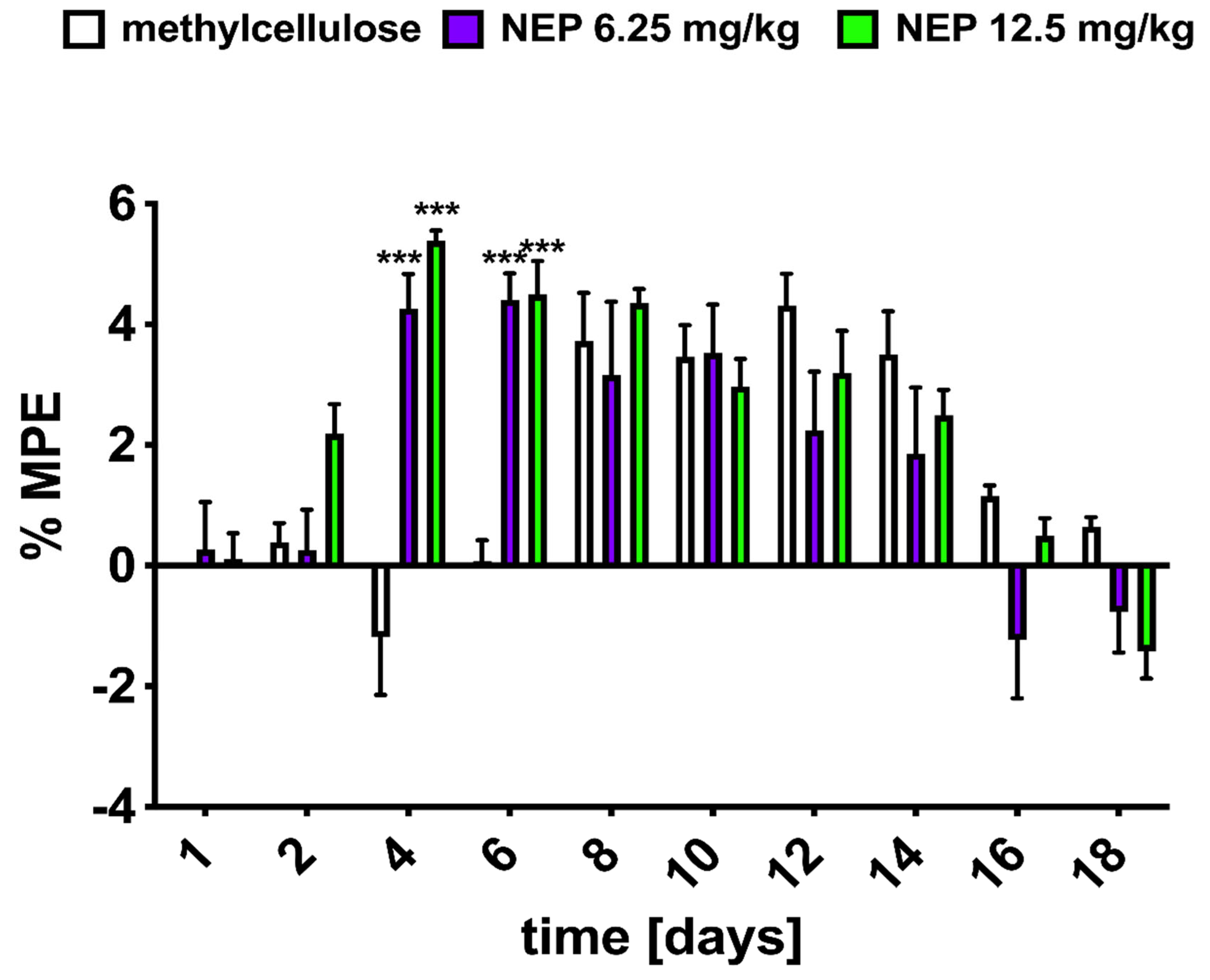
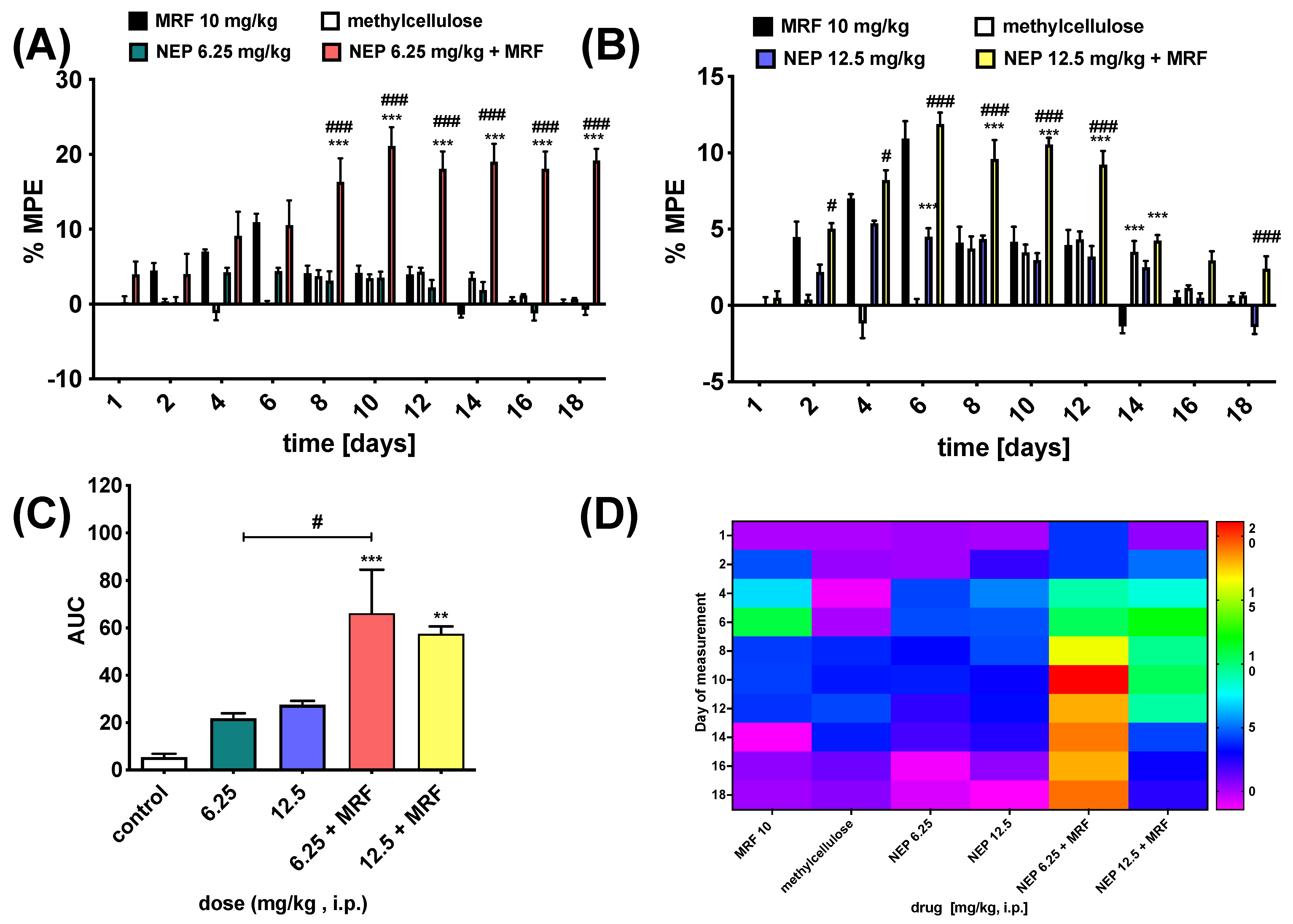
2.4. NEP Affects MRF-Induced Analgesia and Tolerance
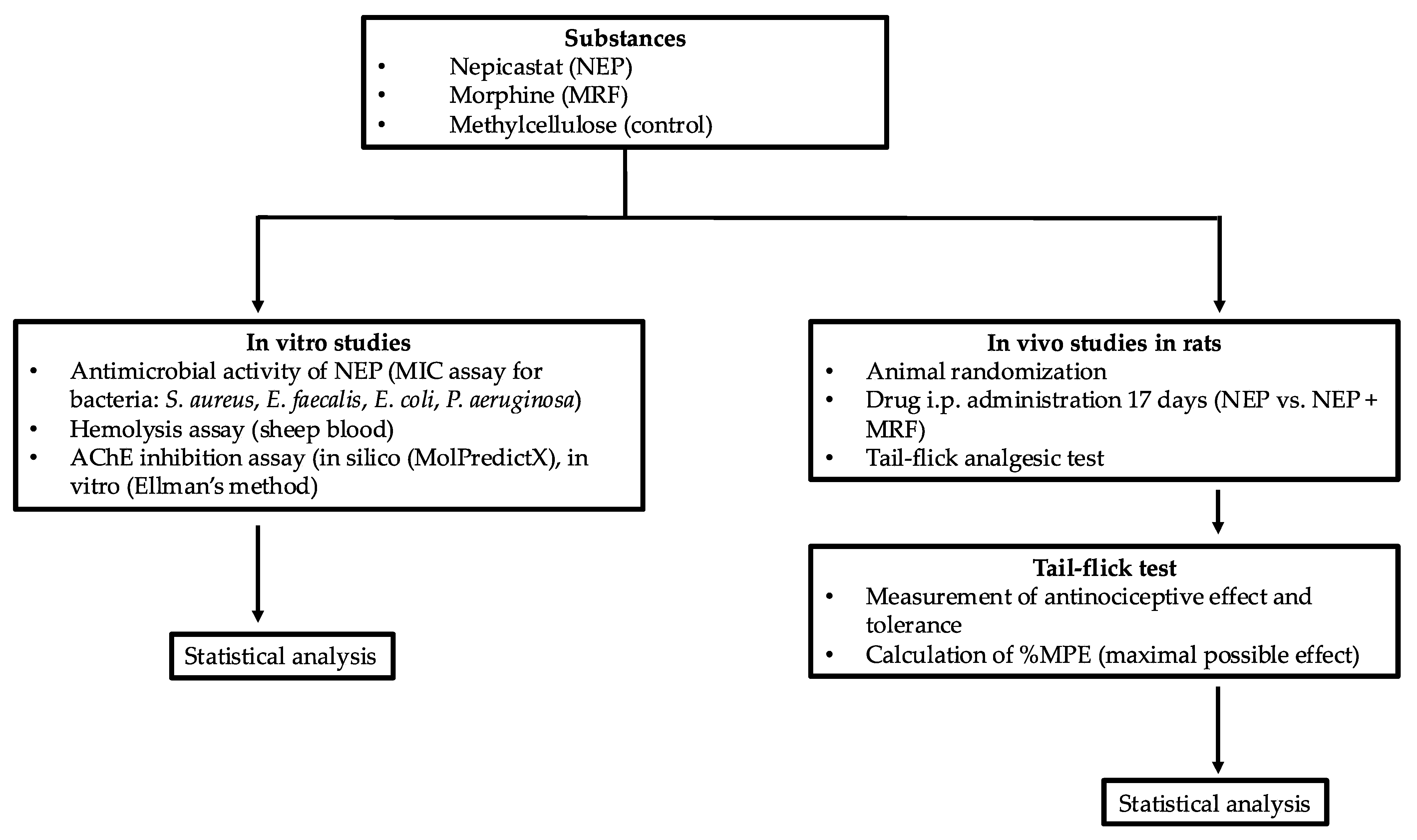
3. Materials and Methods
3.1. Chemicals, Microorganisms, and Cell Cultures
3.2. Determination of Minimum Inhibitory Concentrations (MICs)
3.3. Hemolysis Assay
3.4. Activity Against AChE Enzyme
3.4.1. Computational-Aided Prediction of NEP Biological Activity
3.4.2. In Vitro AChE Inhibition Assay
3.5. Behavioral Studies
3.5.1. Animals
3.5.2. Analgesia and Tolerance Induced by Morphine in Rats and NEP Influence
Tail-Flick Test
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biaggioni, I. Dopamine Beta-Hydroxylase Deficiency. 4 September 2003 [Updated 26 September 2024]. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1116/ (accessed on 17 February 2025).
- Gonzalez-Lopez, E.; Vrana, K.E. Dopamine beta-hydroxylase and its genetic variants in human health and disease. J. Neurochem. 2020, 152, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Senard, J.M.; Rouet, P. Dopamine beta-hydroxylase deficiency. Orphanet J. Rare Dis. 2006, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, G.; Rao, F.; Zhang, K.; Wang, L.; Rodriguez-Flores, J.L.; Sanchez, A.P.; Mahata, M.; Taupenot, L.; Sun, P.; et al. Human dopamine beta-hydroxylase (DBH) regulatory polymorphism that influences enzymatic activity, autonomic function, and blood pressure. J. Hypertens. 2010, 28, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.J.; Stolk, J.M. Depression, hypertension, and serum dopamine-beta-hydroxylase activity. Psychosom. Med. 1978, 40, 107–115. [Google Scholar] [CrossRef]
- Ohlstein, E.H.; Kruse, L.I.; Ezekiel, M.; Sherman, S.S.; Erickson, R.; DeWolf, W.E., Jr.; Berkowitz, B.A. Cardiovascular effects of a new potent dopamine beta-hydroxylase inhibitor in spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1987, 241, 554–559. [Google Scholar] [CrossRef]
- Matuzas, W.; Meltzer, H.Y.; Uhlenhuth, E.H.; Glass, R.M.; Tong, C. Plasma dopamine-beta-hydroxylase in depressed patients. Biol. Psychiatry 1982, 17, 1415–1424. [Google Scholar]
- Eshel, Y.; Korczyn, A.D.; Paran, E.; Cristal, N.; Rabinowitz, R.; Gitter, S. Serum dopamine beta-hydroxylase activity in acute cardiac disease. Chest 1978, 74, 522–525. [Google Scholar] [CrossRef]
- Skorobogatykh, K.; Azimova, J.; Sergeev, A.; Klimov, E.; Tabeeva, G.; Fokina, N.; Korobeynikova, L.; Kokaeva, Z. EHMTI-0233. Clinical characteristics of migraine patients with dopaminergic gene polymorphisms RS1611115. J. Headache Pain 2014, 15 (Suppl. S1), B35. [Google Scholar] [CrossRef]
- Gotoh, F.; Kanda, T.; Sakai, F.; Yamamoto, M.; Takeoka, T. Serum dopamine-beta-hydroxylase activity in migraine. Arch. Neurol. 1976, 33, 656–657. [Google Scholar] [CrossRef]
- Sanna, M.D.; Mello, T.; Masini, E.; Galeotti, N. Activation of ERK/CREB pathway in noradrenergic neurons contributes to hypernociceptive phenotype in H4 receptor knockout mice after nerve injury. Neuropharmacology 2018, 128, 340–350. [Google Scholar] [CrossRef]
- Stanley, W.C.; Li, B.; Bonhaus, D.W.; Johnson, L.G.; Lee, K.; Porter, S.; Walker, K.; Martinez, G.; Eglen, R.M.; Whiting, R.L.; et al. Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase. Br. J. Pharmacol. 1997, 121, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Maccioni, P.; Vargiolu, D.; Loi, B.; Lobina, C.; Zaru, A.; Carai, M.A.; Gessa, G.L. The dopamine β-hydroxylase inhibitor, nepicastat, reduces different alcohol-related behaviors in rats. Alcohol Clin. Exp. Res. 2014, 38, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.P.; Epps, S.A.; Grice, T.W.; Weinshenker, D. The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 2013, 38, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Karamanakos, P.N.; Pappas, P.; Stephanou, P.; Marselos, M. Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Pharmacol. Toxicol. 2001, 88, 106–110. [Google Scholar] [CrossRef]
- Devoto, P.; Flore, G.; Saba, P.; Frau, R.; Gessa, G.L. Selective inhibition of dopamine-beta-hydroxylase enhances dopamine release from noradrenergic terminals in the medial prefrontal cortex. Brain Behav. 2015, 5, e00393. [Google Scholar] [CrossRef]
- Hegde, S.S.; Friday, K.F. Dopamine-beta-hydroxylase inhibition: A novel sympatho-modulatory approach for the treatment of congestive heart failure. Curr. Pharm. Des. 1998, 4, 469–479. [Google Scholar] [CrossRef]
- Stanley, W.C.; Lee, K.; Johnson, L.G.; Whiting, R.L.; Eglen, R.M.; Hegde, S.S. Cardiovascular effects of nepicastat (RS-25560-197), a novel dopamine beta-hydroxylase inhibitor. J. Cardiovasc. Pharmacol. 1998, 31, 963–970. [Google Scholar] [CrossRef]
- Frączek, K.; Kowalczyk, A.; Pekala, M.; Kasarello, K.; Sygitowicz, G.; Sulejczak, D.; Zaremba, M.; Konop, M.; Frankowska, M.; Filip, M.; et al. The positive and negative outcome of morphine and disulfiram subacute co-administration in rats in the absence of ethanol challenge. Pharmaceutics 2020, 13, 29. [Google Scholar] [CrossRef]
- Klegeris, A.; Korkina, L.G.; Greenfield, S.A. A possible interaction between acetylcholinesterase and dopamine molecules during autoxidation of the amine. Free Radic. Biol. Med. 1995, 18, 223–230. [Google Scholar]
- Soininen, H.; Pitkänen, A.; Halonen, T.; Riekkinen, P.J. Dopamine-beta-hydroxylase and acetylcholinesterase activities of cerebrospinal fluid in Alzheimer’s disease. Acta Neurol. Scand. 1984, 70, 29–34. [Google Scholar] [CrossRef]
- Docobo-Perez, F.; Lopez-Cerero, L.; Lopez-Rojas, R.; Egea, P.; Dominguez-Herrera, J.; Rodriguez-Bano, J.; Pascual, A.; Pachón, J. Inoculum effect on the efficacies of amoxicillin-clavulanate, piperacillin-tazobactam, and imipenem against extended-spectrum beta-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli in an experimental murine sepsis model. Antimicrob. Agents Chemother. 2013, 57, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Inoculum effect. Rev. Infect. Dis. 1989, 11, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rio-Marques, L.; Hartke, A.; Bizzini, A. The effect of inoculum size on selection of in vitro resistance to vancomycin, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2014, 20, 539–543. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Lowe, G.; Stike, R.; Pollack, M.; Bosley, J.; O’Brien, P.; Hake, A.; Landis, G.; Billings, N.; Gordon, P.; Manzella, S.; et al. Nursing blood specimen collection techniques and hemolysis rates in an emergency department: Analysis of venipuncture versus intravenous catheter collection techniques. J. Emerg. Nurs. 2008, 34, 26–32. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the hemolysis assay for the assessment of cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Rees, T.M.; Brimijoin, S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today 2003, 39, 75–83. [Google Scholar] [CrossRef]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 48, 471–480. [Google Scholar] [CrossRef]
- Stern, N.; Gacs, A.; Tátrai, E.; Flachner, B.; Hajdú, I.; Dobi, K.; Bágyi, I.; Dormán, G.; Lőrincz, Z.; Cseh, S.; et al. Dual inhibitors of AChE and BACE-1 for reducing Aβ in Alzheimer’s disease: From In Silico to In Vivo. Int. J. Mol. Sci. 2022, 23, 13098. [Google Scholar] [CrossRef]
- Gajendra, K.; Pratap, G.K.; Poornima, D.V.; Shantaram, M.; Ranjita, G. Natural acetylcholinesterase inhibitors: A multi-targeted therapeutic potential in Alzheimer’s disease. Eur. J. Med. Chem. Rep. 2024, 11, 100154. [Google Scholar] [CrossRef]
- Van Greunen, D.G.; Cordier, W.; Nell, M.; van der Westhuyzen, C.; Steenkamp, V.; Panayides, J.L.; Riley, D.L. Targeting Alzheimer’s disease by investigating previously unexplored chemical space surrounding the cholinesterase inhibitor donepezil. Eur. J. Med. Chem. 2017, 127, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.; Alotibi, I.; Alghamdi, A.A.; Ahmad, A.; Jamal, Q.M.S.; Srivastava, S. Computational approaches to evaluate the acetylcholinesterase binding interaction with taxifolin for the management of Alzheimer’s disease. Molecules 2024, 29, 674. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, K.P.; Arumugam, A.; Kandasamy, R.; Alagarsamy, S. In silico method for prediction of maximum binding affinity and ligand-protein interacion studies on Alzheimer’s disease. Int. J. Res. Grathaalayah 2020, 8, 326–370. [Google Scholar]
- Pompermaier, A.; Cole Varela, A.C.; Mozzato, M.T.; Soares, S.M.; Fortuna, M.; Alves, C.; Tamagno, W.A.; Barcellos, L.J.G. Impaired initial development and behavior in zebrafish exposed to environmentally relevant concentrations of widely used pesticides. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109328. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J. DBH Inhbitors for Treating or Preventing Memory Loss. World Patent 2017/100623A1, 15 June 2017. [Google Scholar]
- Tiemann, L.; Heitmann, H.; Schulz, E.; Baumkoẗter, J.; Ploner, M. Dopamine precursor depletion influences pain affect rather than pain sensation. PLoS ONE 2014, 9, e96167. [Google Scholar] [CrossRef]
- Jaaskelainen, S.K.; Rinne, J.O.; Forssell, H.; Tenovuo, O.; Kaasinen, V.; Sonninen, P.; Bergman, J. Role of the dopaminergic system in chronic pain—A fluorodopa-PET study. Pain 2001, 90, 257–260. [Google Scholar] [CrossRef]
- Wood, P.B.; Patterson, J.C., II; Sunderland, J.J.; Tainter, K.H.; Glabus, M.F.; Lilien, D.L. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: A pilot study. J. Pain 2007, 8, 51–58. [Google Scholar] [CrossRef]
- Taylor, A.M.W.; Becker, S.; Schweinhardt, P.; Cahill, C. Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 2016, 157, 1194–1198. [Google Scholar] [CrossRef]
- Taylor, B.K.; Joshi, C.; Uppal, H. Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res. 2003, 987, 135–143. [Google Scholar] [CrossRef]
- Kernbaum, S.; Hauchecorne, J. Administration of levodopa for relief of herpes zoster pain. JAMA 1981, 246, 132–134. [Google Scholar] [CrossRef]
- Holman, A.J.; Myers, R.R. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005, 52, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Bushnell, M.C.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, S.; Lu, X.; Tao, F. Role of descending dopaminergic pathways in pain modulation. Curr. Neuropharmacol. 2019, 17, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Ceko, M.; Louis-Foster, M.; Elfassy, N.M.; Leyton, M.; Shir, Y.; Schweinhardt, P. Dopamine and pain sensitivity: Neither sulpiride nor acute phenylalanine and tyrosine depletion have effects on thermal pain sensations in healthy volunteers. PLoS ONE 2013, 8, e80766. [Google Scholar] [CrossRef]
- Strickland, J.C.; Gipson, C.D.; Dunn, K.E. Dopamine Supersensitivity: A Novel Hypothesis of Opioid-Induced Neurobiological Mechanisms Underlying Opioid-Stimulant Co-use and Opioid Relapse. Front. Psychiatry 2022, 13, 835816. [Google Scholar] [CrossRef]
- Dai, W.L.; Xiong, F.; Yan, B.; Cao, Z.Y.; Liu, W.T.; Liu, J.H.; Yu, B.Y. Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord. Sci. Rep. 2016, 6, 38746. [Google Scholar] [CrossRef]
- Kosten, T.R.; George, T.P. The neurobiology of opioid dependence: Implications for treatment. Sci. Pract. Perspect. 2002, 1, 13–20. [Google Scholar] [CrossRef]
- Sun, G.-L.; Song, Z.-J.; Peng, X.-H.; Chen, P.-P.; Song, Y.; Qin, X.; Hua, R.; Zhang, Y.-M. Projection-specific dopamine neurons in the ventral tegmental area participated in morphine-induced hyperalgesia and anti-nociceptive tolerance in male mice. J. Psychopharmacol. 2021, 35, 59. [Google Scholar] [CrossRef]
- Johnson, D.W.; Glick, S.D. Dopamine release and metabolism in nucleus accumbens and striatum of morphine-tolerant and nontolerant rats. Pharmacol. Biochem. Behav. 1993, 46, 341–347. [Google Scholar] [CrossRef]
- Nevo, I.; Avidor-Reiss, T.; Levy, R.; Bayewitch, M.; Vogel, Z. Acute and chronic activation of the mu-opioid receptor with the endogenous ligand endomorphin differentially regulates adenylyl cyclase isozymes. Neuropharmacology 2000, 39, 364–371. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Mohamed, L.H.; Ahmed, H.N. Modification of morphine-induced analgesia, tolerance and dependence by bromocriptine. Eur. J. Pharmacol. 1989, 170, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Michael-Titus, A.; Bousselmame, R.; Costentin, J. Stimulation of dopamine D2 receptors induces an analgesia involving an opioidergic but non enkephalinergic link. Eur. J. Pharmacol. 1990, 187, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Serafin, P.; Kowalczyk, P.; Mollica, A.; Stefanucci, A.; Laskowska, A.K.; Zawadzka, M.; Kramkowski, K.; Kleczkowska, P. Evaluation of antimicrobial activities against various E. coli strains of a novel hybrid peptide-LENART01. Molecules 2023, 28, 4955. [Google Scholar] [CrossRef] [PubMed]
- Scotti, M.T.; Albuquerque, E.F.; da Silva, C.G., Jr.; Menezes, R.P.B.; Herrera-Acevedo, C.; Sousa, N.F.; Calado, L.F.; Alves, E.H.P.; Scotti, L. MolPredictX: A pioneer mobile app version for online biological activity predictions by machine learning models. Methods Mol. Biol. 2025, 2834, 351–371. [Google Scholar]
- Ellman, G.L.; DianeCourtney, K.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Szeleszczuk, Ł.; Pisklak, D.M.; Grodner, B. Thiamine and thiamine pyrophosphate as non-competitive inhibitors of acetylcholinesterase—Experimental and theoretical investigations. Molecules 2025, 30, 412. [Google Scholar] [CrossRef]
| Tested Bacteria | ||||
|---|---|---|---|---|
| Staphylococcus aureus ATCC 6538 | Enterococcus faecalis ATCC 29212 | Escherichia coli ATCC 25922 | Pseudomonas aeruginosa ATCC 15442 | |
| Nepicastat (NEP; µg/mL) | 64 | 128 | 128 | >521 |
| Ciprofloxacin (ctrl; µg/mL) | 0.125 | 1.0 | 0.008 | 0.06 |
| Acetylcholinesterase (AChE) | ||||
|---|---|---|---|---|
| Predicted outcome | Probability | Probability active | Probability inactive | Predicted reliability |
| Active | 0.6 | 0.6 | 0.4 | reliable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jas, R.; Bauer, M.; Grodner, B.; Kończak, W.; Frączek, K.; Laskowska, A.K.; Milczarek, M.; Kamysz, W.; Kleczkowska, P. Exploring Nepicastat Activity: Beyond DβH. Int. J. Mol. Sci. 2025, 26, 4356. https://doi.org/10.3390/ijms26094356
Jas R, Bauer M, Grodner B, Kończak W, Frączek K, Laskowska AK, Milczarek M, Kamysz W, Kleczkowska P. Exploring Nepicastat Activity: Beyond DβH. International Journal of Molecular Sciences. 2025; 26(9):4356. https://doi.org/10.3390/ijms26094356
Chicago/Turabian StyleJas, Rafal, Marta Bauer, Błażej Grodner, Weronika Kończak, Karolina Frączek, Anna K. Laskowska, Małgorzata Milczarek, Wojciech Kamysz, and Patrycja Kleczkowska. 2025. "Exploring Nepicastat Activity: Beyond DβH" International Journal of Molecular Sciences 26, no. 9: 4356. https://doi.org/10.3390/ijms26094356
APA StyleJas, R., Bauer, M., Grodner, B., Kończak, W., Frączek, K., Laskowska, A. K., Milczarek, M., Kamysz, W., & Kleczkowska, P. (2025). Exploring Nepicastat Activity: Beyond DβH. International Journal of Molecular Sciences, 26(9), 4356. https://doi.org/10.3390/ijms26094356







