N-Acetylcysteine Attenuates Aβ-Mediated Oxidative Stress, Blood–Brain Barrier Leakage, and Renal Dysfunction in 5xFAD Mice
Abstract
1. Introduction
2. Results
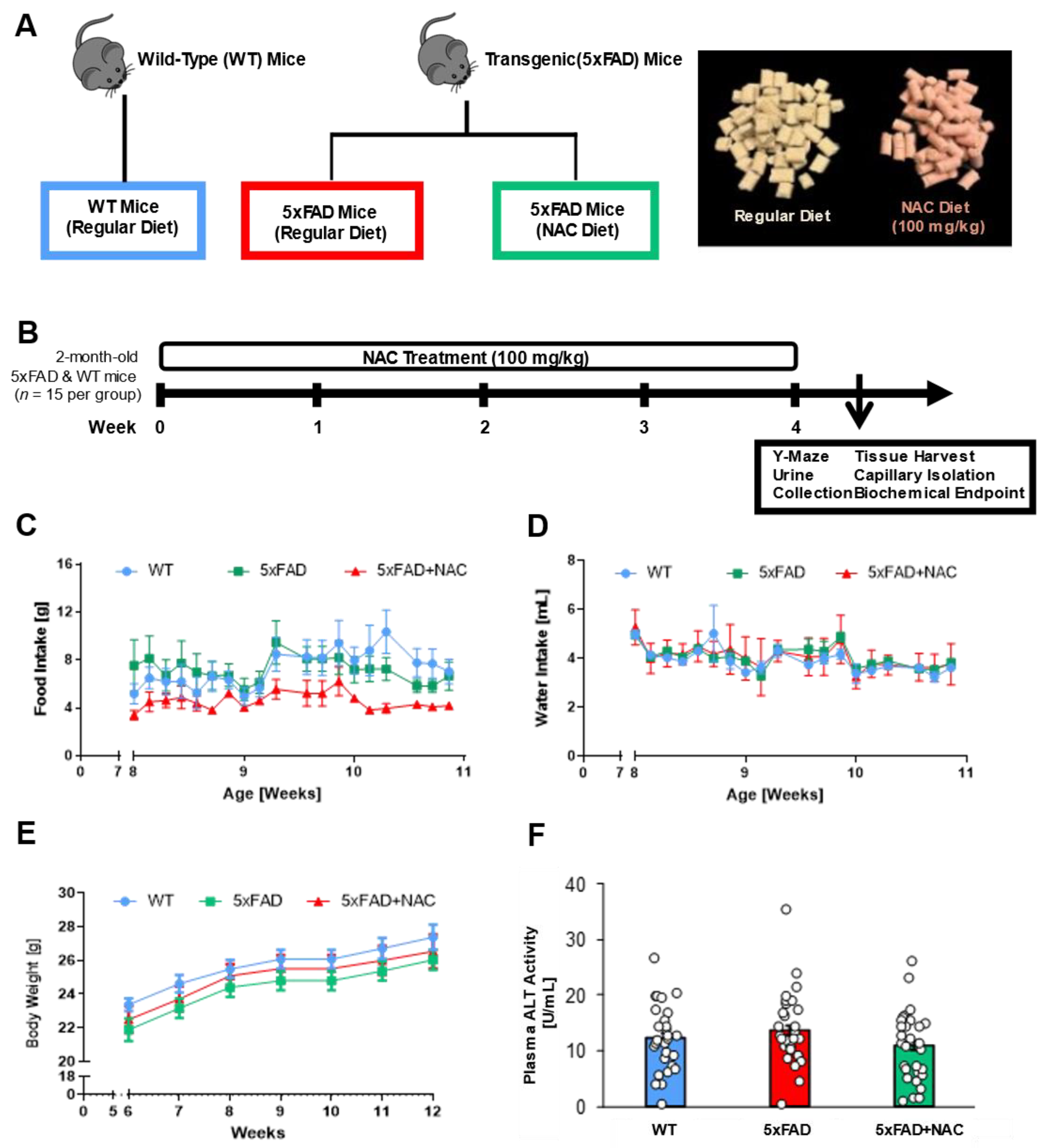
2.1. NAC Effect on Food Intake, Water Intake, Body Weight, and Plasma ALT Levels
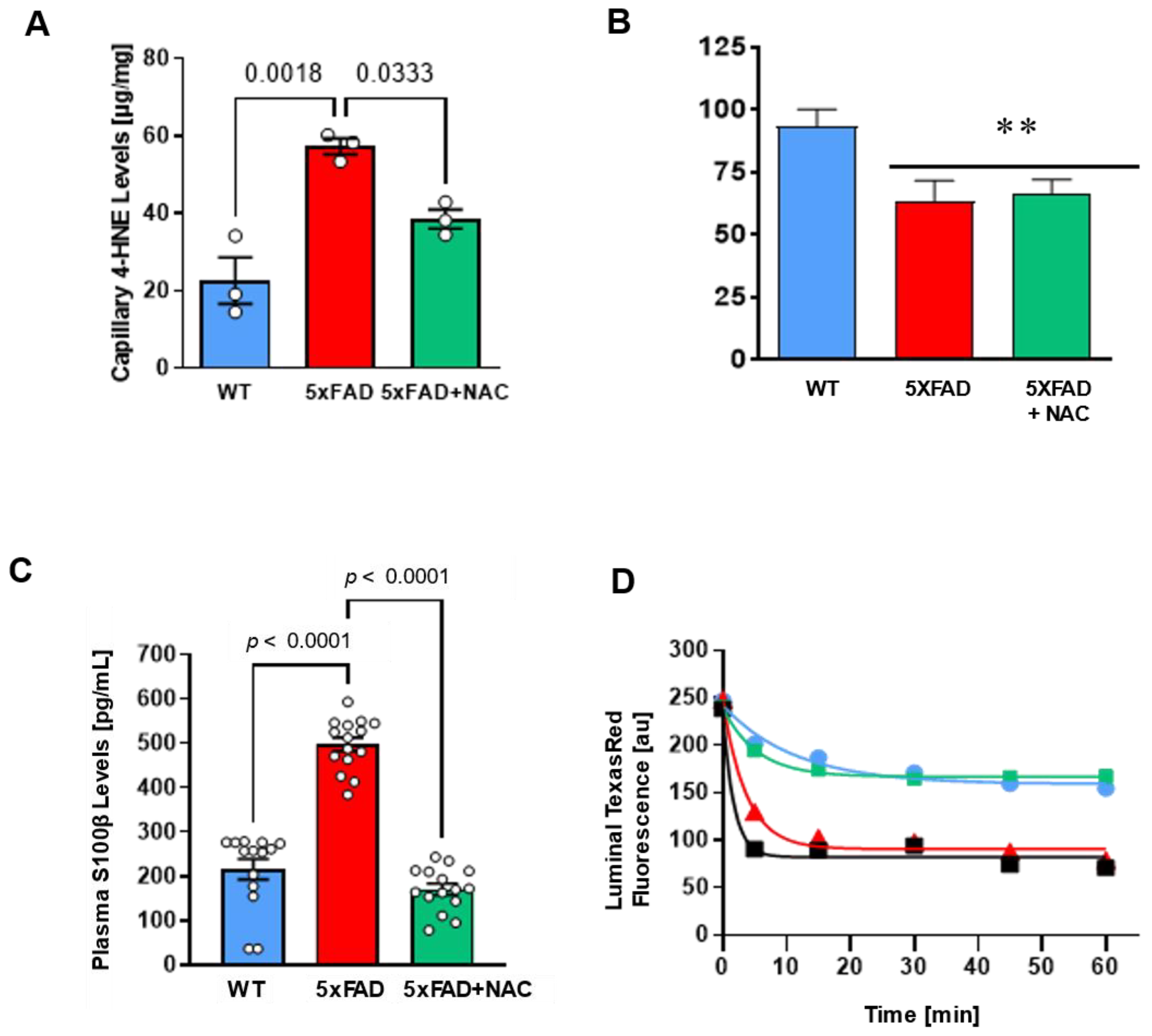
2.2. NAC Effect on Brain Lipid Peroxidation Levels
2.3. NAC Effect on Blood–Brain Barrier Function
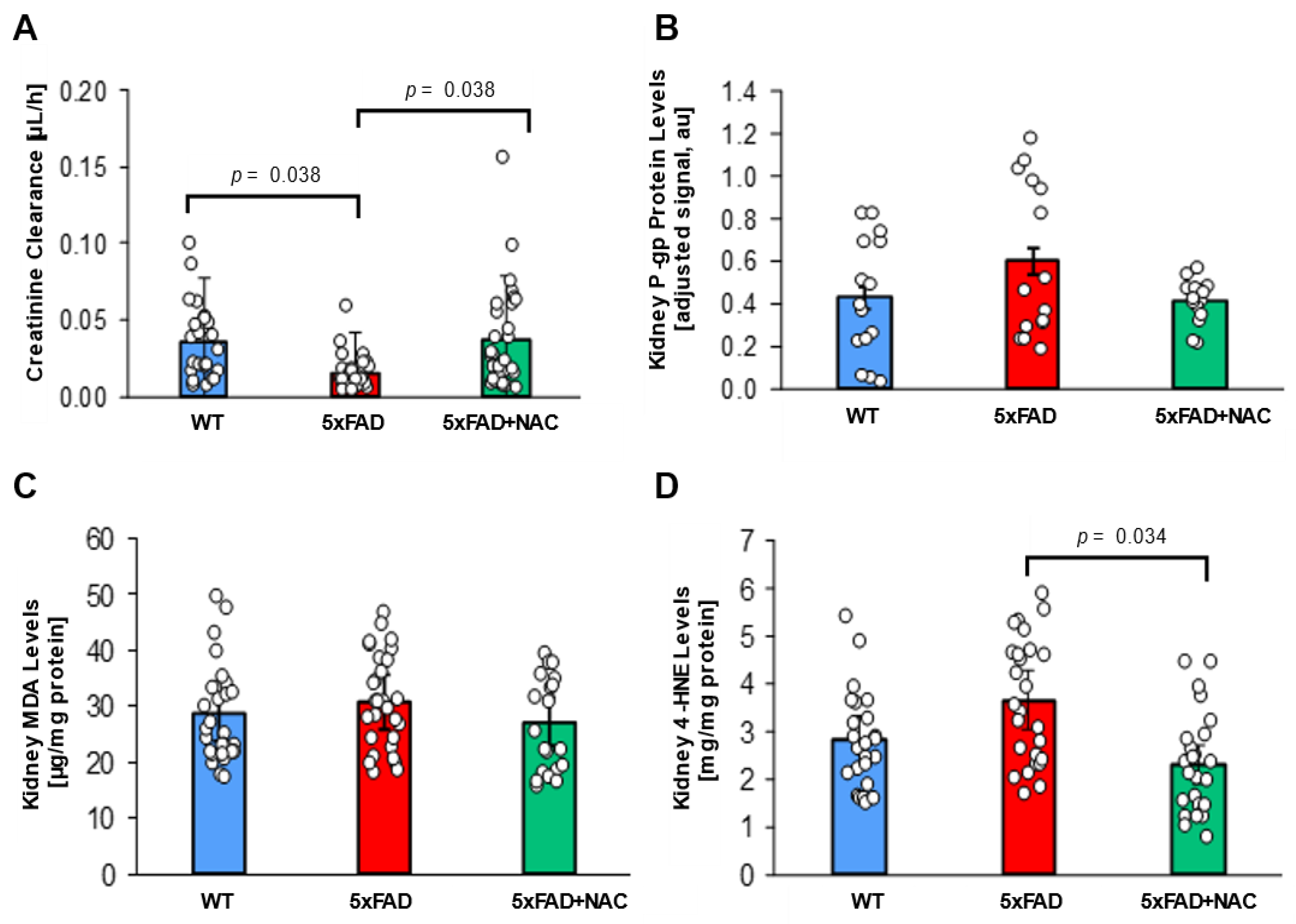
2.4. NAC Effect on Renal Function
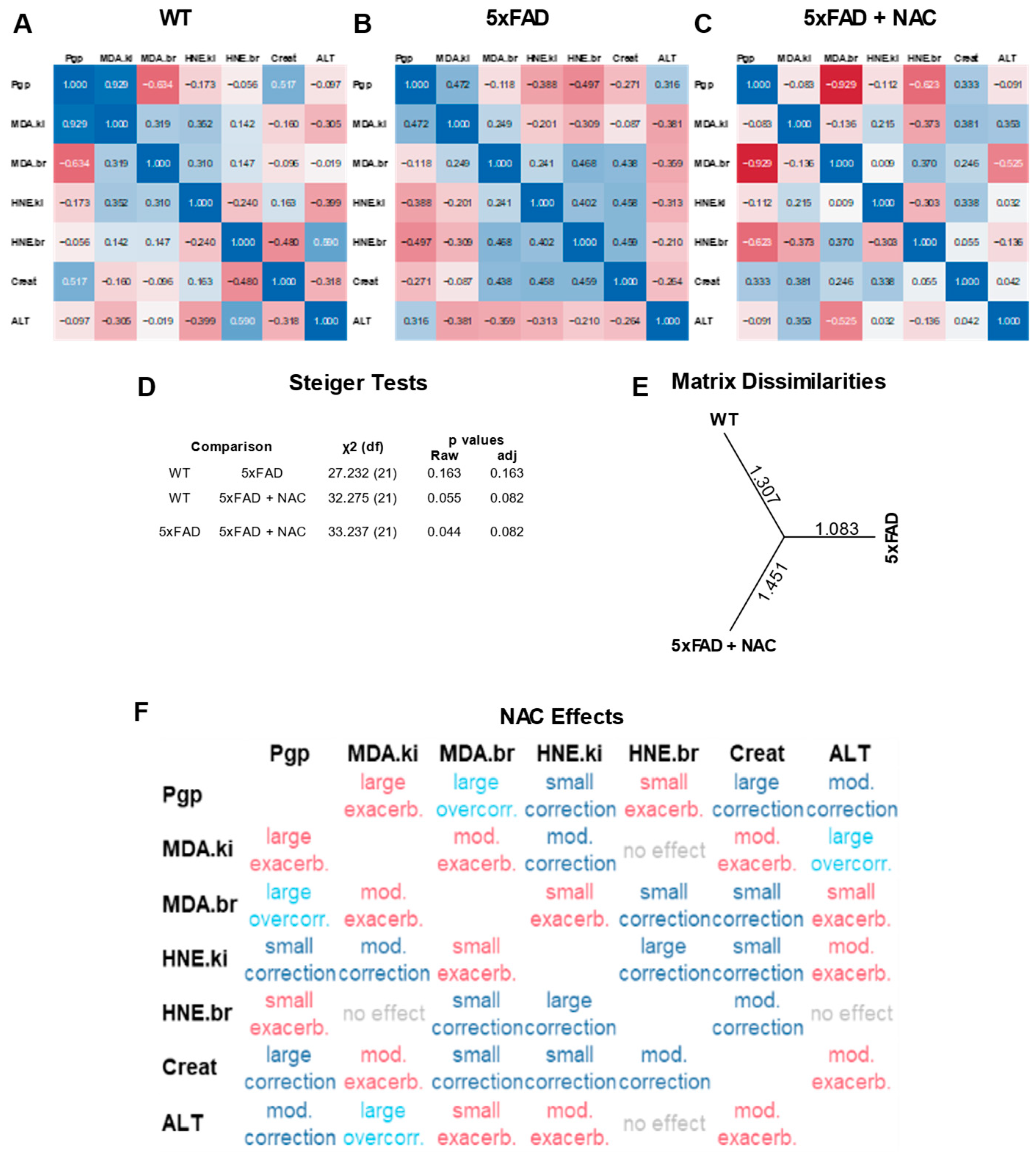
2.5. Multivariate Correlation Analysis of Protein Normalized Endpoints
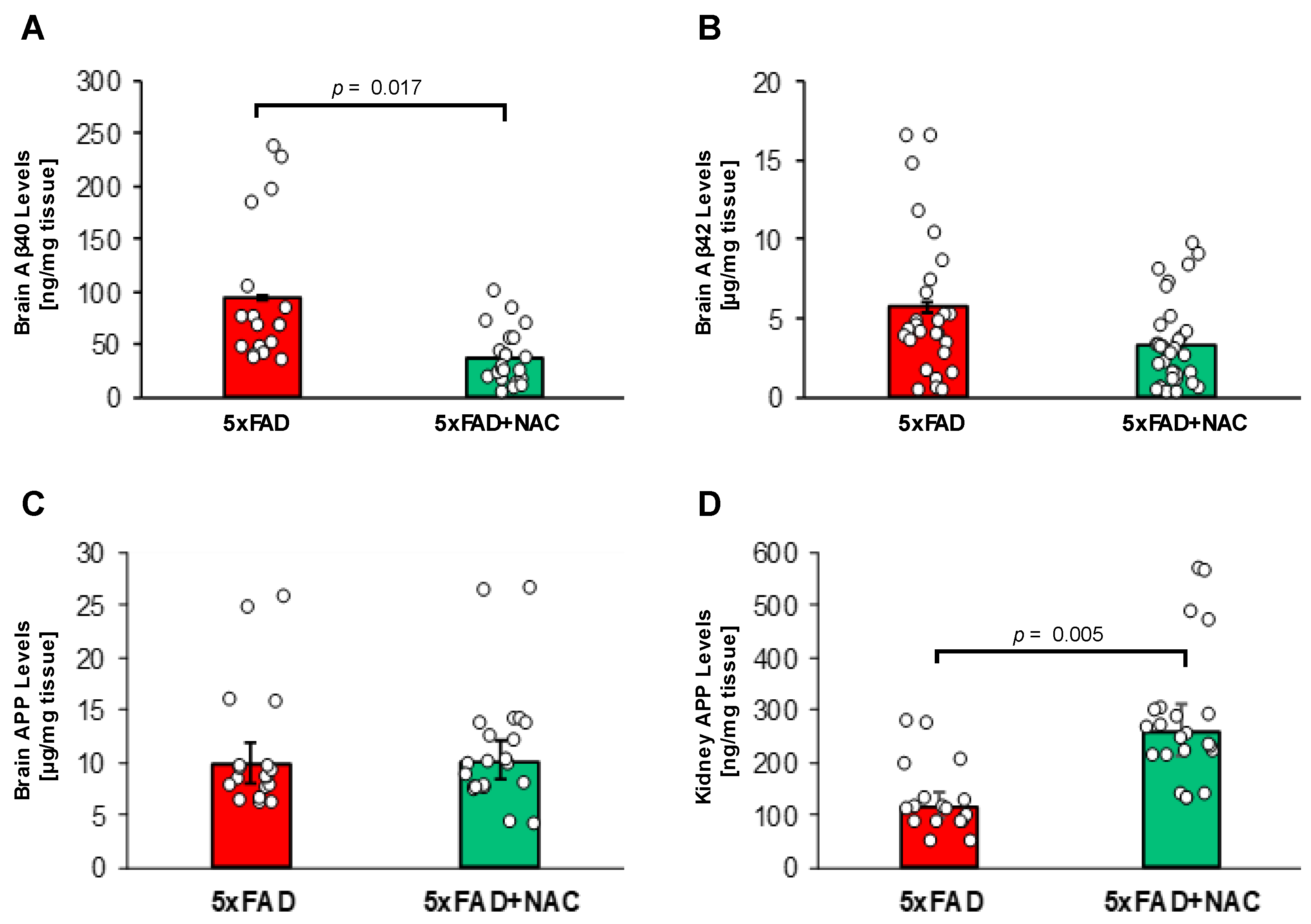
2.6. NAC Effect on Brain Aβ Levels
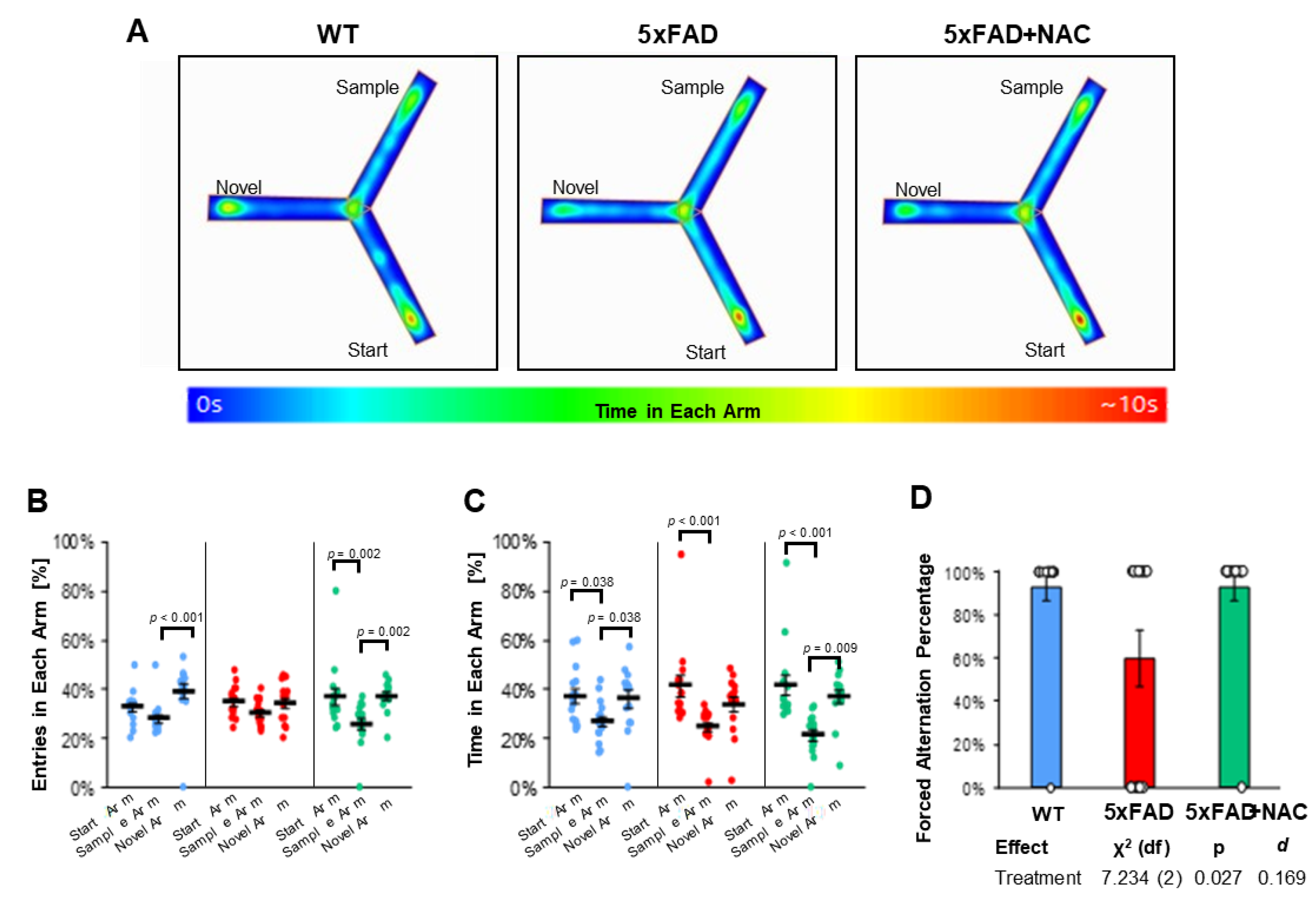
2.7. NAC Effect on Cognition
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. NAC Feeding Study
4.4. Y-Maze Test
4.5. Urine Collection
4.6. Blood and Tissue Collection
4.7. Brain Capillary Isolation
4.8. P-gp Transport Assay
4.9. Capillary Leakage Assay
4.10. Crude Kidney Membrane Isolation
4.11. Western Blotting
4.12. Aβ Extraction
4.13. Aβ40 and Aβ42 ELISA
4.14. S100β ELISA
4.15. Brain Homogenization for TBARs Assay and 4-HNE and APP ELISAs
4.16. TBARS Assay
4.17. APP ELISA
4.18. 4-HNE ELISA
4.19. ALT Assay
4.20. Creatinine Clearance
4.21. General Statistical Analysis
4.22. Multivariate Correlation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxynonenal |
| AD | Alzheimer’s disease |
| ALT | alanine transaminase |
| APP | amyloid precursor protein |
| Aβ | amyloid beta |
| ELISA | enzyme-linked immunosorbent assay |
| MDA | malonaldehyde |
| NAC | N-acetylcysteine |
| NBD-CSA | N-ε (4-nitrobenzofurazan-7-yl)-D-Lys8]-cyclosporin A |
| P-gp | P-glycoprotein |
| PSEN1 | presenilin 1 |
| TBARS | thiobarbituric acid reactive substances |
| WT | wild-type |
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef]
- Li, T.; Hu, W.; Han, Q.; Wang, Y.; Ma, Z.; Chu, J.; He, Q.; Feng, Z.; Sun, N.; Shenet, Y. Trajectories of quality of life and cognition in different multimorbidity patterns: Evidence from SHARE. Arch. Gerontol. Geriatr. 2024, 117, 105219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, H.; Zhang, M.; Li, J. Healthcare Utilization and Mortality After Hospice Live Discharge Among Medicare Patients With and Without Alzheimer’s Disease and Related Dementias. J. Gen. Intern. Med. 2023, 38, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.; Leeuwenburgh, C. Molecular mechanisms of oxidative stress in aging: Free radicals, aging, antioxidants and disease. In Handbook of Oxidants and Antioxidants in Exercise; Sen, C.K., Packer, L., HÌnninen, O., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1999; Volume 30, pp. 881–926. [Google Scholar]
- Pluta, R.; Amek, M.U. Brain ischemia and ischemic blood–brain barrier as etiological factors in sporadic Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zuo, L.; Wang, F.; Chen, Z.; Hu, Y.; Wang, Y.; Wang, X.; Zhang, W. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: A cross-sectional study. BMC Neurol. 2014, 14, 113. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar]
- Butterfield, D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Carrano, A.; Hoozemans, J.J.; van der Vies, S.M.; Rozemuller, A.J.; van Horssen, J.; de Vries, H.E. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid. Redox Signal 2011, 15, 1167–1178. [Google Scholar] [CrossRef]
- Carrano, A.; Hoozemans, J.J.; van der Vies, S.M.; van Horssen, J.; de Vries, H.E.; Rozemuller, A.J. Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neurodegener. Dis. 2012, 10, 329–331. [Google Scholar] [CrossRef]
- Zhao, R.; Han, X.; Jiang, S.; Zhao, W.; Liu, J.; Zhang, H.; Mao, X.; Zhang, M.; Lei, L.; You, H. Association of Dietary and Supplement Intake of Antioxidants with Risk of Dementia: A Meta-Analysis of Cohort Studies. J. Alzheimers Dis. 2024, 99, S35–S50. [Google Scholar] [CrossRef]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C.; Cache County Study Group. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Wilson, R.S.; Scherr, P.A. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 2002, 287, 3230–3237. [Google Scholar] [CrossRef]
- Zhao, R.; Han, X.; Zhang, H.; Liu, J.; Zhang, M.; Zhao, W.; Jiang, S.; Li, R.; Cai, H.; You, H. Association of vitamin E intake in diet and supplements with risk of dementia: A meta-analysis. Front. Aging Neurosci. 2022, 14, 955878. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.B.; Pavia, D.; Agnew, J.E.; Lopez-Vidriero, M.T.; Lauque, D.; Clarke, S.W. Effect of oral N-acetylcysteine on mucus clearance. Br. J. Dis. Chest 1985, 79, 262–266. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Hara, Y.; McKeehan, N.; Dacks, P.A.; Fillit, H.M. Evaluation of the Neuroprotective Potential of N-Acetylcysteine for Prevention and Treatment of Cognitive Aging and Dementia. J. Prev. Alzheimers Dis. 2017, 4, 201–206. [Google Scholar] [CrossRef]
- Remington, R.; Lortie, J.J.; Hoffmann, H.; Page, R.; Morrell, C.; Shea, T.B. A Nutritional Formulation for Cognitive Performance in Mild Cognitive Impairment: A Placebo-Controlled Trial with an Open-Label Extension. J. Alzheimers Dis. 2015, 48, 591–595. [Google Scholar] [CrossRef] [PubMed]
- NCT00597376. Study of the Effects of Cerefolin NAC on Inflammation Blood Markers in Older Individuals with Memory Complaints. Available online: https://clinicaltrials.gov/study/NCT00597376 (accessed on 22 January 2025).
- NCT01320527. A Clinical Trial of a Vitamin/Nutriceutical Formulation for Alzheimer’s Disease. Available online: https://clinicaltrials.gov/study/NCT01320527 (accessed on 22 January 2025).
- NCT01370954 NAC-003, P.L.U.S. Program (Progress Through Learning Understanding & Support). Available online: https://clinicaltrials.gov/study/NCT01370954 (accessed on 22 January 2025).
- NCT01745198. Effect of Cerefolin®/CerefolinNAC® on Biomarker Measurements. Available online: https://clinicaltrials.gov/study/NCT01745198 (accessed on 22 January 2025).
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Eldik, L.V.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS ONE 2010, 5, e12974. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef]
- Xiao, Q.; Yan, P.; Ma, X.; Liu, H.; Perez, R.; Zhu, A.; Gonzales, E.; Tripoli, D.L.; Czerniewski, L.; Ballabio, A.; et al. Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Aβ Generation and Amyloid Plaque Pathogenesis. J. Neurosci. 2015, 35, 12137–12151. [Google Scholar] [CrossRef]
- Cruz Hernández, J.C.; Bracko, O.; Kersbergen, C.J.; Muse, V.; Haft-Javaherian, M.; Berg, M.; Park, L.; Vinarcsik, L.K.; Ivasyk, I.; Rivera, D.A.; et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 2019, 22, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Rosa-Neto, P.; Hsiung, G.Y.; Sadovnick, A.D.; Masellis, M.; Black, S.E.; Jia, J.; Gauthier, S. Early-onset familial Alzheimer’s disease (EOFAD). Can. J. Neurol. Sci. 2012, 39, 436–445. [Google Scholar] [CrossRef]
- Giannoni, P.; Arango-Lievano, M.; Neves, I.D.; Rousset, M.C.; Baranger, K.; Rivera, S.; Jeanneteau, F.; Claeysen, S.; Marchi, N. Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol. Dis. 2016, 88, 107–117. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef]
- Xiang, Y.; Bu, X.L.; Liu, Y.H.; Zhu, C.; Shen, L.L.; Jiao, S.S.; Zhu, X.Y.; Giunta, B.; Tan, J.; Songet, W.H.; et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. 2015, 130, 487–499. [Google Scholar] [CrossRef]
- Tian, D.Y.; Cheng, Y.; Zhuang, Z.Q.; He, C.Y.; Pan, Q.G.; Tang, M.Z.; Hu, X.L.; Shen, Y.Y.; Wang, Y.-R.; Chenet, S.-H.; et al. Physiological clearance of amyloid-beta by the kidney and its therapeutic potential for Alzheimer’s disease. Mol. Psychiatry 2021, 26, 6074–6082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Xiang, Y.; Wang, Y.R.; Jiao, S.S.; Wang, Q.H.; Bu, X.L.; Zhu, C.; Yao, X.Q.; Giunta, B.; Tan, J.; et al. Association Between Serum Amyloid-Beta and Renal Functions: Implications for Roles of Kidney in Amyloid-Beta Clearance. Mol. Neurobiol. 2015, 52, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Wu, S.; Masson, P.; Kelly, P.J.; Duthie, F.A.; Whiteley, W.; Parker, D.; Gillespie, D.; Webster, A.C. Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med. 2016, 14, 206. [Google Scholar] [CrossRef]
- Vroon, D.H.; Israili, Z. Aminotransferases. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; H Walker, K., Hall, W.D., Hurst, W., Eds.; Butterworth Publishers, a division of Reed Publishing: Oxford, UK, 1990; Chapter 99. [Google Scholar]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase-old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925–935. [Google Scholar] [CrossRef]
- Rejc, L.; Gómez-Vallejo, V.; Joya, A.; Arsequell, G.; Egimendia, A.; Castellnou, P.; Ríos-Anglada, X.; Cossío, U.; Baz, Z.; Iglesias, L.; et al. Longitudinal evaluation of neuroinflammation and oxidative stress in a mouse model of Alzheimer disease using positron emission tomography. Alzheimers Res. Ther. 2022, 14, 80. [Google Scholar] [CrossRef]
- Wendt, S.; Johnson, S.; Weilinger, N.L.; Groten, C.; Sorrentino, S.; Frew, J.; Yang, L.; Choi, H.B.; Nygaard, H.B.; MacVicar, B.A. Simultaneous imaging of redox states in dystrophic neurites and microglia at Aβ plaques indicate lysosome accumulation not microglia correlate with increased oxidative stress. Redox Biol. 2022, 56, 102448. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Kim, J.; Huang, Z.; Goo, N.; Bae, H.J.; Jeong, Y.; Park, H.J.; Park, H.J.; Cai, M.; Cho, K.; Jung, S.Y.; et al. Theracurmin Ameliorates Cognitive Dysfunctions in 5XFAD Mice by Improving Synaptic Function and Mitigating Oxidative Stress. Biomol. Ther. 2019, 27, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, D.K.; Suh, Y.H.; Chang, K.A. Long-Term Treatment of Cuban Policosanol Attenuates Abnormal Oxidative Stress and Inflammatory Response via Amyloid Plaques Reduction in 5xFAD Mice. Antioxidants 2021, 10, 1321. [Google Scholar] [CrossRef]
- Shin, S.W.; Kim, D.H.; Jeon, W.K.; Han, J.S. 4-Hydroxynonenal Immunoreactivity Is Increased in the Frontal Cortex of 5XFAD Transgenic Mice. Biomedicines 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Gao, Y.; Zhuang, X.; Lin, Y.; He, W.; Wang, Y.; Chen, W.; Chen, T.; Huang, X.; Yang, R.; et al. Bazhu Decoction, a Traditional Chinese Medical Formula, Ameliorates Cognitive Deficits in the 5xFAD Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1391. [Google Scholar] [CrossRef]
- Tang, J.J.; Huang, L.F.; Deng, J.L.; Wang, Y.M.; Guo, C.; Peng, X.N.; Liu, Z.; Gao, J.M. Cognitive enhancement and neuroprotective effects of OABL, a sesquiterpene lactone in 5xFAD Alzheimer’s disease mice model. Redox Biol. 2022, 50, 102229. [Google Scholar] [CrossRef] [PubMed]
- Wong-Ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Tieleman, D.P.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef]
- Lehner, C.; Gehwolf, R.; Tempfer, H.; Krizbai, I.; Hennig, B.; Bauer, H.C.; Bauer, H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid. Redox Signal 2011, 15, 1305–1323. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, H.R.; Park, J.H.; Hoe, H.S. Idebenone Decreases Aβ Pathology by Modulating RAGE/Caspase-3 Signaling and the Aβ Degradation Enzyme NEP in a Mouse Model of AD. Biology 2021, 10, 938. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; Bharate, S.S.; Kumar, V.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B.; Kaddoumi, A. Crocus sativus Extract Tightens the Blood-Brain Barrier, Reduces Amyloid β Load and Related Toxicity in 5XFAD Mice. ACS Chem. Neurosci. 2017, 8, 1756–1766. [Google Scholar] [CrossRef]
- Rabe, M.; Schaefer, F. Non-Transgenic Mouse Models of Kidney Disease. Nephron 2016, 133, 53–61. [Google Scholar] [CrossRef]
- Sears, S.M.; Sharp, C.N.; Krueger, A.; Oropilla, G.B.; Saforo, D.; Doll, M.A.; Megyesi, J.; Beverly, L.J.; Siskind, L.J. C57BL/6 mice require a higher dose of cisplatin to induce renal fibrosis and CCL2 correlates with cisplatin-induced kidney injury. Am. J. Physiol. Renal Physiol. 2020, 319, F674–F685. [Google Scholar] [CrossRef]
- Matsuki, H.; Mandai, S.; Shiwaku, H.; Koide, T.; Takahashi, N.; Yanagi, T.; Inaba, S.; Ida, S.; Fujiki, T.; Mori, Y.; et al. Chronic kidney disease causes blood-brain barrier breakdown via urea-activated matrix metalloproteinase-2 and insolubility of tau protein. Aging 2023, 15, 10972–10995. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hasegawa, Y.; Uekawa, K.; Kim-Mitsuyama, S. Chronic kidney disease accelerates cognitive impairment in a mouse model of Alzheimer’s disease, through angiotensin II. Exp. Gerontol. 2017, 87, 108–112. [Google Scholar] [CrossRef]
- Manna, J.; Dunbar, G.L.; Maiti, P. Curcugreen Treatment Prevented Splenomegaly and Other Peripheral Organ Abnormalities in 3xTg and 5xFAD Mouse Models of Alzheimer’s Disease. Antioxidants 2021, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Lucas, J.A.; Younkin, L.H.; Younkin, S.G.; Graff-Radford, N.R. Serum creatinine levels correlate with plasma amyloid Beta protein. Alzheimer Dis. Assoc. Disord. 2002, 16, 187–190. [Google Scholar] [CrossRef]
- Vinothkumar, G.; Kedharnath, C.; Krishnakumar, S.; Sreedhar, S.; Preethikrishnan, K.; Dinesh, S.; Sundaram, A.; Balakrishnan, D.; Shivashekar, G.; Sureshkumar; et al. Abnormal amyloid β. BBA Clin. 2017, 8, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bobot, M.; Guedj, E.; Resseguier, N.; Faraut, J.; Garrigue, P.; Nail, V.; Hache, G.; Gonzalez, S.; McKay, N.; Vial, R.; et al. Increased Blood-Brain Barrier Permeability and Cognitive Impairment in Patients With ESKD. Kidney Int. Rep. 2024, 9, 2988–2995. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Zhou, J.; Coles, L.D.; Kartha, R.V.; Mishra, U.; Lund, T.C.; Cloyd, J.C. Intravenous administration of stable-labeled N-acetylcysteine demonstrates an indirect mechanism for boosting glutathione and improving redox status. Pharmacokin Pharmacod Drug Transport Metab. 2015, 104, 2619–2626. [Google Scholar] [CrossRef]
- Borgström, L.; Kågedal, B.; Paulsen, O. Pharmacokinetics of N-acetylcysteine in man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar] [CrossRef]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail? Oxid. Med. Cell Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef]
- Gualtieri, P.; Frank, G.; Cianci, R.; Ciancarella, L.; Romano, L.; Ortoman, M.; Bigioni, G.; Nicoletti, F.; Falco, M.I.; La Placa, G.; et al. Exploring the Efficacy and Safety of Nutritional Supplements in Alzheimer’s Disease. Nutrients 2025, 17, 922. [Google Scholar] [CrossRef] [PubMed]
- Bowers, Z.; Maiti, P.; Bourcier, A.; Morse, J.; Jenrow, K.; Rossignol, J.; Dunbar, G.L. Tart Cherry Extract and Omega Fatty Acids Reduce Behavioral Deficits, Gliosis, and Amyloid-Beta Deposition in the 5xFAD Mouse Model of Alzheimer’s Disease. Brain Sci. 2021, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.J.; Kim, S.; Kim, J.J.; Jang, G.Y.; Moon, M.; Kim, H.D. Crude Saponin from Platycodon grandiflorum Attenuates Aβ-Induced Neurotoxicity via Antioxidant, Anti-Inflammatory and Anti-Apoptotic Signaling Pathways. Antioxidants 2021, 10, 1968. [Google Scholar] [CrossRef]
- Cho, E.; Youn, K.; Kwon, H.; Jeon, J.; Cho, W.S.; Park, S.J.; Son, S.H.; Jang, D.S.; Shin, C.Y.; Moon, M.; et al. Eugenitol ameliorates memory impairments in 5XFAD mice by reducing Aβ plaques and neuroinflammation. Biomed. Pharmacother. 2022, 148, 112763. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Miao, Z.; Jiang, X.; Zheng, Y.; Yang, G. Quercitrin improved cognitive impairment through inhibiting inflammation induced by microglia in Alzheimer’s disease mice. Neuroreport 2022, 33, 327–335. [Google Scholar] [CrossRef]
- Whitmore, C.A.; Haynes, J.R.; Behof, W.J.; Rosenberg, A.J.; Tantawy, M.N.; Hachey, B.C.; Wadzinski, B.E.; Spiller, B.W.; Peterson, T.E.; Paffenroth, K.C.; et al. Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2022, 15, 742. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, N.; Jeon, S.H.; Gee, M.S.; Ju, Y.J.; Jung, M.J.; Cho, J.S.; Lee, Y.; Lee, S.; Kil Lee, J. Hesperidin Improves Memory Function by Enhancing Neurogenesis in a Mouse Model of Alzheimer’s Disease. Nutrients 2022, 14, 3125. [Google Scholar] [CrossRef]
- Wijesinghe, P.; Whitmore, C.A.; Campbell, M.; Li, C.; Tsuyuki, M.; To, E.; Haynes, J.; Pham, W.; Matsubara, J.A. Ergothioneine, a dietary antioxidant improves amyloid beta clearance in the neuroretina of a mouse model of Alzheimer’s disease. Front. Neurosci. 2023, 17, 1107436. [Google Scholar] [CrossRef]
- Upadhyay, P.; Gupta, S. Dual mode of Triphala in the reversal of cognition through gut restoration in antibiotic mediated prolonged dysbiosis condition in 5XFAD mice. Exp. Neurol. 2023, 367, 114473. [Google Scholar] [CrossRef]
- Lymperopoulos, D.; Dedemadi, A.G.; Voulgari, M.L.; Georgiou, E.; Dafnis, I.; Mountaki, C.; Panagopoulou, E.A.; Karvelas, M.; Chiou, A.; Karathanos, V.T.; et al. Corinthian Currants Promote the Expression of Paraoxonase-1 and Enhance the Antioxidant Status in Serum and Brain of 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules 2024, 14, 426. [Google Scholar] [CrossRef]
- Nam, Y.; Ji, Y.J.; Shin, S.J.; Park, H.H.; Yeon, S.H.; Kim, S.Y.; Son, R.H.; Jang, G.Y.; Kim, H.D.; Moon, M. Platycodon grandiflorum root extract inhibits Aβ deposition by breaking the vicious circle linking oxidative stress and neuroinflammation in Alzheimer’s disease. Biomed. Pharmacother. 2024, 177, 117090. [Google Scholar] [CrossRef] [PubMed]
- Gladen-Kolarsky, N.; Monestime, O.; Bollen, M.; Choi, J.; Yang, L.; Magaña, A.A.; Maier, C.S.; Soumyanath, A.; Gray, N.E. Withania somnifera (Ashwagandha) Improves Spatial Memory, Anxiety and Depressive-like Behavior in the 5xFAD Mouse Model of Alzheimer’s Disease. Antioxidants 2024, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Varada, S.; Chamberlin, S.R.; Bui, L.; Brandes, M.S.; Gladen-Kolarsky, N.; Harris, C.J.; Hack, W.; Neff, C.J.; Brumbach, B.H.; Soumyanath, A.; et al. Oral Asiatic Acid Improves Cognitive Function and Modulates Antioxidant and Mitochondrial Pathways in Female 5xFAD Mice. Nutrients 2025, 17, 729. [Google Scholar] [CrossRef]
- Schramm, U.; Fricker, G.; Wenger, R.; Miller, D.S. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am. J. Physiol. 1995, 268, F46–F52. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Poon, H.F.; Dogrukol-Ak, D.; Drake, J.; Banks, W.A.; Eyerman, E.; Butterfield, D.A.; Morley, J.E. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef]
- Hartz, A.M.S.; Zhong, Y.; Shen, A.N.; Abner, E.L.; Bauer, B. Preventing P-gp Ubiquitination Lowers Aβ Brain Levels in an Alzheimer’s Disease Mouse Model. Front. Aging Neurosci. 2018, 10, 186. [Google Scholar] [CrossRef]
- Wolf, A.; Bauer, B.; Abner, E.L.; Ashkenazy-Frolinger, T.; Hartz, A.M. A Comprehensive Behavioral Test Battery to Assess Learning and Memory in 129S6/Tg2576 Mice. PLoS ONE 2016, 11, e0147733. [Google Scholar] [CrossRef]
- Hartz, A.M.; Zhong, Y.; Wolf, A.; LeVine, H.; Miller, D.S.; Bauer, B. Aβ40 Reduces P-Glycoprotein at the Blood-Brain Barrier through the Ubiquitin-Proteasome Pathway. J. Neurosci. 2016, 36, 1930–1941. [Google Scholar] [CrossRef]
- Hartz, A.M.; Bauer, B.; Fricker, G.; Miller, D.S. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol. Pharmacol. 2004, 66, 387–394. [Google Scholar] [CrossRef]
- Bauer, B.; Hartz, A.M.; Lucking, J.R.; Yang, X.; Pollack, G.M.; Miller, D.S. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2008, 28, 1222–1234. [Google Scholar] [CrossRef]
- Hartz, A.M.; Bauer, B.; Soldner, E.L.; Wolf, A.; Boy, S.; Backhaus, R.; Mihaljevic, I.; Bogdahn, U.; Klünemann, H.H.; Schuierer, G.; et al. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 2012, 43, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow. Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lenth, R.V.; Banfai, B.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; et al. emmeans: Estimated Marginal Means, aka Least-Squares Means 2022. Available online: https://cran.r-project.org/web/packages/emmeans/ (accessed on 22 January 2025).
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Model Adequacy Checking. In Introduction to Linear Regression Analysis, 6th ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 134–176. [Google Scholar]
- Steiger, J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980, 87, 245–251. [Google Scholar] [CrossRef]


| Group | k (min−1) |
|---|---|
| WT | 0.08 ± 0.01 |
| 5xFAD | 0.26 ± 0.03 |
| 5xFAD + NAC | 0.18 ± 0.03 |
| 100 mM Mannitol | 0.58 ± 0.12 |
| Category | Score |
|---|---|
| Large Exacerbation | −3.0 |
| Moderate Exacerbation | −2.0 |
| Small Exacerbation | −1.0 |
| No Effect | 0.0 |
| Small Correction | 1.0 |
| Moderate Correction | 2.0 |
| Large Correction | 3.0 |
| Small Overcorrection | 0.5 |
| Moderate Overcorrection | 1.0 |
| Large Overcorrection | 1.5 |
| Effect | χ2 (df) | p | R2 |
|---|---|---|---|
| Treatment | 0.236 (2) | 0.888 | 0.030 |
| Arm | 27.488 (2) | <0.001 | 0.195 |
| Treatment × Arm | 11.552 (4) | 0.021 | 0.031 |
| Omnibus | 40.212 (8) | <0.001 | 0.195 |
| Effect | χ2 (df) | p | R2 |
|---|---|---|---|
| Treatment | 0.252 (2) | 0.881 | 0.015 |
| Arm | 7.001 (2) | 0.030 | 0.259 |
| Treatment × Arm | 4.464 (4) | 0.347 | 0.014 |
| Omnibus | 38.777 (8) | <0.001 | 0.259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ontawong, A.; Nehra, G.; Maloney, B.J.; Vaddhanaphuti, C.S.; Bauer, B.; Hartz, A.M.S. N-Acetylcysteine Attenuates Aβ-Mediated Oxidative Stress, Blood–Brain Barrier Leakage, and Renal Dysfunction in 5xFAD Mice. Int. J. Mol. Sci. 2025, 26, 4352. https://doi.org/10.3390/ijms26094352
Ontawong A, Nehra G, Maloney BJ, Vaddhanaphuti CS, Bauer B, Hartz AMS. N-Acetylcysteine Attenuates Aβ-Mediated Oxidative Stress, Blood–Brain Barrier Leakage, and Renal Dysfunction in 5xFAD Mice. International Journal of Molecular Sciences. 2025; 26(9):4352. https://doi.org/10.3390/ijms26094352
Chicago/Turabian StyleOntawong, Atcharaporn, Geetika Nehra, Bryan J. Maloney, Chutima S. Vaddhanaphuti, Björn Bauer, and Anika M. S. Hartz. 2025. "N-Acetylcysteine Attenuates Aβ-Mediated Oxidative Stress, Blood–Brain Barrier Leakage, and Renal Dysfunction in 5xFAD Mice" International Journal of Molecular Sciences 26, no. 9: 4352. https://doi.org/10.3390/ijms26094352
APA StyleOntawong, A., Nehra, G., Maloney, B. J., Vaddhanaphuti, C. S., Bauer, B., & Hartz, A. M. S. (2025). N-Acetylcysteine Attenuates Aβ-Mediated Oxidative Stress, Blood–Brain Barrier Leakage, and Renal Dysfunction in 5xFAD Mice. International Journal of Molecular Sciences, 26(9), 4352. https://doi.org/10.3390/ijms26094352






