Respiratory Function Improvement and Lifespan Extension Following Immunotherapy with NP001 Support the Concept That Amyotrophic Lateral Sclerosis (ALS) Is an Immuno-Neurologic Disease
Abstract
1. Introduction
2. Results
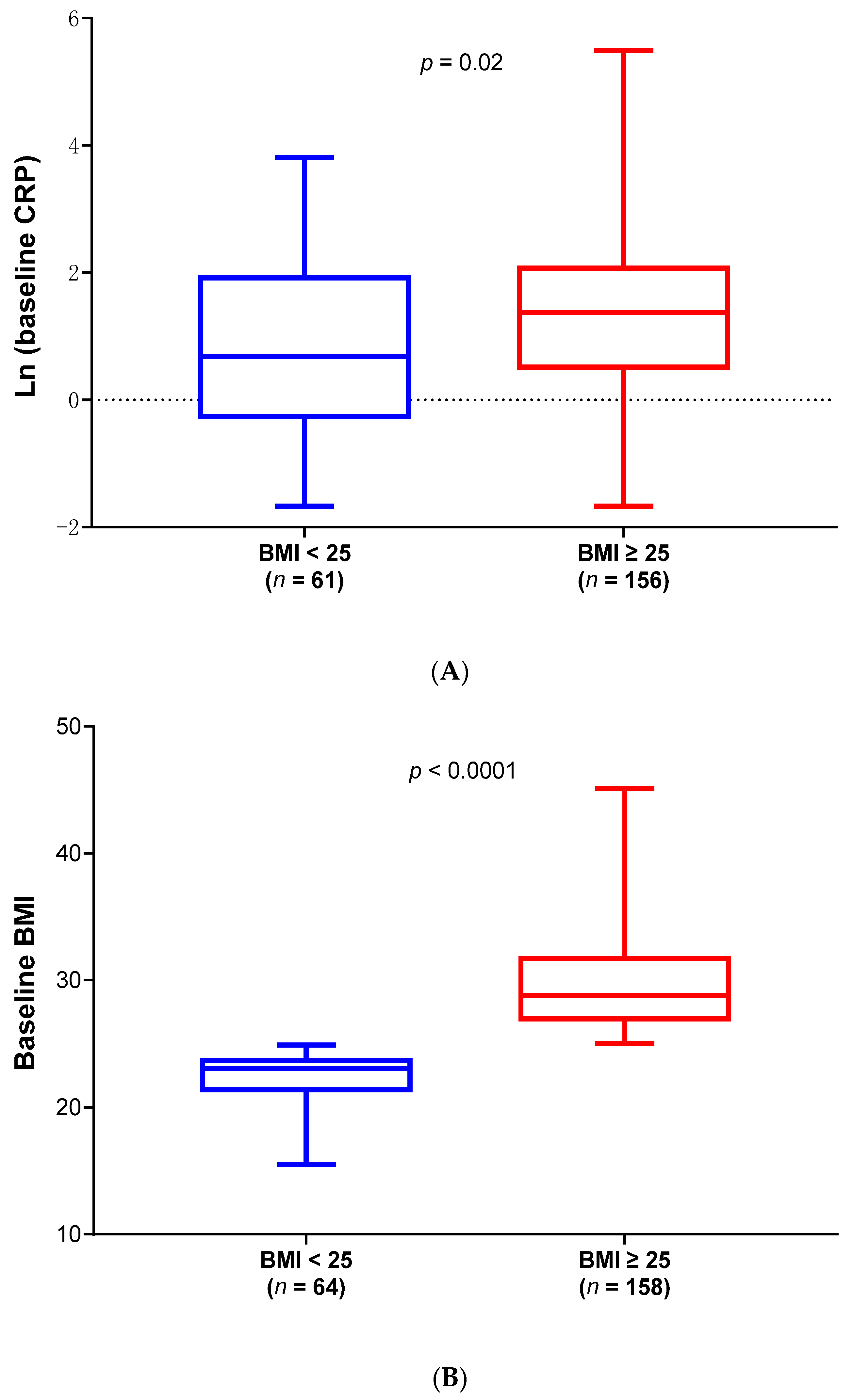
2.1. Differences of Demographics and Clinical Characteristics Between Low and High BMI ALS Patients in Phase 2A and 2B Modified Intend-to-Treat (mITT) Population
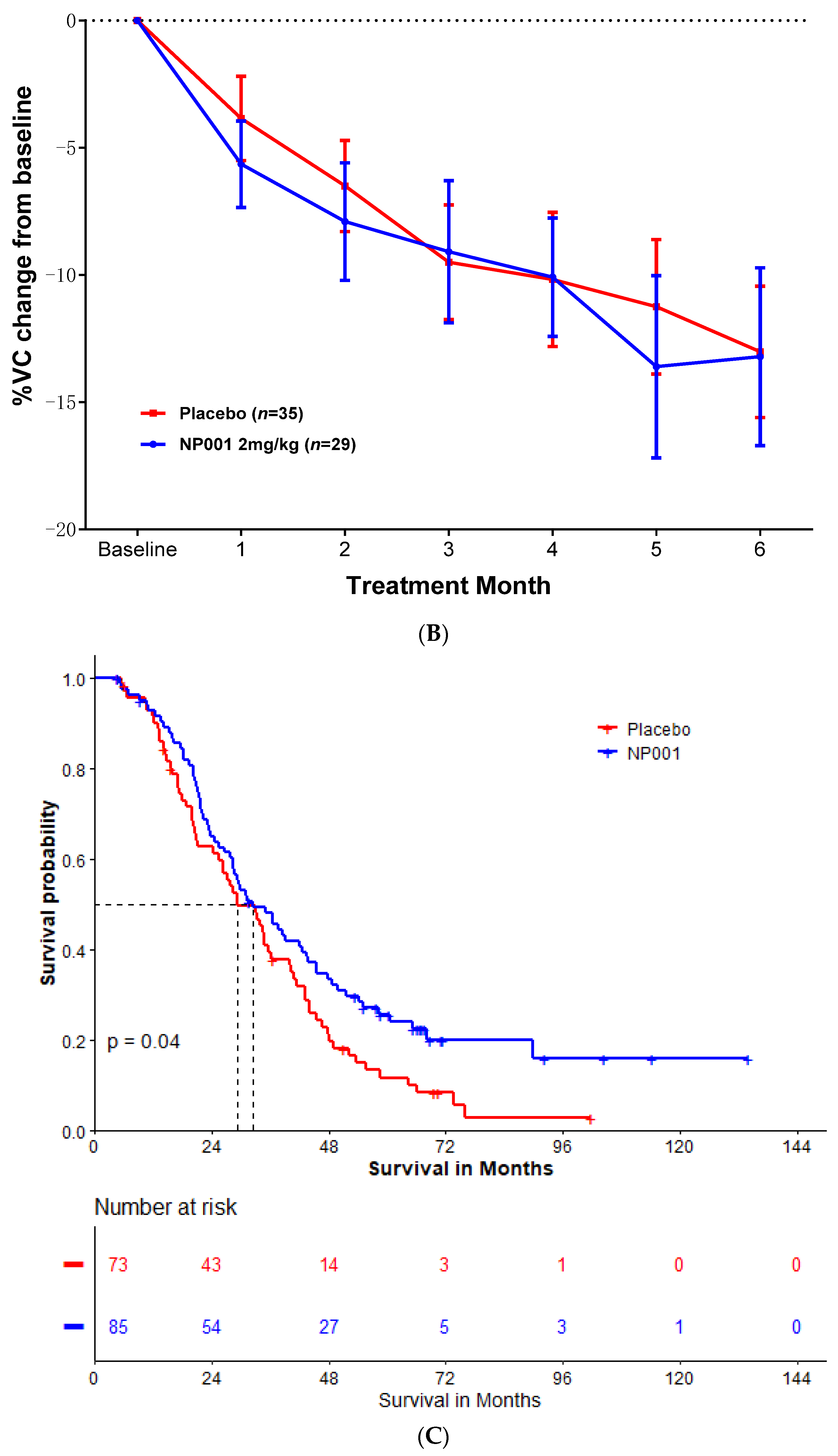
2.2. Effects of NP001 on VC and OS Are Significant and Beneficial in ALS Patients with BMI ≥ 25, but Not in Patients with BMI < 25 with Similar Demographics
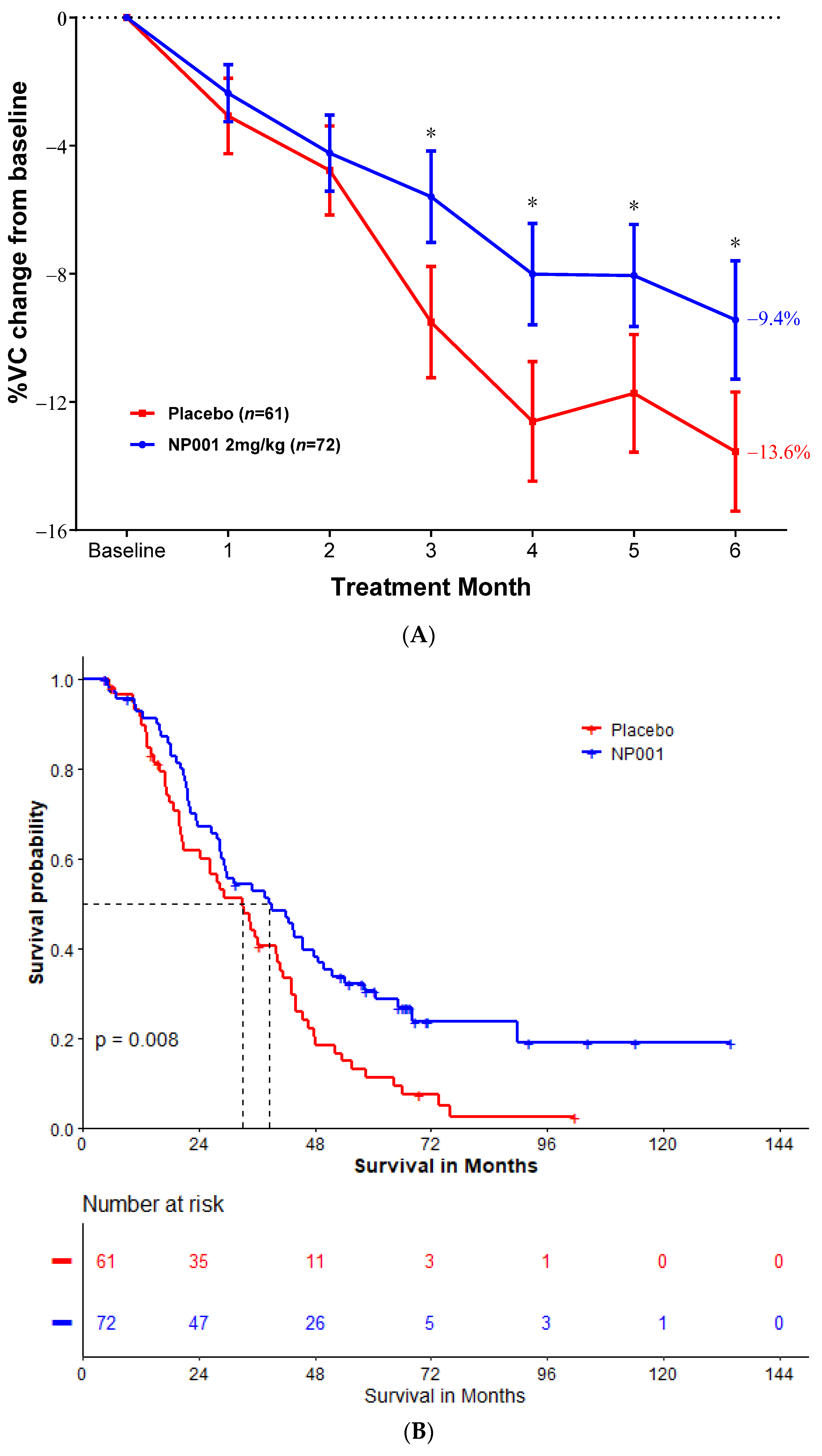
2.3. OS and Percent of VC Change in mITT Population with High BMI and Age ≤ 65 Years Old at Baseline
3. Discussion
4. Materials and Methods
4.1. Clinical Trials Overview
4.2. Description of ALS Phase 2A and 2B Trials and Participants
4.3. Analysis of Clinical Outcome Data
4.4. Phase 2A and 2B Survival Analyses
4.5. Statistical Analyses
5. Conclusions
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goyal, N.A.; Berry, J.D.; Windebank, A.; Staff, N.P.; Maragakis, N.J.; van den Berg, L.H.; Genge, A.; Miller, R.; Baloh, R.H.; Kern, R.; et al. Addressing heterogeneity in amyotrophic lateral sclerosis CLINICAL TRIALS. Muscle Nerve 2020, 62, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Block, G.; Katz, J.S.; Barohn, R.J.; Gopalakrishnan, V.; Cudkowicz, M.; Zhang, J.R.; McGrath, M.S.; Ludington, E.; Appel, S.H.; et al. Randomized phase 2 trial of NP001-a novel immune regulator: Safety and early efficacy in ALS. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e100. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Zhang, R.; Bracci, P.M.; Azhir, A.; Barohn, R.; Bedlack, R.; Benatar, M.; Berry, J.D.; Cudkowicz, M.; Kasarskis, E.J.; et al. Phase 2B randomized controlled trial of NP001 in amyotrophic lateral sclerosis: Pre-specified and post hoc analyses. Muscle Nerve 2022, 66, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- McGrath, M.S.; Zhang, R.; Bracci, P.M.; Azhir, A.; Forrest, B.D. Systemic Innate Immune System Restoration as a Therapeutic Approach for Neurodegenerative Disease: Effects of NP001 on Amyotrophic Lateral Sclerosis (ALS) Progression. Biomedicines 2024, 12, 2362. [Google Scholar] [CrossRef]
- Grassano, M.; Manera, U.; De Marchi, F.; Cugnasco, P.; Matteoni, E.; Daviddi, M.; Solero, L.; Bombaci, A.; Palumbo, F.; Vasta, R.; et al. The role of peripheral immunity in ALS: A population-based study. Ann. Clin. Transl. Neurol. 2023, 10, 1623–1632. [Google Scholar] [CrossRef]
- Cao, W.; Cao, Z.; Tian, Y.; Zhang, L.; Wang, W.; Tang, L.; Xu, C.; Fan, D. Neutrophils Are Associated with Higher Risk of Incident Amyotrophic Lateral Sclerosis in a BMI- and Age-Dependent Manner. Ann. Neurol. 2023, 94, 942–954. [Google Scholar] [CrossRef]
- Giese, T.; McGrath, M.S.; Stumm, S.; Schempp, H.; Elstner, E.; Meuer, S.C. Differential effects on innate versus adaptive immune responses by WF10. Cell. Immunol. 2004, 229, 149–158. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.H.; Jang, J.H.; Kim, C.; Kim, K.; Suh, Y.G.; Joe, Y.; Chung, H.T.; Cha, Y.N.; Surh, Y.J. Role of heme oxygenase-1 in potentiation of phagocytic activity of macrophages by taurine chloramine: Implications for the resolution of zymosan A-induced murine peritonitis. Cell. Immunol. 2018, 327, 36–46. [Google Scholar] [CrossRef]
- Miller, R.G.; Zhang, R.; Block, G.; Katz, J.; Barohn, R.; Kasarskis, E.; Forshew, D.; Gopalakrishnan, V.; McGrath, M.S. NP001 regulation of macrophage activation markers in ALS: A phase I clinical and biomarker study. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 601–609. [Google Scholar] [CrossRef]
- Forrest, B.D.; Goyal, N.A.; Fleming, T.R.; Bracci, P.M.; Brett, N.R.; Khan, Z.; Robinson, M.; Azhir, A.; McGrath, M. The Effectiveness of NP001 on the Long-Term Survival of Patients with Amyotrophic Lateral Sclerosis. Biomedicines 2024, 12, 2367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bracci, P.M.; Azhir, A.; Forrest, B.D.; McGrath, M.S. Macrophage-Targeted Sodium Chlorite (NP001) Slows Progression of Amyotrophic Lateral Sclerosis (ALS) through Regulation of Microbial Translocation. Biomedicines 2022, 10, 2907. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Siokas, V.; Sokratous, M.; Tsouris, Z.; Aloizou, A.M.; Florou, D.; Dastamani, M.; Mentis, A.A.; Brotis, A.G. Body mass index and survival from amyotrophic lateral sclerosis: A meta-analysis. Neurol. Clin. Pract. 2018, 8, 437–444. [Google Scholar] [CrossRef]
- Goutman, S.A.; Boss, J.; Iyer, G.; Habra, H.; Savelieff, M.G.; Karnovsky, A.; Mukherjee, B.; Feldman, E.L. Body mass index associates with amyotrophic lateral sclerosis survival and metabolomic profiles. Muscle Nerve 2023, 67, 208–216. [Google Scholar] [CrossRef]
- Cohen, E.; Margalit, I.; Shochat, T.; Goldberg, E.; Krause, I. Markers of Chronic Inflammation in Overweight and Obese Individuals and the Role of Gender: A Cross-Sectional Study of a Large Cohort. J. Inflamm. Res. 2021, 14, 567–573. [Google Scholar] [CrossRef]
- Liqiang, S.; Fang-Hui, L.; Minghui, Q.; Haichun, C. Threshold effect and sex characteristics of the relationship between chronic inflammation and BMI. BMC Endocr. Disord. 2023, 23, 175. [Google Scholar] [CrossRef] [PubMed]
- Molani Gol, R.; Rafraf, M. Association between abdominal obesity and pulmonary function in apparently healthy adults: A systematic review. Obes. Res. Clin. Pract. 2021, 15, 415–424. [Google Scholar] [CrossRef]
- Jung, D.H.; Shim, J.Y.; Ahn, H.Y.; Lee, H.R.; Lee, J.H.; Lee, Y.J. Relationship of body composition and C-reactive protein with pulmonary function. Respir. Med. 2010, 104, 1197–1203. [Google Scholar] [CrossRef]
- McNeill, J.N.; Lau, E.S.; Zern, E.K.; Nayor, M.; Malhotra, R.; Liu, E.E.; Bhat, R.R.; Brooks, L.C.; Farrell, R.; Sbarbaro, J.A.; et al. Association of obesity-related inflammatory pathways with lung function and exercise capacity. Respir. Med. 2021, 183, 106434. [Google Scholar] [CrossRef]
- Cui, C.; Sun, J.; Pawitan, Y.; Piehl, F.; Chen, H.; Ingre, C.; Wirdefeldt, K.; Evans, M.; Andersson, J.; Carrero, J.J.; et al. Creatinine and C-reactive protein in amyotrophic lateral sclerosis, multiple sclerosis and Parkinson’s disease. Brain Commun. 2020, 2, fcaa152. [Google Scholar] [CrossRef]
- Beers, D.R.; Zhao, W.; Neal, D.W.; Thonhoff, J.R.; Thome, A.D.; Faridar, A.; Wen, S.; Wang, J.; Appel, S.H. Elevated acute phase proteins reflect peripheral inflammation and disease severity in patients with amyotrophic lateral sclerosis. Sci. Rep. 2020, 10, 15295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; de Carvalho, M. SVC Is a Marker of Respiratory Decline Function, Similar to FVC, in Patients With ALS. Front. Neurol. 2019, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M. A Package for Survival Analysis in R. R Package Version 3.7-0. 2024. Available online: https://cran.r-project.org/web/packages/survival/ (accessed on 9 December 2024).
- Kassambara, A.; Kassambara, M.; Biecek, P. Survminer: Drawing Survival Curves using ‘ggplot2’. R Package Version 0.4.9. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 9 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 9 December 2024).

| BMI < 25 | BMI ≥ 25 | Overall | |
|---|---|---|---|
| Characteristics | (n = 64) | (n = 158) | (n = 222) |
| Sex, n (%) | |||
| Female | 22 (34.4%) | 50 (31.6%) | 72 (32.4%) |
| Male | 42 (65.6%) | 108 (68.4%) | 150 (67.6%) |
| Age at baseline, year | 56.3 ± 11.4 | 55.7 ± 10.3 | 55.9 ± 10.6 |
| Site of ALS onset, n (%) | |||
| Bulbar | 14 (21.9%) | 18 (11.4%) | 32 (14.4%) |
| Limb | 50 (78.1%) | 140 (88.6%) | 190 (86.6%) |
| El Escorial criteria for ALS, n (%) | |||
| Definite | 27 (42.2%) | 68 (43.0%) | 95 (42.8%) |
| Possible | 1 (1.6%) * | 16 (10.1%) | 17 (7.7%) |
| Probable | 34 (53.1%) | 62 (39.2%) | 96 (43.2%) |
| Probable Laboratory Supported | 2 (3.1%) | 12 (7.6%) | 14 (6.3%) |
| Concurrent riluzole use, n (%) | |||
| Yes | 43 (67.2%) | 106 (67.1%) | 149 (67.1%) |
| No | 21 (32.8%) | 52 (32.9%) | 73 (32.9%) |
| ALSFRS-R score at baseline, mean ± SD | 36.7 ± 5.7 | 37.8 ± 5.2 | 37.5 ± 5.4 |
| Vital capacity at baseline, mean ± SD | 96.6 ± 20.5 | 93.1 ± 19.9 | 94.1 ± 20.1 |
| Months since ALS symptom onset 2, mean ± SD | 17.65 ± 8.59 | 18.12 ± 8.10 | 17.98 ± 8.23 |
| NP001 2 mg/kg | Placebo | Overall | |
|---|---|---|---|
| Characteristics | (n = 85) | (n = 73) | (n = 158) |
| Sex, n (%) | |||
| Female | 28 (32.9%) | 22 (30.1%) | 50 (31.6%) |
| Male | 57 (67.1%) | 51 (69.9%) | 108 (68.4%) |
| Age at baseline, year | 55.5 ± 10.5 | 55.9 ± 10.1 | 55.7 ± 10.3 |
| Site of ALS onset, n (%) | |||
| Bulbar | 8 (9.4%) | 10 (13.7%) | 18 (11.4%) |
| Limb | 77 (90.6%) | 63 (86.3%) | 140 (88.6%) |
| El Escorial criteria for ALS, n (%) | |||
| Definite | 35 (41.2%) | 33 (45.2%) | 68 (43.0%) |
| Possible | 9 (10.6%) | 7 (9.6%) | 16 (10.1%) |
| Probable | 35 (41.2%) | 27 (37.0%) | 62 (39.2%) |
| Probable Laboratory Supported | 6 (7.1%) | 6 (8.2%) | 12 (7.6%) |
| Concurrent riluzole use, n (%) | |||
| Yes | 59 (69.4%) | 47 (64.4%) | 106 (67.1%) |
| No | 26 (30.6%) | 26 (35.6%) | 52 (32.9%) |
| ALSFRS-R score at baseline, mean ± SD | 38.4 ± 4.9 | 37.2 ± 5.5 | 37.8 ± 5.2 |
| Vital capacity at baseline, mean ± SD | 95.6 ± 20.0 | 90.1 ± 19.5 | 93.1 ± 19.9 |
| Months since ALS symptom onset 2, mean ± SD | 18.03 ± 7.90 | 18.22 ± 8.39 | 18.12 ± 8.10 |
| BMI, mean ± SD | 29.7 ± 3.7 | 29.9 ± 4.2 | 29.8 ± 3.9 |
| NP001 2 mg/kg | Placebo | Overall | |
|---|---|---|---|
| Characteristics | (n = 72) | (n = 61) | (n = 133) |
| Sex, n (%) | |||
| Female | 23 (31.9%) | 17 (27.9%) | 40 (30.1%) |
| Male | 49 (68.1%) | 44 (72.1%) | 93 (69.9%) |
| Age at baseline, year | 52.8 ± 9.0 | 53.1 ± 8.5 | 53.0 ± 8.7 |
| Site of ALS onset, n (%) | |||
| Bulbar | 6 (8.3%) | 9 (14.8%) | 15 (11.3%) |
| Limb | 66 (91.7%) | 52 (85.2%) | 118 (88.7%) |
| El Escorial criteria for ALS, n (%) | |||
| Definite | 31 (43.1%) | 30 (49.2%) | 61 (45.9%) |
| Possible | 7 (9.7%) | 7 (11.5%) | 14 (10.5%) |
| Probable | 29 (40.3%) | 20 (32.8%) | 49 (36.8%) |
| Probable Laboratory Supported | 5 (6.9%) | 4 (6.6%) | 9 (6.8%) |
| Concurrent riluzole use, n (%) | |||
| Yes | 48 (66.7%) | 38 (62.3%) | 86 (64.7%) |
| No | 24 (33.3%) | 23 (37.7%) | 47 (35.3%) |
| ALSFRS-R score at baseline, mean ± SD | 38.6 ± 4.8 | 37.0 ± 5.5 | 37.8 ± 5.2 |
| Vital capacity at baseline, mean ± SD | 96.7 ± 20.5 | 91.4 ± 18.7 | 94.3 ± 19.8 |
| Months since ALS symptom onset 2, mean ± SD | 18.73 ± 8.08 | 18.00 ± 8.36 | 18.39 ± 8.19 |
| BMI, mean ± SD | 29.7 ± 3.7 | 30.1 ± 4.3 | 29.8 ± 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Azhir, A.; McGrath, M.S. Respiratory Function Improvement and Lifespan Extension Following Immunotherapy with NP001 Support the Concept That Amyotrophic Lateral Sclerosis (ALS) Is an Immuno-Neurologic Disease. Int. J. Mol. Sci. 2025, 26, 4349. https://doi.org/10.3390/ijms26094349
Zhang R, Azhir A, McGrath MS. Respiratory Function Improvement and Lifespan Extension Following Immunotherapy with NP001 Support the Concept That Amyotrophic Lateral Sclerosis (ALS) Is an Immuno-Neurologic Disease. International Journal of Molecular Sciences. 2025; 26(9):4349. https://doi.org/10.3390/ijms26094349
Chicago/Turabian StyleZhang, Rongzhen, Ari Azhir, and Michael S. McGrath. 2025. "Respiratory Function Improvement and Lifespan Extension Following Immunotherapy with NP001 Support the Concept That Amyotrophic Lateral Sclerosis (ALS) Is an Immuno-Neurologic Disease" International Journal of Molecular Sciences 26, no. 9: 4349. https://doi.org/10.3390/ijms26094349
APA StyleZhang, R., Azhir, A., & McGrath, M. S. (2025). Respiratory Function Improvement and Lifespan Extension Following Immunotherapy with NP001 Support the Concept That Amyotrophic Lateral Sclerosis (ALS) Is an Immuno-Neurologic Disease. International Journal of Molecular Sciences, 26(9), 4349. https://doi.org/10.3390/ijms26094349







