Galactooligosaccharides Attenuate Behavioural, Haematological and Immunological Abnormalities and Influence Gut Microbiota in Rats with Amygdala Hyperactivation Induced by Electrical Stimulation
Abstract
1. Introduction
2. Results
2.1. Locomotor Activity
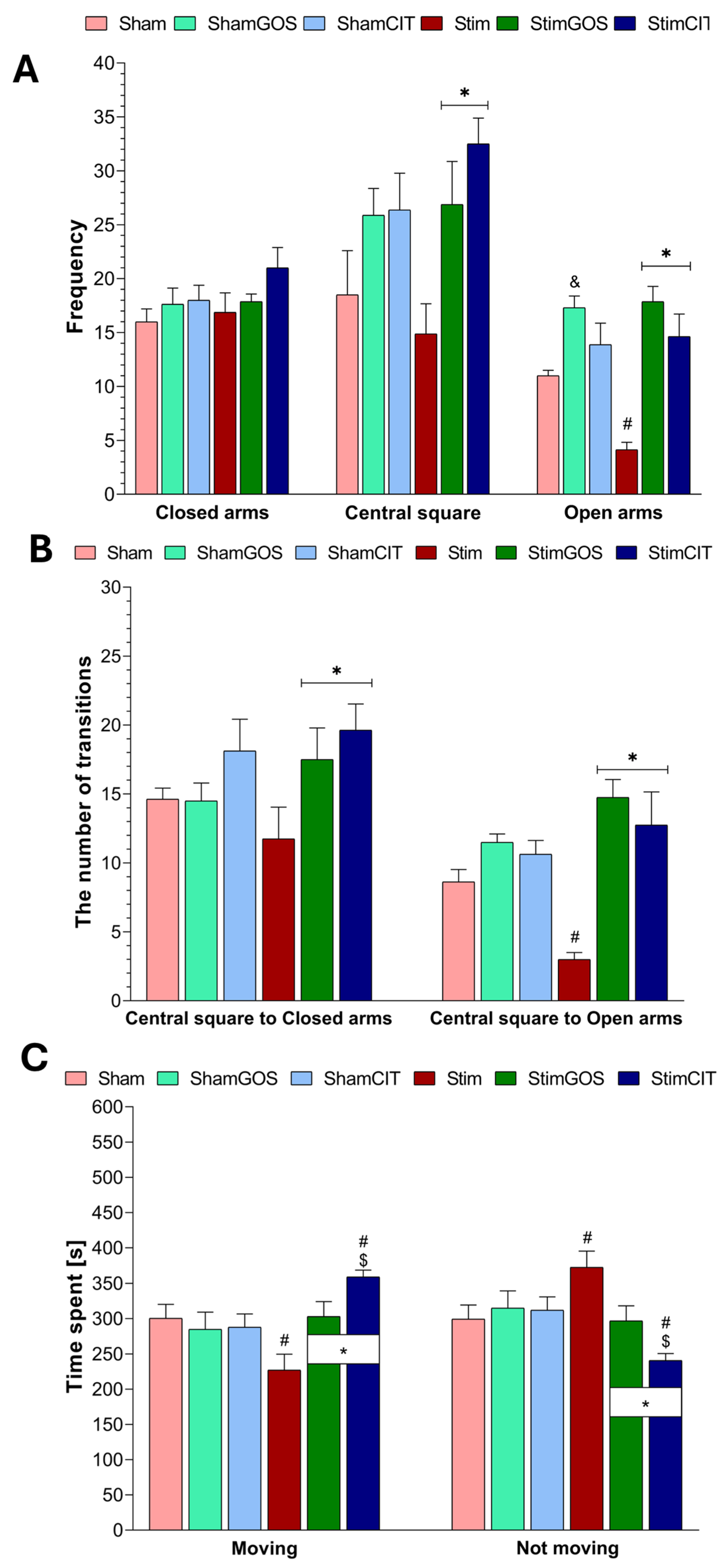
2.2. The Anxiety Level
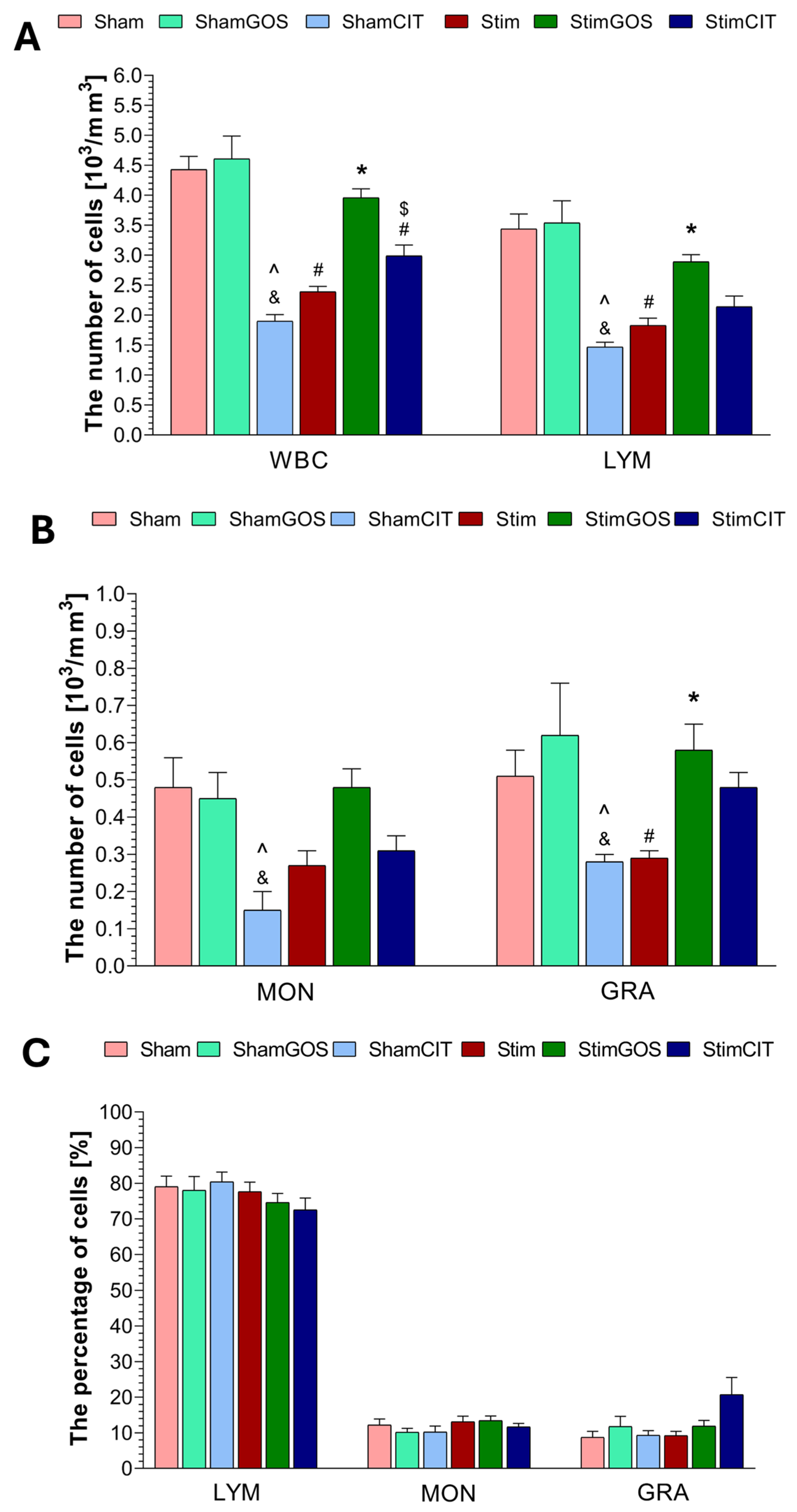
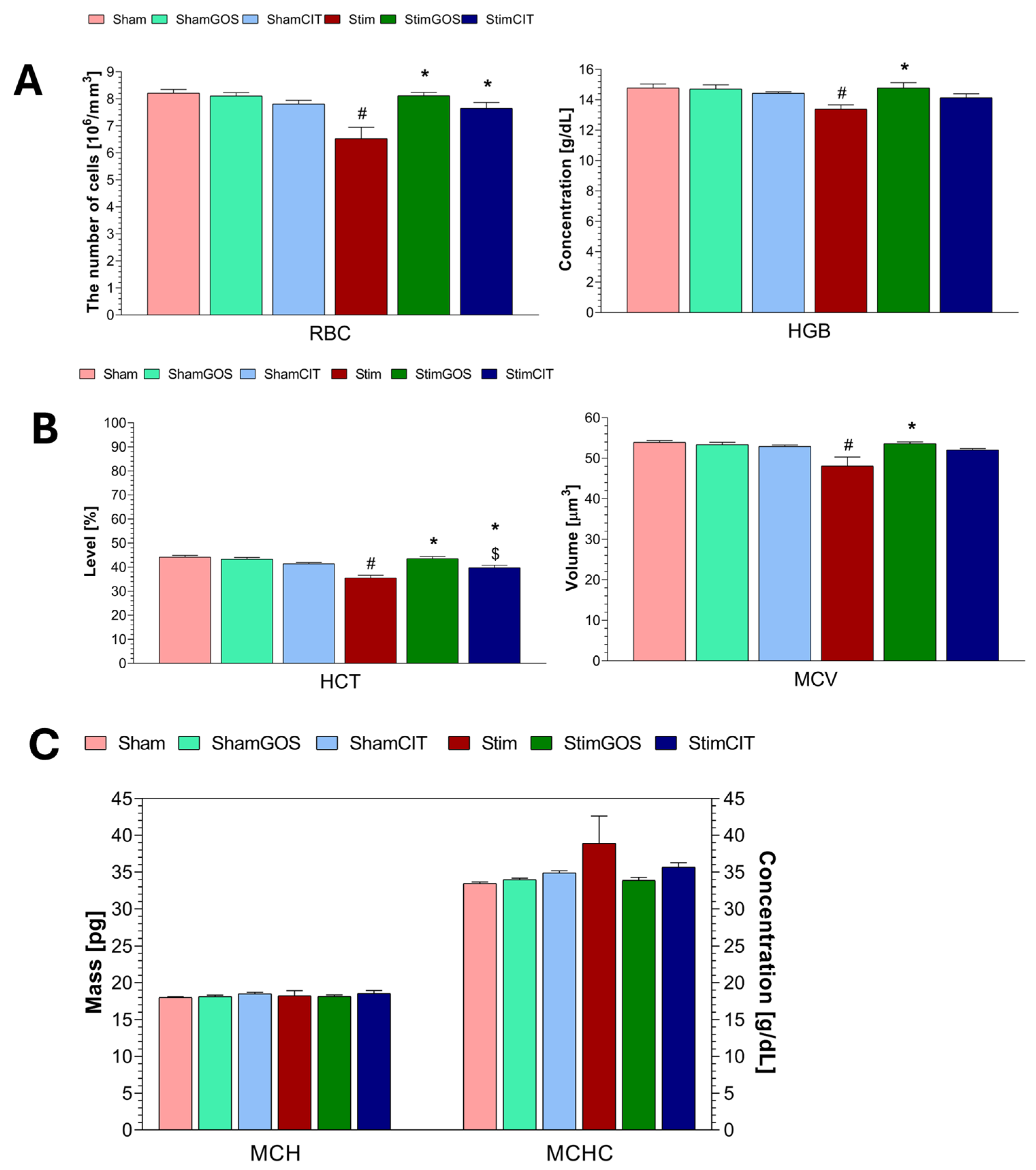
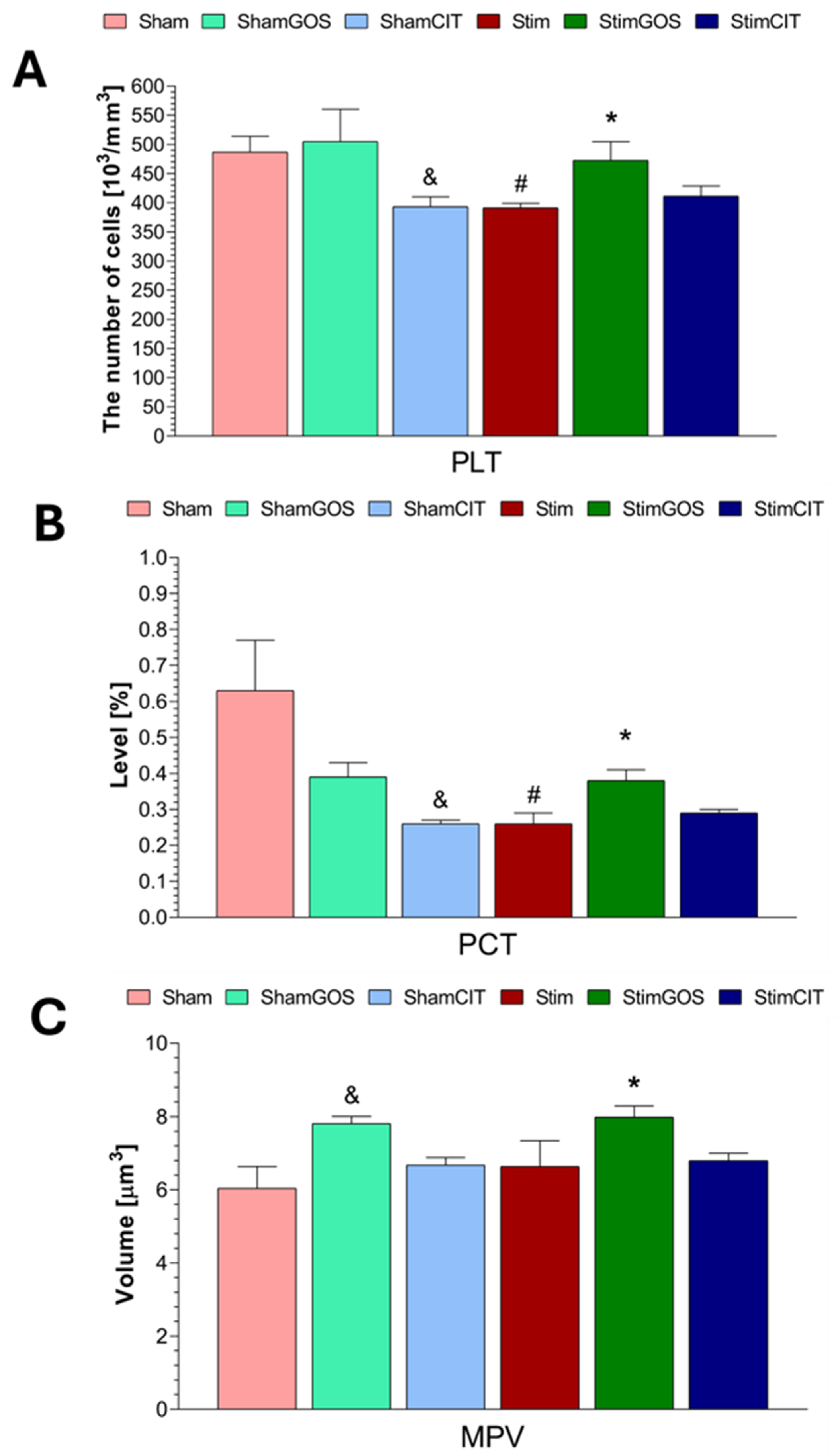
2.3. Haematological Parameters
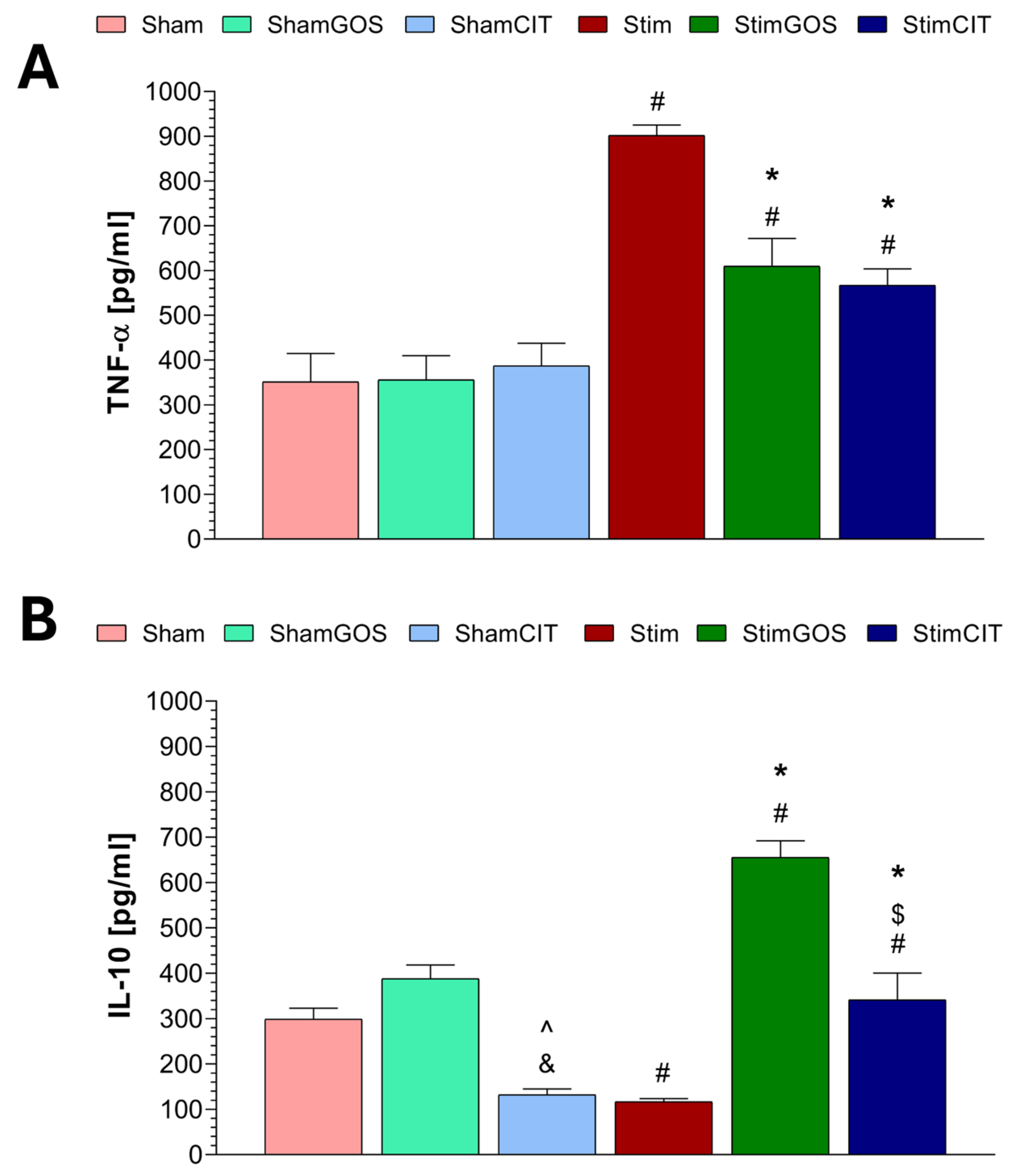
2.4. Plasma Immunological Markers
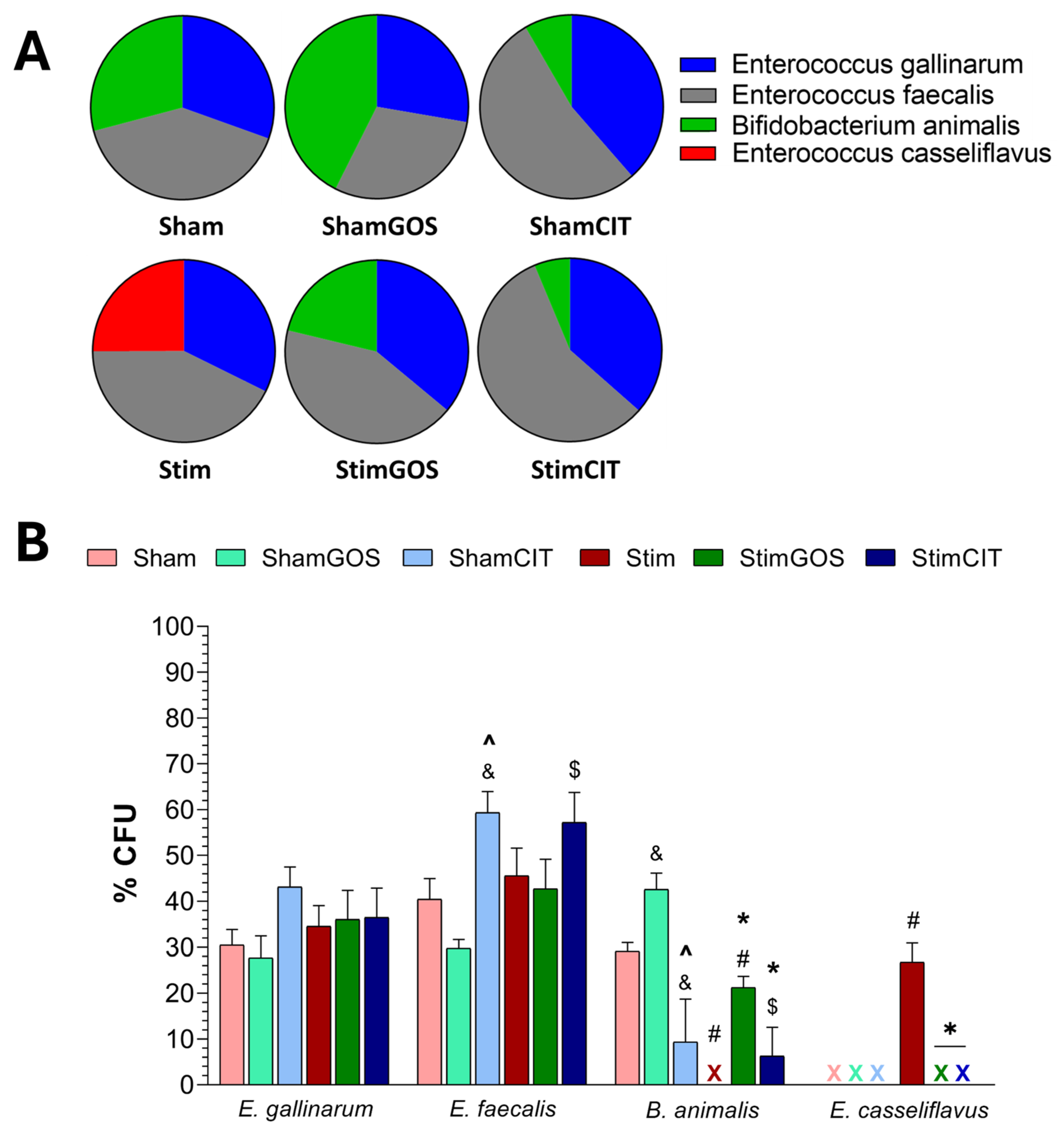
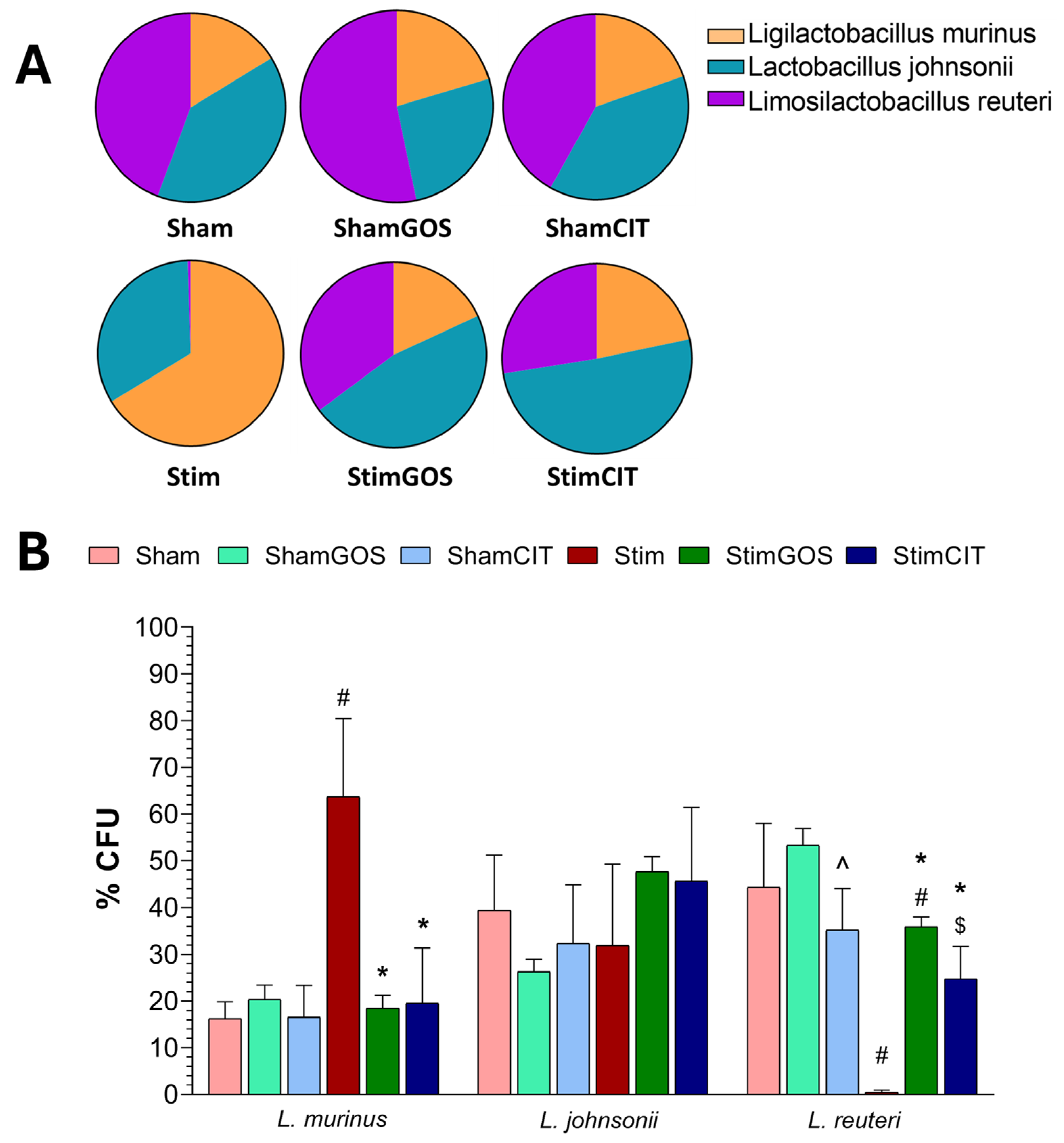
2.5. Changes in Gut Microbiota
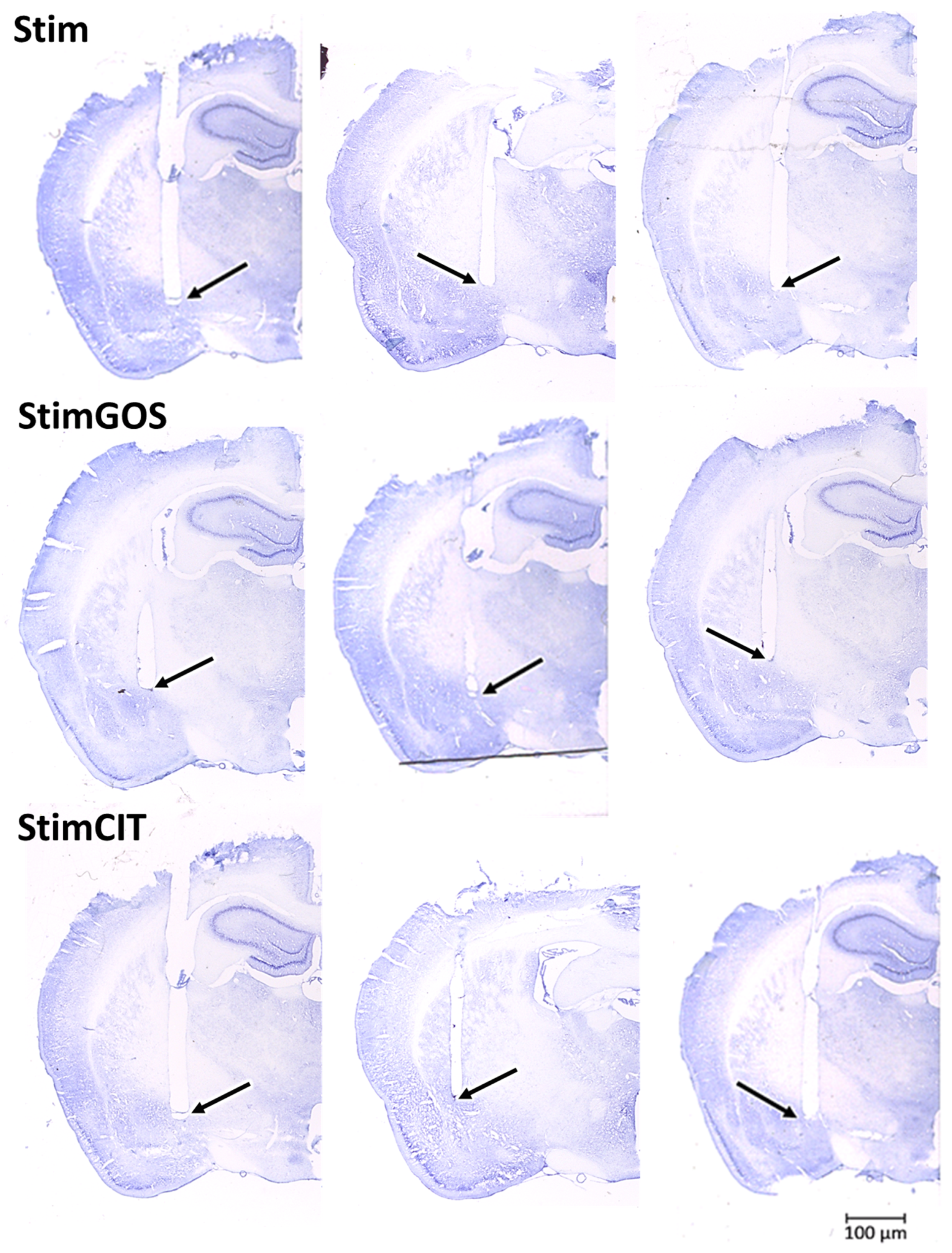
2.6. Histological Verification of the Electrode Implantation
3. Discussion
3.1. The Impact of Amygdala Hyperactivation and Prebiotic Supplementation on Behavioural Performance—Locomotor Activity and Anxiety-like Behaviour
3.2. The Impact of Amygdala Hyperactivation and Prebiotic Supplementation on Haematological Parameters
3.3. The Impact of Amygdala Hyperactivation and Prebiotic Supplementation on Plasma Immunological Markers
3.4. The Impact of Amygdala Hyperactivation and Prebiotic Supplementation on Gut Microbiota Composition
3.5. Comparison of Galactooligosaccharides’ and Citalopram’s Therapeutic Potential
4. Materials and Methods
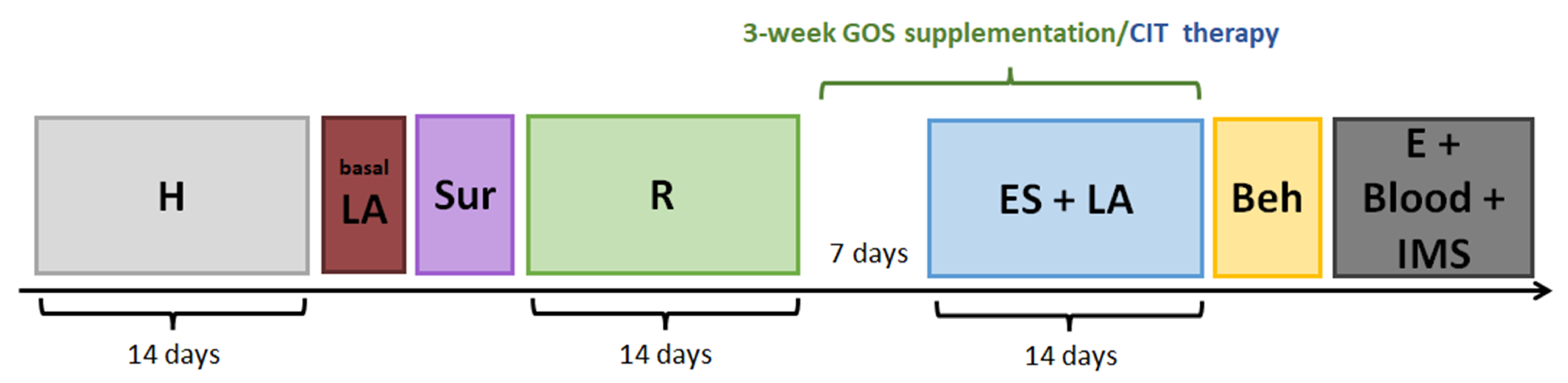
4.1. Animals and Experimental Approach
4.2. Stereotaxic Surgery for Stimulating Electrode Implantation
4.3. Pre-Stimulation Screening and Experimental Groups
4.4. GOS Supplementation and CIT Therapy
4.5. Long-Term Electrical Stimulation of the CeA in Actometers
4.6. Locomotor Activity
4.7. The Anxiety Level Assessment
4.8. Euthanasia and Blood Sample Collection
4.9. Haematological Analysis
4.10. Measurement of the Plasma Immunological Marker Concentration
4.11. Histological Verification of Electrode Implantation
4.12. Gut Microbiota Analysis
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMB | ambulatory movement |
| Amg | amygdala |
| BLA | basolateral amygdala |
| BNST | bed nucleus of stria terminalis |
| BSM | bifidus selective medium |
| CeA | central nucleus of the amygdala |
| CFU | colony-forming unit |
| CIT | citalopram |
| CNS | central nervous system |
| EPM | elevated plus maze |
| FOS | fructooligosaccharide |
| GABA | gamma-aminobutyric acid |
| GOS | galactooligosaccharide |
| GRA | granulocyte |
| HCT | haematocrit |
| HGB | haemoglobin concentration |
| HOR | horizontal movements |
| HPA | hypothalamic–pituitary–adrenal axis |
| IL | interleukin |
| LPS | lipopolysaccharide |
| LYM | lymphocyte |
| MCH | mean corpuscular haemoglobin |
| MCHC | mean corpuscular haemoglobin concentration |
| MCV | mean corpuscular volume |
| MON | monocyte |
| MPV | mean platelet volume |
| PCT | plateletcrit |
| PLT | platelet |
| PTSD | post-traumatic stress disorder |
| RBC | red blood cell |
| SSR | selective serotonin reuptake inhibitor |
| TNF | tumour necrosis factor |
| VER | vertical movement |
| WBC | white blood cell |
References
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO Prevalence Estimates of Mental Disorders in Conflict Settings: A Systematic Review and Meta-Analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, R.; Russo, S.J.; Müller, M.B. Neurobiology and Systems Biology of Stress Resilience. Physiol. Rev. 2024, 104, 1205–1263. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Hoban, A.E.; Ventura-Silva, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. Bioessays 2018, 40, 1700172. [Google Scholar] [CrossRef] [PubMed]
- Sharvin, B.L.; Aburto, M.R.; Cryan, J.F. Decoding the Neurocircuitry of Gut Feelings: Region-Specific Microbiome-Mediated Brain Alterations. Neurobiol. Dis. 2023, 179, 106033. [Google Scholar] [CrossRef]
- Yeh, L.-F.; Zuo, S.; Liu, P.-W. Molecular Diversity and Functional Dynamics in the Central Amygdala. Front. Mol. Neurosci. 2024, 17, 1364268. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Becker, M.; Mothes-Lasch, M.; Miltner, W.H.R.; Straube, T. Loud and Angry: Sound Intensity Modulates Amygdala Activation to Angry Voices in Social Anxiety Disorder. Soc. Cogn. Affect. Neurosci. 2017, 12, 409–416. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, T.T.; Yin, G.; Cui, R.; Zhao, G.; Yang, W. Stress-Induced Functional Alterations in Amygdala: Implications for Neuropsychiatric Diseases. Front. Neurosci. 2018, 12, 367. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. Pharmacological Treatment of Anxiety Disorders: The Role of the HPA Axis. Front. Psychiatry 2020, 11, 443. [Google Scholar] [CrossRef]
- Svingen, E. PTSD and Crime Propensity: Stress Systems, Brain Structures, and the Nature of the Relationship. Heliyon 2023, 9, e18381. [Google Scholar] [CrossRef]
- Perreault, M.L.; Seeman, P.; Szechtman, H. Kappa-Opioid Receptor Stimulation Quickens Pathogenesis of Compulsive Checking in the Quinpirole Sensitization Model of Obsessive-Compulsive Disorder (OCD). Behav. Neurosci. 2007, 121, 976–991. [Google Scholar] [CrossRef] [PubMed]
- Myślińska, D.; Plucińska, K.; Glac, W.; Wrona, D. Blood Natural Killer Cell Cytotoxicity Enhancement Correlates with an Increased Activity in Brain Motor Structures Following Chronic Stimulation of the Bed Nucleus of the Stria Terminalis in Rats. Brain Res. Bull. 2012, 87, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, R.; Binko, M.; Nobrega, J.N.; Hamani, C. Deep Brain Stimulation in Animal Models of Fear, Anxiety, and Posttraumatic Stress Disorder. Neuropsychopharmacology 2016, 41, 2810–2817. [Google Scholar] [CrossRef]
- Jerzemowska, G.; Plucińska, K.; Piwka, A.; Podlacha, M.; Orzeł-Gryglewska, J. Behavioral Reaction and C-Fos Expression after Opioids Injection into the Pedunculopontine Tegmental Nucleus and Electrical Stimulation of the Ventral Tegmental Area. Int. J. Mol. Sci. 2022, 24, 512. [Google Scholar] [CrossRef]
- Adkins, A.M.; Colby, E.M.; Kim, W.-K.; Wellman, L.L.; Sanford, L.D. Stressor Control and Regional Inflammatory Responses in the Brain: Regulation by the Basolateral Amygdala. J. Neuroinflamm. 2023, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of Gut Microbiota and Brain via Immune and Neuroendocrine Signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-Microglia Connections via the Gut-Brain Axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Morgadinho, J.; Halverson, T. Approach to the Diagnosis and Management of Dysbiosis. Front. Nutr. 2024, 11, 1330903. [Google Scholar] [CrossRef]
- Warren, A.; Nyavor, Y.; Beguelin, A.; Frame, L.A. Dangers of the Chronic Stress Response in the Context of the Microbiota-Gut-Immune-Brain Axis and Mental Health: A Narrative Review. Front. Immunol. 2024, 15, 1365871. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Lingrand, L.; Maillard, M.; Feuz, B.; Tompkins, T.A. The Effects of Psychobiotics on the Microbiota-Gut-Brain Axis in Early-Life Stress and Neuropsychiatric Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110142. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.E.; Tzortzis, G.; Burnet, P.W.J. Prebiotic Feeding Elevates Central Brain Derived Neurotrophic Factor, N-Methyl-D-Aspartate Receptor Subunits and D-Serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic Administration Normalizes Lipopolysaccharide (LPS)-Induced Anxiety and Cortical 5-HT2A Receptor and IL1-β Levels in Male Mice. Brain Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Johnstone, N.; Dart, S.; Knytl, P.; Nauta, A.; Hart, K.; Cohen Kadosh, K. Nutrient Intake and Gut Microbial Genera Changes after a 4-Week Placebo Controlled Galacto-Oligosaccharides Intervention in Young Females. Nutrients 2021, 13, 4384. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Cao, X.; Chen, S.; Jiang, X.; Li, D.; Chen, G. Dietary Galacto-Oligosaccharides Ameliorate Atopic Dermatitis-like Skin Inflammation and Behavioral Deficits by Modulating Gut Microbiota-Brain-Skin Axis. J. Agric. Food Chem. 2024, 72, 7954–7968. [Google Scholar] [CrossRef] [PubMed]
- Vaghef-Mehrabany, E.; Ranjbar, F.; Asghari-Jafarabadi, M.; Hosseinpour-Arjmand, S.; Ebrahimi-Mameghani, M. Calorie Restriction in Combination with Prebiotic Supplementation in Obese Women with Depression: Effects on Metabolic and Clinical Response. Nutr. Neurosci. 2021, 24, 339–353. [Google Scholar] [CrossRef]
- Wilms, E.; An, R.; Smolinska, A.; Stevens, Y.; Weseler, A.R.; Elizalde, M.; Drittij, M.-J.; Ioannou, A.; van Schooten, F.J.; Smidt, H.; et al. Galacto-Oligosaccharides Supplementation in Prefrail Older and Healthy Adults Increased Faecal Bifidobacteria, but Did Not Impact Immune Function and Oxidative Stress. Clin. Nutr. 2021, 40, 3019–3031. [Google Scholar] [CrossRef]
- Mysonhimer, A.R.; Cannavale, C.N.; Bailey, M.A.; Khan, N.A.; Holscher, H.D. Prebiotic Consumption Alters Microbiota but Not Biological Markers of Stress and Inflammation or Mental Health Symptoms in Healthy Adults: A Randomized, Controlled, Crossover Trial. J. Nutr. 2023, 153, 1283–1296. [Google Scholar] [CrossRef]
- Nimmons, D.; Aker, N.; Burnand, A.; Jordan, K.P.; Cooper, C.; Davies, N.; Manthorpe, J.; Chew-Graham, C.A.; Kingstone, T.; Petersen, I.; et al. Clinical Effectiveness of Pharmacological and Non-Pharmacological Treatments for the Management of Anxiety in Community Dwelling People Living with Dementia: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2024, 157, 105507. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Walker, L.C. A Balancing Act: The Role of pro- and Anti-Stress Peptides within the Central Amygdala in Anxiety and Alcohol Use Disorders. J. Neurochem. 2021, 157, 1615–1643. [Google Scholar] [CrossRef]
- Bohnsack, J.P.; Zhang, H.; Pandey, S.C. EZH2-Dependent Epigenetic Reprogramming in the Central Nucleus of Amygdala Regulates Adult Anxiety in Both Sexes after Adolescent Alcohol Exposure. Transl. Psychiatry 2024, 14, 197. [Google Scholar] [CrossRef]
- Sladky, R.; Kargl, D.; Haubensak, W.; Lamm, C. An Active Inference Perspective for the Amygdala Complex. Trends Cogn. Sci. 2024, 28, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, N.; Takahashi, D.; Sugimura, Y.K.; Kato, F.; Amano, T.; Minami, M. Activation of the Neural Pathway from the Dorsolateral Bed Nucleus of the Stria Terminalis to the Central Amygdala Induces Anxiety-like Behaviors. Eur. J. Neurosci. 2018, 48, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Asok, A.; Draper, A.; Hoffman, A.F.; Schulkin, J.; Lupica, C.R.; Rosen, J.B. Optogenetic Silencing of a Corticotropin-Releasing Factor Pathway from the Central Amygdala to the Bed Nucleus of the Stria Terminalis Disrupts Sustained Fear. Mol. Psychiatry 2018, 23, 914–922. [Google Scholar] [CrossRef]
- Paliwal, N.P.; Taksande, B.G.; Jain, S.P.; Borikar, S.P. Possible Involvement of GABAergic System on Central Amygdala Mediated Anxiolytic Effect of Agmatine in Rats. Int. J. Neurosci. 2024, 134, 1346–1356. [Google Scholar] [CrossRef]
- Li, Q.; Luo, T.; Jiang, X.; Wang, J. Anxiolytic Effects of 5-HT1A Receptors and Anxiogenic Effects of 5-HT₂C Receptors in the Amygdala of Mice. Neuropharmacology 2012, 62, 474–484. [Google Scholar] [CrossRef] [PubMed]
- de los Angeles Marinzalda, M.; Pérez, P.A.; Gargiulo, P.A.; Casarsa, B.S.; Bregonzio, C.; Baiardi, G. Fear-Potentiated Behaviour Is Modulated by Central Amygdala Angiotensin II AT1 Receptors Stimulation. Biomed. Res. Int. 2014, 2014, 183248. [Google Scholar]
- Yu, Z.; Kisner, A.; Bhatt, A.; Polter, A.M.; Marvar, P.J. Central Amygdala Angiotensin Type 1 Receptor (Agtr1) Expressing Neurons Contribute to Fear Extinction. Neuropharmacology 2023, 229, 109460. [Google Scholar] [CrossRef]
- Rosso, M.; Wirz, R.; Loretan, A.V.; Sutter, N.A.; Pereira da Cunha, C.T.; Jaric, I.; Würbel, H.; Voelkl, B. Reliability of Common Mouse Behavioural Tests of Anxiety: A Systematic Review and Meta-Analysis on the Effects of Anxiolytics. Neurosci. Biobehav. Rev. 2022, 143, 104928. [Google Scholar] [CrossRef]
- Rao, R.M.; Sadananda, M. Influence of State and/or Trait Anxieties of Wistar Rats in an Anxiety Paradigm. Ann. Neurosci. 2016, 23, 44–50. [Google Scholar] [CrossRef]
- Shang, C.; Chen, Z.; Liu, A.; Li, Y.; Zhang, J.; Qu, B.; Yan, F.; Zhang, Y.; Liu, W.; Liu, Z.; et al. Divergent Midbrain Circuits Orchestrate Escape and Freezing Responses to Looming Stimuli in Mice. Nat. Commun. 2018, 9, 1232. [Google Scholar] [CrossRef]
- Pernía-Andrade, A.J.; Wenger, N.; Esposito, M.S.; Tovote, P. Circuits for State-Dependent Modulation of Locomotion. Front. Hum. Neurosci. 2021, 15, 745689. [Google Scholar] [CrossRef] [PubMed]
- Szklany, K.; Wopereis, H.; de Waard, C.; van Wageningen, T.; An, R.; van Limpt, K.; Knol, J.; Garssen, J.; Knippels, L.M.J.; Belzer, C.; et al. Supplementation of Dietary Non-Digestible Oligosaccharides from Birth Onwards Improve Social and Reduce Anxiety-like Behaviour in Male BALB/c Mice. Nutr. Neurosci. 2020, 23, 896–910. [Google Scholar] [CrossRef]
- Chung, Y.; Wu, J.-L.; Huang, W.-C. Effects of Prebiotics on Intestinal Physiology, Neuropsychological Function, and Exercise Capacity of Mice with Sleep Deprivation. Food Res. Int. 2023, 165, 112568. [Google Scholar] [CrossRef]
- de Paiva, I.H.R.; Maciel, L.M.; da Silva, R.S.; Mendonça, I.P.; de Souza, J.R.B.; Peixoto, C.A. Prebiotics Modulate the Microbiota-Gut-Brain Axis and Ameliorate Anxiety and Depression-like Behavior in HFD-Fed Mice. Food Res. Int. 2024, 182, 114153. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical Trial: The Effects of a Trans-Galactooligosaccharide Prebiotic on Faecal Microbiota and Symptoms in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Dobrin, N.; Costea, D.; Glavan, L.-A.; Covache-Busuioc, R.-A.; Dumitrascu, D.-I.; Bratu, B.-G.; Costin, H.-P.; Ciurea, A.V. Mind, Mood and Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 3340. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Osman, A.; Hofford, R.S.; Meckel, K.R.; Dave, Y.A.; Zeldin, S.M.; Shipman, A.L.; Lucerne, K.E.; Trageser, K.J.; Oguchi, T.; Kiraly, D.D. Dietary Polyphenols Drive Dose-Dependent Behavioral and Molecular Alterations to Repeated Morphine. Sci. Rep. 2023, 13, 12223. [Google Scholar] [CrossRef]
- Arslanova, A.; Tarasova, A.; Alexandrova, A.; Novoselova, V.; Shaidullov, I.; Khusnutdinova, D.; Grigoryeva, T.; Yarullina, D.; Yakovleva, O.; Sitdikova, G. Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis. Life 2021, 11, 764. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, P.; Zhang, X.; Yang, Y.; Yang, M.; Wang, T.; Lu, H.; Tian, H.; Wang, H.; Liu, J. Inulin Ameliorates Schizophrenia via Modulation of the Gut Microbiota and Anti-Inflammation in Mice. Food Funct. 2021, 12, 1156–1175. [Google Scholar] [CrossRef]
- Rackayová, V.; Flatt, E.; Braissant, O.; Grosse, J.; Capobianco, D.; Mastromarino, P.; McMillin, M.; DeMorrow, S.; McLin, V.A.; Cudalbu, C. Probiotics Improve the Neurometabolic Profile of Rats with Chronic Cholestatic Liver Disease. Sci. Rep. 2021, 11, 2269. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Yıldız, S.; Korucu, T.; Gundogan, B.; Sunbul, Z.E.; Korkmaz, H.; Atmaca, M. Frequency of Anemia in Chronic Psychiatry Patients. Neuropsychiatr. Dis. Treat. 2015, 11, 2737–2741. [Google Scholar] [CrossRef]
- Le, C.H.H. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003-2012). PLoS ONE 2016, 11, e0166635. [Google Scholar] [CrossRef] [PubMed]
- Wysokiński, A.; Szczepocka, E. Red Blood Cells Parameters in Patients with Acute Schizophrenia, Unipolar Depression and Bipolar Disorder. Psychiatr. Danub. 2018, 30, 323–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wei, Z.; Mei, J.; Li, Q.; Zhen, X.; Zhang, Y. Elevated Serum Platelet Count Inhibits the Effects of Brain Functional Changes on Cognitive Function in Patients with Mild Cognitive Impairment: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Aging Neurosci. 2023, 15, 1088095. [Google Scholar] [CrossRef]
- Gasche, C.; Lomer, M.C.E.; Cavill, I.; Weiss, G. Iron, Anaemia, and Inflammatory Bowel Diseases. Gut 2004, 53, 1190–1197. [Google Scholar] [CrossRef]
- Wei, Y.; Feng, J.; Ma, J.; Chen, D.; Xu, H.; Yin, L.; Chen, J. Characteristics of Platelet-Associated Parameters and Their Predictive Values in Chinese Patients with Affective Disorders. BMC Psychiatry 2022, 22, 150. [Google Scholar] [CrossRef]
- Almis, B.H.; Aksoy, I. Mean Platelet Volume Level in Patients with Generalized Anxiety Disorder. Psychiatry Clin. Psychopharmacol. 2018, 28, 43–47. [Google Scholar] [CrossRef]
- Wang, J.-M.; Yang, K.-D.; Wu, S.-Y.; Zou, X.-G.; Liao, Y.-S.; Yang, B.; Xie, B.-N.; Huang, Y.; Li, S.-J.; Ma, H.-J. Platelet Parameters, C-Reactive Protein, and Depression: An Association Study. Int. J. Gen. Med. 2022, 15, 243–251. [Google Scholar] [CrossRef]
- Rust, C.; Malan-Muller, S.; van den Heuvel, L.L.; Tonge, D.; Seedat, S.; Pretorius, E.; Hemmings, S.M.J. Platelets Bridging the Gap between Gut Dysbiosis and Neuroinflammation in Stress-Linked Disorders: A Narrative Review. J. Neuroimmunol. 2023, 382, 578155. [Google Scholar] [CrossRef]
- Foley, É.M.; Parkinson, J.T.; Mitchell, R.E.; Turner, L.; Khandaker, G.M. Peripheral Blood Cellular Immunophenotype in Depression: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2023, 28, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Han, L.-L.; Chen, L.; Dong, Z.-L. Impact of Acute and Chronic Stress Hormone on Male Albino Rat Brain. Int. J. Clin. Exp. Pathol. 2015, 8, 6841–6846. [Google Scholar] [PubMed]
- Kishor, B.; Rai, P.; Bharatia, R.; Kumar, S.; Gupta, S.K.; Sinha, A. Adaptogenic Potential of Oxitard in Experimental Chronic Stress and Chronic Unpredictable Stress Induced Dysfunctional Homeostasis in Rodents. J. Ayurveda Integr. Med. 2017, 8, 169–176. [Google Scholar] [CrossRef]
- McDonald, L.T.; Lopez, M.F.; Helke, K.L.; McCrackin, M.A.; Cray, J.J.; Becker, H.C.; LaRue, A.C. Early Blood Profile of C57BL/6 Mice Exposed to Chronic Unpredictable Stress. Front. Psychiatry 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Im, E.; Ae, A.; Bn, U.; Po, U. Immuno-Modulatory Properties of Prebiotics Extracted from Vernonia Amygdalina. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 11–17. [Google Scholar]
- Sanguri, S.; Gupta, D. Prebiotic Mannan Oligosaccharide Pretreatment Improves Mice Survival Against Lethal Effects of Gamma Radiation by Protecting GI Tract and Hematopoietic Systems. Front. Oncol. 2021, 11, 677781. [Google Scholar] [CrossRef]
- Rehman, H.-U.; Nasir, M.; Abdul Jabbar, M.; Ayaz, M.; Ali, M.A.; Imran, M.; Gondal, T.A.; Tufail, T.; Aslam, F.; Lal, S.; et al. Effects of Galacto-Oligosaccharide Prebiotics in Blood Profile of Severely Acute Malnourished Children. Cell. Mol. Biol. 2020, 66, 37–44. [Google Scholar] [CrossRef]
- Ooi, L.-G.; Bhat, R.; Rosma, A.; Yuen, K.-H.; Liong, M.-T. A Synbiotic Containing Lactobacillus Gasseri [Corrected] CHO-220 and Inulin Improves Irregularity of Red Blood Cells. J. Dairy. Sci. 2010, 93, 4535–4544. [Google Scholar] [CrossRef]
- Kooshki, A.; Akbarzadeh, R.; Amin, B.; Tofighiyan, T.; Foroumandi, E. Synbiotic Supplement for Treatment of Iron Deficiency Anaemia in Haemodialysis Patients: A Randomized Controlled Trial. Nephrology 2023, 28, 234–239. [Google Scholar] [CrossRef]
- Squillace, S.; Salvemini, D. Toll-like Receptor-Mediated Neuroinflammation: Relevance for Cognitive Dysfunctions. Trends Pharmacol. Sci. 2022, 43, 726–739. [Google Scholar] [CrossRef]
- Kuijer, E.J.; Steenbergen, L. The Microbiota-Gut-Brain Axis in Hippocampus-Dependent Learning and Memory: Current State and Future Challenges. Neurosci. Biobehav. Rev. 2023, 152, 105296. [Google Scholar] [CrossRef] [PubMed]
- Bemark, M.; Pitcher, M.J.; Dionisi, C.; Spencer, J. Gut-Associated Lymphoid Tissue: A Microbiota-Driven Hub of B Cell Immunity. Trends Immunol. 2024, 45, 211–223. [Google Scholar] [CrossRef]
- Sanguri, S.; Gupta, D. Mannan Oligosaccharide Requires Functional ETC and TLR for Biological Radiation Protection to Normal Cells. BMC Cell Biol. 2018, 19, 9. [Google Scholar] [CrossRef]
- Inagaki, T.K.; Muscatell, K.A.; Irwin, M.R.; Cole, S.W.; Eisenberger, N.I. Inflammation Selectively Enhances Amygdala Activity to Socially Threatening Images. Neuroimage 2012, 59, 3222–3226. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater Amygdala Activity and Dorsomedial Prefrontal-Amygdala Coupling Are Associated with Enhanced Inflammatory Responses to Stress. Brain Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef]
- Swartz, J.R.; Carranza, A.F.; Knodt, A.R.; Irwin, M.R.; Hostinar, C.E. Associations between Peripheral Inflammatory Markers and Amygdala Activity and Connectivity in Response to Emotional Faces in Adolescents. Brain Behav. Immun. 2022, 105, 98–108. [Google Scholar] [CrossRef]
- Czerwiec, K.; Ruciński, J.; Piwko, G.; Myślińska, D.; Kosiński, A. Mutual Relations between the Amygdala and Pro-Inflammatory Cytokines: IL-1β and IL-6. Eur. J. Transl. Clin. Med. 2022, 5, 40–46. [Google Scholar] [CrossRef]
- Kuring, J.K.; Mathias, J.L.; Ward, L.; Tachas, G. Inflammatory Markers in Persons with Clinically-Significant Depression, Anxiety or PTSD: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2023, 168, 279–292. [Google Scholar] [CrossRef]
- Ming, Z.; Criswell, H.E.; Breese, G.R. Evidence for TNFα Action on Excitatory and Inhibitory Neurotransmission in the Central Amygdala: A Brain Site Influenced by Stress. Brain Behav. Immun. 2013, 33, 102–111. [Google Scholar] [CrossRef]
- da Silva, R.S.; Mendonça, I.P.; de Paiva, I.H.R.; de Souza, J.R.B.; Peixoto, C.A. Fructooligosaccharides and Galactooligosaccharides Improve Hepatic Steatosis via Gut Microbiota-Brain Axis Modulation. Int. J. Food Sci. Nutr. 2023, 74, 760–780. [Google Scholar] [CrossRef]
- Wang, G.; Sun, W.; Pei, X.; Jin, Y.; Wang, H.; Tao, W.; Xiao, Z.; Liu, L.; Wang, M. Galactooligosaccharide Pretreatment Alleviates Damage of the Intestinal Barrier and Inflammatory Responses in LPS-Challenged Mice. Food Funct. 2021, 12, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, H.; Qian, J.; Han, J.; Zhang, Y.; Li, Y.; Zhang, M.; Cong, J. Compound Prebiotics as Prophylactic and Adjunctive Treatments Ameliorate DSS-Induced Colitis through Gut Microbiota Modulation Effects. Int. J. Biol. Macromol. 2024, 270, 132362. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the Fecal Microflora Profile and Immune Function by a Novel Trans-Galactooligosaccharide Mixture (B-GOS) in Healthy Elderly Volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [CrossRef]
- Lalitsuradej, E.; Sirilun, S.; Sittiprapaporn, P.; Sivamaruthi, B.S.; Pintha, K.; Tantipaiboonwong, P.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Chaiyasut, C. The Effects of Synbiotics Administration on Stress-Related Parameters in Thai Subjects-A Preliminary Study. Foods 2022, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Ayechu-Muruzabal, V.; van de Kaa, M.; Mukherjee, R.; Garssen, J.; Stahl, B.; Pieters, R.J.; Van’t Land, B.; Kraneveld, A.D.; Willemsen, L.E.M. Modulation of the Epithelial-Immune Cell Crosstalk and Related Galectin Secretion by DP3-5 Galacto-Oligosaccharides and β-3’Galactosyllactose. Biomolecules 2022, 12, 384. [Google Scholar] [CrossRef]
- Gao, R.; Tian, S.; Wang, J.; Zhu, W. Galacto-Oligosaccharides Improve Barrier Function and Relieve Colonic Inflammation via Modulating Mucosa-Associated Microbiota Composition in Lipopolysaccharides-Challenged Piglets. J. Anim. Sci. Biotechnol. 2021, 12, 92. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, D.; Wang, Y.; Niu, L.; Jiang, H. Short-Chain Fatty Acids Reduce Oligodendrocyte Precursor Cells Loss by Inhibiting the Activation of Astrocytes via the SGK1/IL-6 Signalling Pathway. Neurochem. Res. 2022, 47, 3476–3489. [Google Scholar] [CrossRef]
- Younus, Z.; Goyal, S.M.; Singh, V.; Ikram, A.; Imran, M. Genomic-Based Characterization of Enterococcus Spp.: An Emerging Pathogen Isolated from Human Gut. Mol. Biol. Rep. 2021, 48, 5371–5376. [Google Scholar] [CrossRef]
- Katagiri, S.; Shiba, T.; Tohara, H.; Yamaguchi, K.; Hara, K.; Nakagawa, K.; Komatsu, K.; Watanabe, K.; Ohsugi, Y.; Maekawa, S.; et al. Re-Initiation of Oral Food Intake Following Enteral Nutrition Alters Oral and Gut Microbiota Communities. Front. Cell Infect. Microbiol. 2019, 9, 434. [Google Scholar] [CrossRef]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-Derived Probiotic Lactobacillus Reuteri Demonstrate Antimicrobial Activities Targeting Diverse Enteric Bacterial Pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Mikami, Y.; Miyamoto, K.; Kamada, N.; Sato, T.; Mizuno, S.; Naganuma, M.; Teratani, T.; Aoki, R.; Fukuda, S.; et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus Murinus in Mice. Cell Rep. 2017, 20, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Liu, J.; Peng, X.; Cai, Y.; Peng, M.; Li, X.; Tan, Z.; Deng, N. Association of Short-Chain Fatty Acids with Gut Microbiota and Lipid Metabolism in Mice with Diarrhea Induced by High-Fat Diet in a Fatigued State. Mol. Nutr. Food Res. 2023, 67, e2300452. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Freeborn, J.; Venna, V.R.; Roos, S.; Rhoads, J.M.; Liu, Y. Lactobacillus Reuteri Effects on Maternal Separation Stress in Newborn Mice. Pediatr. Res. 2021, 90, 980–988. [Google Scholar] [CrossRef]
- Mazzoli, A.; Spagnuolo, M.S.; De Palma, F.; Petecca, N.; Di Porzio, A.; Barrella, V.; Troise, A.D.; Culurciello, R.; De Pascale, S.; Scaloni, A.; et al. Limosilactobacillus Reuteri DSM 17938 Relieves Inflammation, Endoplasmic Reticulum Stress, and Autophagy in Hippocampus of Western Diet-Fed Rats by Modulation of Systemic Inflammation. Biofactors 2024, 50, 1236–1250. [Google Scholar] [CrossRef]
- Mosquera, F.E.C.; Lizcano Martinez, S.; Liscano, Y. Effectiveness of Psychobiotics in the Treatment of Psychiatric and Cognitive Disorders: A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 1352. [Google Scholar] [CrossRef]
- Sjöstedt, P.; Enander, J.; Isung, J. Serotonin Reuptake Inhibitors and the Gut Microbiome: Significance of the Gut Microbiome in Relation to Mechanism of Action, Treatment Response, Side Effects, and Tachyphylaxis. Front. Psychiatry 2021, 12, 682868. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the Antimicrobial Action of Antidepressants on Gut Commensal Microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Xi, M.; Hao, G.; Yao, Q.; Duan, X.; Ge, W. Galactooligosaccharide Mediates NF-κB Pathway to Improve Intestinal Barrier Function and Intestinal Microbiota. Molecules 2023, 28, 7611. [Google Scholar] [CrossRef]
- Mohammadi-Farani, A.; Farhangian, S.; Shirooie, S. Sex differences in acetylcholinesterase modulation during spatial and fear memory extinction in the amygdala; an animal study in the single prolonged stress model of PTSD. Res. Pharm. Sci. 2022, 17, 686–696. [Google Scholar] [CrossRef]
- Jerzemowska, G.; Podlacha, M.; Orzeł-Gryglewska, J. Amphetamine Injection into the Nucleus Accumbens and Electrical Stimulation of the Ventral Tegmental Area in Rats After Novelty Test-Behavioral and Neurochemical Correlates. Int. J. Mol. Sci. 2024, 26, 182. [Google Scholar] [CrossRef] [PubMed]
- Karnia, M.J.; Myślińska, D.; Dzik, K.P.; Flis, D.J.; Podlacha, M.; Kaczor, J.J. BST Stimulation Induces Atrophy and Changes in Aerobic Energy Metabolism in Rat Skeletal Muscles-The Biphasic Action of Endogenous Glucocorticoids. Int. J. Mol. Sci. 2020, 21, 2787. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, B.; Glac, W.; Listowska, M.; Jerzemowska, G.; Plucińska, K.; Majkutewicz, I.; Badtke, P.; Wrona, D. Subthalamic Deep Brain Stimulation Affects Plasma Corticosterone Concentration and Peripheral Immunity Changes in Rat Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2021, 16, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Gronier, B.; Savignac, H.M.; Di Miceli, M.; Idriss, S.M.; Tzortzis, G.; Anthony, D.; Burnet, P.W.J. Increased Cortical Neuronal Responses to NMDA and Improved Attentional Set-Shifting Performance in Rats Following Prebiotic (B-GOS®) Ingestion. Eur. Neuropsychopharmacol. 2018, 28, 211–224. [Google Scholar] [CrossRef]
- Kao, A.C.-C.; Chan, K.W.; Anthony, D.C.; Lennox, B.R.; Burnet, P.W. Prebiotic Reduction of Brain Histone Deacetylase (HDAC) Activity and Olanzapine-Mediated Weight Gain in Rats, Are Acetate Independent. Neuropharmacology 2019, 150, 184–191. [Google Scholar] [CrossRef]
- Spitzer, S.O.; Tkacz, A.; Savignac, H.M.; Cooper, M.; Giallourou, N.; Mann, E.O.; Bannerman, D.M.; Swann, J.R.; Anthony, D.C.; Poole, P.S.; et al. Postnatal Prebiotic Supplementation in Rats Affects Adult Anxious Behaviour, Hippocampus, Electrophysiology, Metabolomics, and Gut Microbiota. iScience 2021, 24, 103113. [Google Scholar] [CrossRef]
- Tottey, W.; Feria-Gervasio, D.; Gaci, N.; Laillet, B.; Pujos, E.; Martin, J.-F.; Sebedio, J.-L.; Sion, B.; Jarrige, J.-F.; Alric, M.; et al. Colonic transit time is a driven force of the gut microbiota composition and metabolism: In vitro evidence. J. Neurogastroenterol. Motil. 2017, 23, 124–134. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Li, H.; Liu, H.; Wang, D.; Pan, W.; Liu, S.; Xu, Y. Brain Structure and Synaptic Protein Expression Alterations after Antidepressant Treatment in a Wistar-Kyoto Rat Model of Depression. J. Affect. Disord. 2022, 314, 293–302. [Google Scholar] [CrossRef]
- Eroli, F.; Johnell, K.; Leal, M.L.; Adamo, C.; Hilmer, S.; Wastesson, J.W.; Cedazo-Minguez, A.; Maioli, S. Chronic polypharmacy impairs explorative behavior and reduces synaptic functions in young adult mice. Aging 2020, 12, 10147–10161. [Google Scholar] [CrossRef]
- Dunacka, J.; Świątek, G.; Wrona, D. High Behavioral Reactivity to Novelty as a Susceptibility Factor for Memory and Anxiety Disorders in Streptozotocin-Induced Neuroinflammation as a Rat Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 11562. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Aguiar, D.C.; Guimarães, F.S. Animal Models of Anxiety Disorders and Stress. Braz. J. Psychiatry 2013, 35 (Suppl. S2), S101–S111. [Google Scholar] [CrossRef] [PubMed]

| Factors | df | HOR | VER | AMB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | ES | F | p | ES | F | p | ES | ||
| Electrical stimulation of the CeA (Stim_Sham) | 1, 126 | 104.46 | <0.001 | 0.45 | 124.81 | <0.001 | 0.72 | 178.57 | <0.001 | 0.82 |
| Supplementation (GOS_CIT_water) | 2, 126 | 30.69 | 0.002 | 0.33 | 26.11 | <0.001 | 0.29 | 68.22 | <0.001 | 0.52 |
| Time point (basal_7 days after_14 days after stimulation) | 2, 126 | 59.93 | <0.001 | 0.49 | 132.80 | <0.001 | 0.68 | 98.44 | <0.001 | 0.61 |
| Interactions: | ||||||||||
| (1) Stimulation × supplem. | 2, 126 | 37.92 | 0.002 | 0.38 | 23.73 | 0.004 | 0.27 | 61.10 | <0.001 | 0.49 |
| (2) Stimulation × Time point | 2, 126 | 23.84 | 0.003 | 0.27 | 91.95 | <0.001 | 0.59 | 156.83 | <0.001 | 0.71 |
| (3) Supplem. × Time point | 4, 126 | 12.21 | 0.007 | 0.28 | 21.04 | 0.003 | 0.40 | 57.81 | <0.001 | 0.65 |
| (4) Stimulation × supplem. × Time point | 4, 126 | 7.54 | 0.013 | 0.19 | 14.29 | 0.007 | 0.31 | 49.61 | <0.001 | 0.61 |
| Factors | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrical Stimulation of the CeA (Stim_Sham) | Supplementation (GOS_CIT_Water) | Interaction (Stimulation × Supplementation) | |||||||||||
| Variables | df | F | p | ES | df | F | p | ES | df | F | p | ES | |
| Frequency | Central square | 1, 42 | 0.19 | 0.664 | 0.00 | 2, 42 | 8.32 | 0.001 | 0.28 | 2, 42 | 1.12 | 0.337 | 0.05 |
| Closed arms | 1, 42 | 1.29 | 0.262 | 0.03 | 2, 42 | 2.16 | 0.128 | 0.09 | 2, 42 | 0.47 | 0.625 | 0.02 | |
| Open arms | 1, 42 | 2.38 | 0.130 | 0.05 | 2, 42 | 25.39 | <0.001 | 0.55 | 2, 42 | 4.79 | 0.013 | 0.19 | |
| Number of transitions | Central square >> Closed arms | 1, 42 | 0.12 | 0.730 | 0.00 | 2, 42 | 4.45 | 0.018 | 0.17 | 2, 42 | 1.28 | 0.288 | 0.06 |
| Central square >> Open arms | 1, 42 | 0.01 | 0.937 | 0.00 | 2, 42 | 18.38 | <0.001 | 0.47 | 2, 42 | 7.15 | 0.002 | 0.25 | |
| Time spent | Moving | 1, 42 | 0.10 | 0.749 | 0.00 | 2, 42 | 4.45 | 0.018 | 0.17 | 2, 42 | 6.67 | 0.003 | 0.24 |
| Not moving | 1, 42 | 0.11 | 0.749 | 0.00 | 2, 42 | 4.50 | 0.018 | 0.17 | 2, 42 | 6.47 | 0.003 | 0.24 | |
| Groups | Mean GOS or CIT Solutions Intake | Frequency in the Open Arms | r Values |
|---|---|---|---|
| ShamGOS | 29.00 | 17.13 | 0.66 |
| ShamCIT | 28.21 | 13.88 | 0.65 |
| StimGOS | 28.88 | 17.88 | 0.84 * |
| StimCIT | 27.88 | 14.63 | 0.83 * |
| Factors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrical Stimulation of the CeA (Stim_Sham) | Supplementation (GOS_CIT_Water) | Interaction (Stimulation × Supplementation) | ||||||||||
| Variables | df | F | p | ES | df | F | p | ES | df | F | p | ES |
| WBC [103/mm3] | 1, 42 | 9.64 | 0.003 | 0.19 | 2, 42 | 38.02 | <0.001 | 0.64 | 2, 42 | 27.50 | <0.001 | 0.57 |
| LYM [103/mm3] | 1, 42 | 9.18 | 0.004 | 0.18 | 2, 42 | 22.19 | <0.001 | 0.51 | 2, 42 | 14.45 | <0.001 | 0.41 |
| MON [103/mm3] | 1, 42 | 0.01 | 0.919 | 0.00 | 2, 42 | 8.25 | <0.001 | 0.28 | 2, 42 | 5.25 | 0.009 | 0.20 |
| GRA [103/mm3] | 1, 42 | 0.11 | 0.738 | 0.00 | 2, 42 | 5.69 | 0.006 | 0.21 | 2, 42 | 4.41 | 0.018 | 0.17 |
| LYM [%] | 1, 42 | 2.89 | 0.096 | 0.06 | 2, 42 | 0.26 | 0.770 | 0.01 | 2, 42 | 0.56 | 0.557 | 0.03 |
| MON [%] | 1, 42 | 2.59 | 0.115 | 0.06 | 2, 42 | 0.73 | 0.490 | 0.03 | 2, 42 | 0.38 | 0.686 | 0.02 |
| GRA [%] | 1, 42 | 3.58 | 0.065 | 0.08 | 2, 42 | 2.71 | 0.078 | 0.11 | 2, 42 | 3.04 | 0.058 | 0.13 |
| RBC [106/mm3] | 1, 42 | 11.37 | 0.002 | 0.21 | 2, 42 | 5.86 | 0.006 | 0.22 | 2, 42 | 9.12 | <0.001 | 0.30 |
| HGB [g/dL] | 1, 42 | 6.00 | 0.018 | 0.12 | 2, 42 | 3.28 | 0.048 | 0.13 | 2, 42 | 4.07 | 0.024 | 0.16 |
| HCT [%] | 1, 42 | 23.51 | <0.001 | 0.36 | 2, 42 | 10.28 | <0.001 | 0.33 | 2, 42 | 15.61 | <0.001 | 0.43 |
| MCV [µm3] | 1, 42 | 7.16 | 0.010 | 0.15 | 2, 42 | 3.20 | 0.051 | 0.13 | 2, 42 | 5.39 | 0.008 | 0.20 |
| MCH [pg] | 1, 42 | 0.10 | 0.750 | 0.00 | 2, 42 | 0.79 | 0.460 | 0.04 | 2, 42 | 0.05 | 0.953 | 0.00 |
| MCHC [g/dL] | 1, 42 | 2.63 | 0.112 | 0.06 | 2, 42 | 1.07 | 0.353 | 0.05 | 2, 42 | 1.85 | 0.170 | 0.08 |
| PLT [103/mm3] | 1, 42 | 2.22 | 0.144 | 0.05 | 2, 42 | 4.19 | 0.022 | 0.17 | 2, 42 | 1.78 | 0.181 | 0.08 |
| MPV [µm3] | 1, 42 | 0.72 | 0.400 | 0.02 | 2, 42 | 7.20 | 0.002 | 0.26 | 2, 42 | 0.19 | 0.825 | 0.01 |
| PCT [%] | 1, 42 | 5.19 | 0.028 | 0.11 | 2, 42 | 3.70 | 0.033 | 0.15 | 2, 42 | 5.70 | 0.006 | 0.21 |
| Factors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrical Stimulation of the CeA (Stim_Sham) | Supplementation (GOS_CIT_Water) | Interaction (Stimulation × Supplementation) | ||||||||||
| Variables | df | F | p | ES | df | F | p | ES | df | F | p | ES |
| TNF-α [pg/mL] | 1, 42 | 63.86 | <0.001 | 0.60 | 2, 42 | 5.68 | 0.006 | 0.21 | 2, 42 | 7.60 | 0.002 | 0.27 |
| IL-10 [pg/mL] | 1, 42 | 9.38 | 0.004 | 0.18 | 2, 42 | 50.03 | <0.001 | 0.70 | 2, 42 | 22.83 | <0.001 | 0.52 |
| Factors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrical Stimulation of the CeA (Stim_Sham) | Supplementation (GOS_CIT_Water) | Interaction (Stimulation × Supplementation) | ||||||||||
| Variables [% CFU] | df | F | p | ES | df | F | p | ES | df | F | p | ES |
| Enterococcus gallinarum | 1, 23 | 0.20 | 0.661 | 0.01 | 2, 23 | 1.47 | 0.250 | 0.12 | 2, 23 | 1.14 | 0.338 | 0.09 |
| Enterococcus faecalis | 1, 23 | 1.43 | 0.244 | 0.06 | 2, 23 | 8.63 | 0.002 | 0.44 | 2, 23 | 0.99 | 0.389 | 0.08 |
| Bifidobacterium animalis | 1, 23 | 17.73 | <0.001 | 0.44 | 2, 23 | 11.89 | <0.001 | 0.51 | 2, 23 | 3.32 | 0.047 | 0.22 |
| Enterococcus casseliflavus | 1, 23 | 38.03 | <0.001 | 0.62 | 2, 23 | 36.57 | <0.001 | 0.76 | 2, 23 | 36.57 | <0.001 | 0.76 |
| Ligilactobacillus murinus | 1, 23 | 4.43 | 0.046 | 0.16 | 2, 23 | 3.27 | 0.048 | 0.22 | 2, 23 | 4.00 | 0.032 | 0.26 |
| Lactobacillus johnsonii | 1, 23 | 0.84 | 0.368 | 0.04 | 2, 23 | 0.04 | 0.962 | 0.00 | 2, 23 | 0.74 | 0.490 | 0.06 |
| Limosilactobacillus reuteri | 1, 23 | 11.44 | 0.002 | 0.33 | 2, 23 | 4.37 | 0.024 | 0.28 | 2, 23 | 4.20 | 0.028 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruciński, J.; Kurowska-Rucińska, E.; Myślińska, D.; Grembecka, B.; Piekarczyk, N.; Necel, A.; Kosznik-Kwaśnicka, K.; Majkutewicz, I. Galactooligosaccharides Attenuate Behavioural, Haematological and Immunological Abnormalities and Influence Gut Microbiota in Rats with Amygdala Hyperactivation Induced by Electrical Stimulation. Int. J. Mol. Sci. 2025, 26, 4353. https://doi.org/10.3390/ijms26094353
Ruciński J, Kurowska-Rucińska E, Myślińska D, Grembecka B, Piekarczyk N, Necel A, Kosznik-Kwaśnicka K, Majkutewicz I. Galactooligosaccharides Attenuate Behavioural, Haematological and Immunological Abnormalities and Influence Gut Microbiota in Rats with Amygdala Hyperactivation Induced by Electrical Stimulation. International Journal of Molecular Sciences. 2025; 26(9):4353. https://doi.org/10.3390/ijms26094353
Chicago/Turabian StyleRuciński, Jan, Ewelina Kurowska-Rucińska, Dorota Myślińska, Beata Grembecka, Natalia Piekarczyk, Agnieszka Necel, Katarzyna Kosznik-Kwaśnicka, and Irena Majkutewicz. 2025. "Galactooligosaccharides Attenuate Behavioural, Haematological and Immunological Abnormalities and Influence Gut Microbiota in Rats with Amygdala Hyperactivation Induced by Electrical Stimulation" International Journal of Molecular Sciences 26, no. 9: 4353. https://doi.org/10.3390/ijms26094353
APA StyleRuciński, J., Kurowska-Rucińska, E., Myślińska, D., Grembecka, B., Piekarczyk, N., Necel, A., Kosznik-Kwaśnicka, K., & Majkutewicz, I. (2025). Galactooligosaccharides Attenuate Behavioural, Haematological and Immunological Abnormalities and Influence Gut Microbiota in Rats with Amygdala Hyperactivation Induced by Electrical Stimulation. International Journal of Molecular Sciences, 26(9), 4353. https://doi.org/10.3390/ijms26094353






