ERC/Mesothelin Is Associated with the Formation of Microvilli on the Mesothelium and Has Limited Functional Relevance Under Physiological Conditions
Abstract
1. Introduction
2. Results
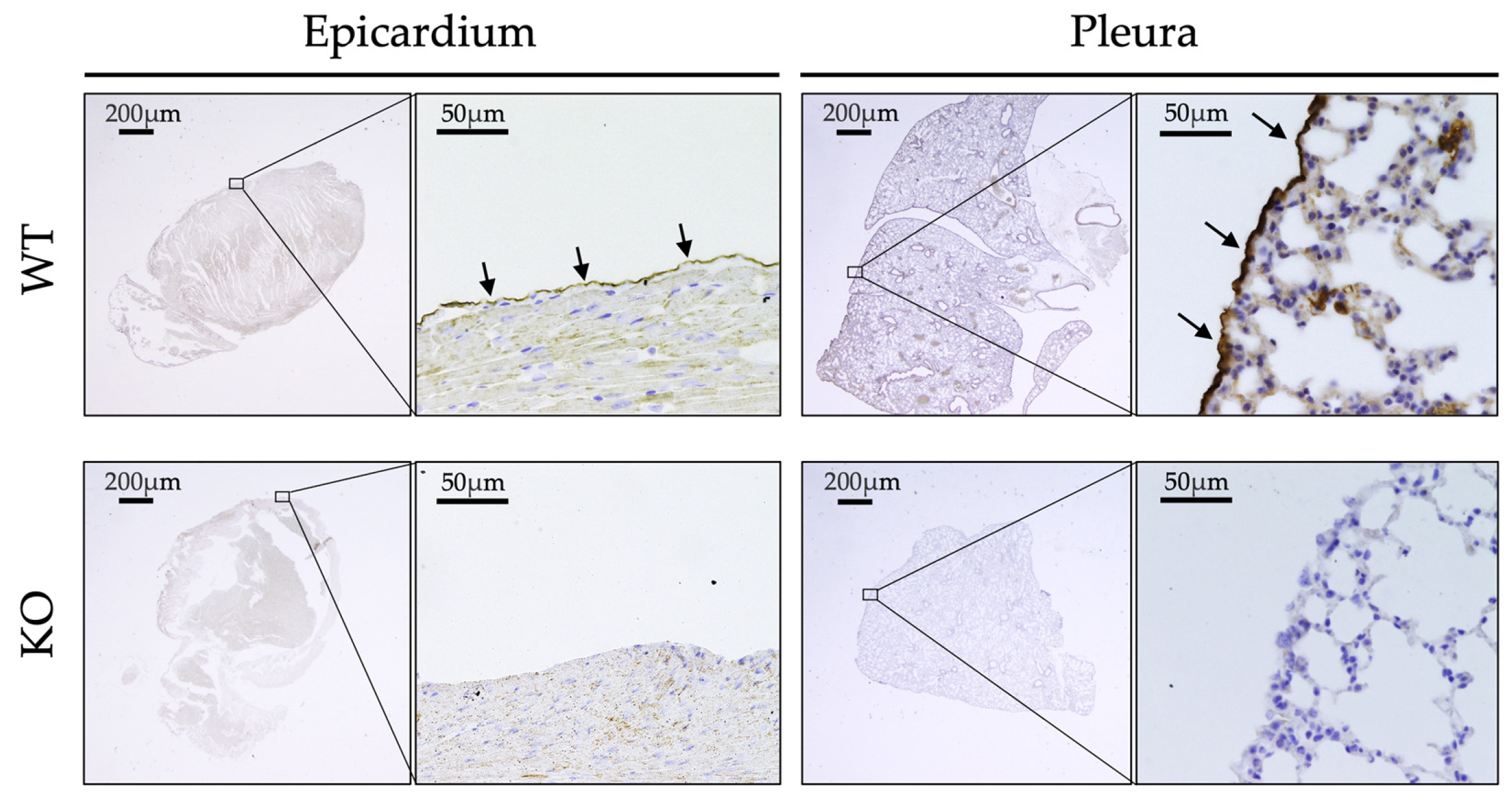
2.1. Expression of ERC in Adult Mice
2.2. Expression of ERC During Developmental Stages of WT Mice
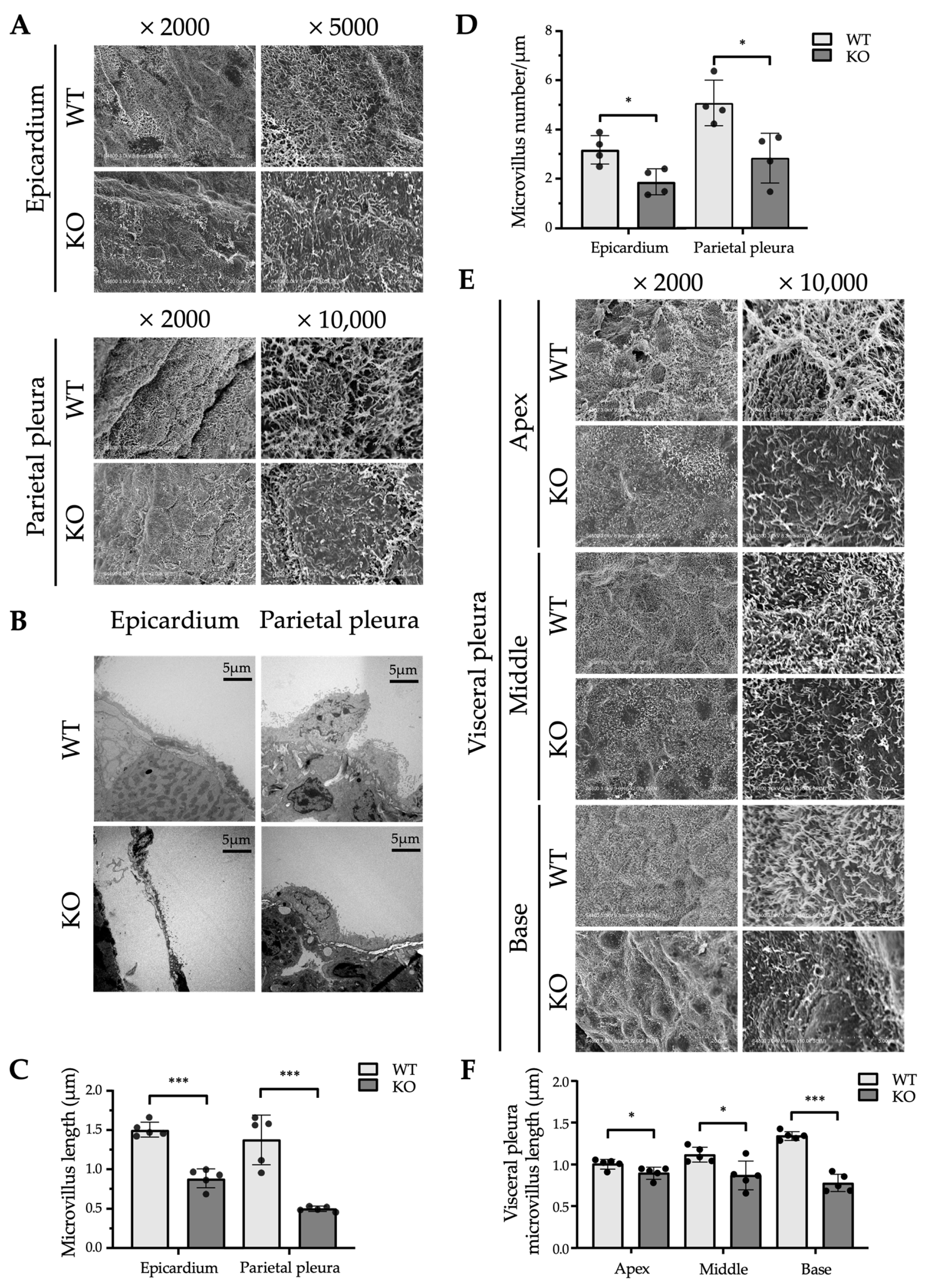
2.3. Ultrastructural Differences in the Mesothelium Between WT and Erc-KO Mice
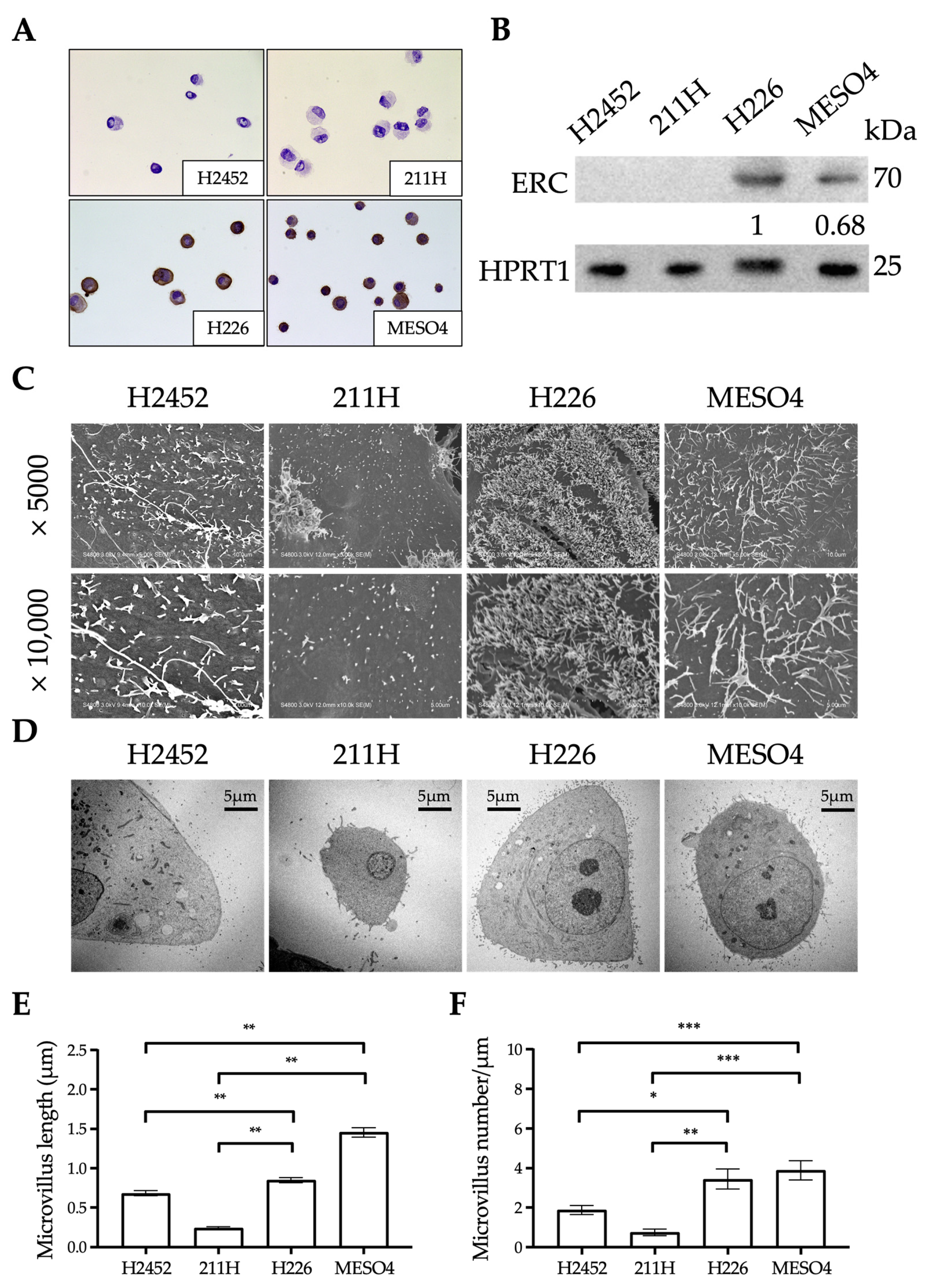
2.4. Ultrastructural Differences in the Mesothelium Between Human Mesothelioma Cell Lines
2.5. Cardiopulmonary Function of WT and Erc-KO Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Preparation
4.2. Cell Lines
4.3. Immunohistochemistry
4.4. Immunocytochemistry
4.5. Western Blotting of Cellular Lysates
4.6. EM and Morphometry
4.7. Assessment of Cardiac Function in WT and Erc-KO Mice
4.8. Assessment of Pulmonary Function in WT and Erc-KO Mice
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B-mode | Brightness mode |
| bpm | Beats per minute |
| E | Embryonic day |
| EM | Electron microscopy |
| ERC/Erc | Expressed in renal cancer |
| FOT | Forced oscillation technique |
| HPRT1 | Hypoxanthine-guanine phosphoribosyltransferase 1 |
| IHC | Immunohistochemistry |
| KO | Knockout |
| M-mode | Motion mode |
| MSLN | Mesothelin |
| ns | Not significant |
| P | Post-delivery day |
| PBS-T | Phosphate-buffered saline with 0.1% Tween-20 |
| PCR | Polymerase chain reaction |
| PFA | Paraformaldehyde |
| PBS | Phosphate-buffered saline |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| Tsc2 | Tuberous sclerosis complex 2 |
| WT | Wild-type |
References
- Hino, O.; Kobayashi, E.; Nishizawa, M.; Kubo, Y.; Kobayashi, T.; Hirayama, Y.; Takai, S.; Kikuchi, Y.; Tsuchiya, H.; Orimoto, K.; et al. Renal carcinogenesis in the Eker rat. J. Cancer Res. Clin. Oncol. 1995, 121, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yokoyama, M.; Kobayashi, E.; Takai, S.; Hino, O. Mapping and determination of the cDNA sequence of the Erc gene preferentially expressed in renal cell carcinoma in the Tsc2 gene mutant (Eker) rat model. Biochem. Biophys. Res. Commun. 2000, 275, 134–140. [Google Scholar] [CrossRef]
- Chang, K.; Pastan, I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef]
- Kojima, T.; Oh-eda, M.; Hattori, K.; Taniguchi, Y.; Tamura, M.; Ochi, N.; Yamaguchi, N. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J. Biol. Chem. 1995, 270, 21984–21990. [Google Scholar] [CrossRef]
- Scholler, N.; Fu, N.; Yang, Y.; Ye, Z.; Goodman, G.E.; Hellström, K.E.; Hellström, I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc. Natl. Acad. Sci. USA 1999, 96, 11531–11536. [Google Scholar] [CrossRef] [PubMed]
- Frierson, H.F., Jr.; Moskaluk, C.A.; Powell, S.M.; Zhang, H.; Cerilli, L.A.; Stoler, M.H.; Cathro, H.; Hampton, G.M. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum. Pathol. 2003, 34, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Ji, H.; Ronnett, B.M. Expression of mesothelin, fascin, and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int. J. Gynecol. Pathol. 2005, 24, 67–72. [Google Scholar]
- Hough, C.D.; Sherman-Baust, C.A.; Pizer, E.S.; Montz, F.J.; Im, D.D.; Rosenshein, N.B.; Cho, K.R.; Riggins, G.J.; Morin, P.J. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000, 60, 6281–6287. [Google Scholar]
- Argani, P.; Iacobuzio-Donahue, C.; Ryu, B.; Rosty, C.; Goggins, M.; Wilentz, R.E.; Murugesan, S.R.; Leach, S.D.; Jaffee, E.; Yeo, C.J.; et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. 2001, 7, 3862–3868. [Google Scholar]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef]
- Chen, S.H.; Hung, W.C.; Wang, P.; Paul, C.; Konstantopoulos, K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci. Rep. 2013, 3, 1870. [Google Scholar] [CrossRef]
- Servais, E.L.; Colovos, C.; Rodriguez, L.; Bograd, A.J.; Nitadori, J.; Sima, C.; Rusch, V.W.; Sadelain, M.; Adusumilli, P.S. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin. Cancer Res. 2012, 18, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Pastan, I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell Biol. 2000, 20, 2902–2906. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, T.S.; Kowalski, B.; Iwamoto, K.; Agadi, E.A.; Liu, Y.; Yang, J.; Asem, M.; Klymenko, Y.; Johnson, J.; Shi, Z.; et al. Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment. Int. J. Mol. Sci. 2021, 22, 12443. [Google Scholar] [CrossRef] [PubMed]
- McGovern, T.K.; Robichaud, A.; Fereydoonzad, L.; Schuessler, T.F.; Martin, J.G. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J. Vis. Exp. 2013, 75, e50172. [Google Scholar] [CrossRef]
- Mariassy, A.T.; Wheeldon, E.B. The pleura: A combined light microscopic, scanning, and transmission electron microscopic study in the sheep. I. Normal pleura. Exp. Lung Res. 1983, 4, 293–314. [Google Scholar] [CrossRef]
- Wang, N.S. The regional difference of pleural mesothelial cells in rabbits. Am. Rev. Respir. Dis. 1974, 110, 623–633. [Google Scholar]
- Usami, N.; Fukui, T.; Kondo, M.; Taniguchi, T.; Yokoyama, T.; Mori, S.; Yokoi, K.; Horio, Y.; Shimokata, K.; Sekido, Y.; et al. Establishment and characterization of four malignant pleural mesothelioma cell lines from Japanese patients. Cancer Sci. 2006, 97, 387–394. [Google Scholar] [CrossRef]
- Zhang, D.; Kobayashi, T.; Kojima, T.; Kanenishi, K.; Hagiwara, Y.; Abe, M.; Okura, H.; Hamano, Y.; Sun, G.; Maeda, M.; et al. Deficiency of the Erc/mesothelin gene ameliorates renal carcinogenesis in Tsc2 knockout mice. Cancer Sci. 2011, 102, 720–727. [Google Scholar] [CrossRef]
- Andrés-Delgado, L.; Mercader, N. Interplay between cardiac function and heart development. Biochim. Biophys. Acta 2016, 1863 Pt B, 1707–1716. [Google Scholar] [CrossRef]
- Chen, F.; De Diego, C.; Chang, M.G.; McHarg, J.L.; John, S.; Klitzner, T.S.; Weiss, J.N. Atrioventricular conduction and arrhythmias at the initiation of beating in embryonic mouse hearts. Dev. Dyn. 2010, 239, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Niblock, M.M.; Perez, A.; Broitman, S.; Jacoby, B.; Aviv, E.; Gilkey, S. In utero development of fetal breathing movements in C57BL6 mice. Respir. Physiol. Neurobiol. 2020, 271, 103288. [Google Scholar] [CrossRef]
- Dixit, R.; Ai, X.; Fine, A. Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development 2013, 140, 4398–4406. [Google Scholar] [CrossRef]
- Waters, C.M.; Chang, J.Y.; Glucksberg, M.R.; DePaola, N.; Grotberg, J.B. Mechanical forces alter growth factor release by pleural mesothelial cells. Am. J. Physiol. 1997, 272 Pt 1, L552–L557. [Google Scholar] [CrossRef]
- Andrews, P.M.; Porter, K.R. The ultrastructural morphology and possible functional significance of mesothelial microvilli. Anat. Rec. 1973, 177, 409–426. [Google Scholar] [CrossRef]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Birnie, K.; Lansley, S.; Herrick, S.E.; Lim, C.B.; Prêle, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef]
- Davenport, M.L.; Sherrill, T.P.; Blackwell, T.S.; Edmonds, M.D. Perfusion and Inflation of the Mouse Lung for Tumor Histology. J. Vis. Exp. 2020, 162, e60605. [Google Scholar] [CrossRef]
- Kojima, M.; Kajino, K.; Momose, S.; Wali, N.; Hlaing, M.T.; Han, B.; Yue, L.; Abe, M.; Fujii, T.; Ikeda, K.; et al. Possible reversibility between epithelioid and sarcomatoid types of mesothelioma is independent of ERC/mesothelin expression. Respir. Res. 2020, 21, 187. [Google Scholar] [CrossRef]
- Ichimura, K.; Kakuta, S.; Kawasaki, Y.; Miyaki, T.; Nonami, T.; Miyazaki, N.; Nakao, T.; Enomoto, S.; Arai, S.; Koike, M.; et al. Morphological process of podocyte development revealed by block-face scanning electron microscopy. J. Cell Sci. 2017, 130, 132–142. [Google Scholar] [CrossRef]
- Mitra, D.; Swaminathan, A.; Mundhe, G.; Rikhy, R. Imaging and quantification of apical microvilli in the syncytial blastoderm of Drosophila embryos. STAR Protoc. 2022, 3, 101736. [Google Scholar] [CrossRef] [PubMed]
- Inazumi, H.; Kuwahara, K.; Nakagawa, Y.; Kuwabara, Y.; Numaga-Tomita, T.; Kashihara, T.; Nakada, T.; Kurebayashi, N.; Oya, M.; Nonaka, M.; et al. NRSF-GNAO1 Pathway Contributes to the Regulation of Cardiac Ca2+ Homeostasis. Circ. Res. 2022, 130, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- Takeshige, T.; Harada, N.; Harada, S.; Ishimori, A.; Katsura, Y.; Sasano, H.; Sandhu, Y.; Matsuno, K.; Makino, F.; Ito, J.; et al. Chitin induces steroid-resistant airway inflammation and airway hyperresponsiveness in mice. Allergol. Int. 2021, 70, 343–350. [Google Scholar] [CrossRef] [PubMed]
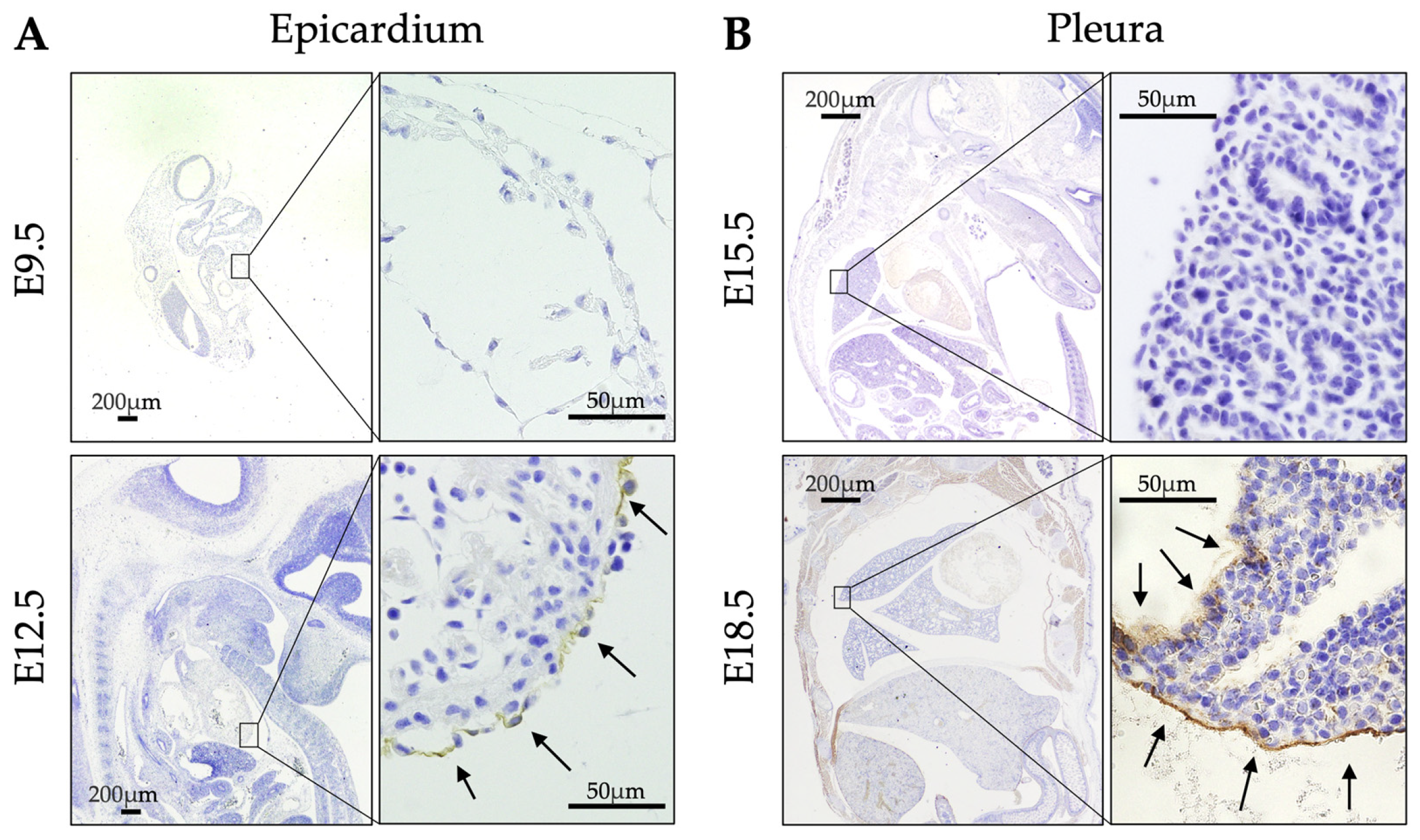

| Stage | E9.5 | E12.5 | E13.5 | E14.5 | E15.5 | E18.5 | P0 | P3 | |
|---|---|---|---|---|---|---|---|---|---|
| Site | |||||||||
| Epicardium | − | + | + | + | + | + | + | + | |
| Pleura | − | − | − | − | − | + | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Kajino, K.; Kobayashi, T.; Sugitani, Y.; Sugihara, M.; Kakuta, S.; Harada, N.; Sasano, H.; Kojima, M.; Abe, M.; et al. ERC/Mesothelin Is Associated with the Formation of Microvilli on the Mesothelium and Has Limited Functional Relevance Under Physiological Conditions. Int. J. Mol. Sci. 2025, 26, 4330. https://doi.org/10.3390/ijms26094330
Yue L, Kajino K, Kobayashi T, Sugitani Y, Sugihara M, Kakuta S, Harada N, Sasano H, Kojima M, Abe M, et al. ERC/Mesothelin Is Associated with the Formation of Microvilli on the Mesothelium and Has Limited Functional Relevance Under Physiological Conditions. International Journal of Molecular Sciences. 2025; 26(9):4330. https://doi.org/10.3390/ijms26094330
Chicago/Turabian StyleYue, Liang, Kazunori Kajino, Toshiyuki Kobayashi, Yoshinobu Sugitani, Masami Sugihara, Soichiro Kakuta, Norihiro Harada, Hitoshi Sasano, Masataka Kojima, Masaaki Abe, and et al. 2025. "ERC/Mesothelin Is Associated with the Formation of Microvilli on the Mesothelium and Has Limited Functional Relevance Under Physiological Conditions" International Journal of Molecular Sciences 26, no. 9: 4330. https://doi.org/10.3390/ijms26094330
APA StyleYue, L., Kajino, K., Kobayashi, T., Sugitani, Y., Sugihara, M., Kakuta, S., Harada, N., Sasano, H., Kojima, M., Abe, M., Lu, R., Otsuji, N., Orimo, A., & Hino, O. (2025). ERC/Mesothelin Is Associated with the Formation of Microvilli on the Mesothelium and Has Limited Functional Relevance Under Physiological Conditions. International Journal of Molecular Sciences, 26(9), 4330. https://doi.org/10.3390/ijms26094330






