Transcriptome Profiling Analysis Reveals Changes in the Antioxidant Defense System, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense Caused by Alkalinity Exposure
Abstract
1. Introduction
2. Results
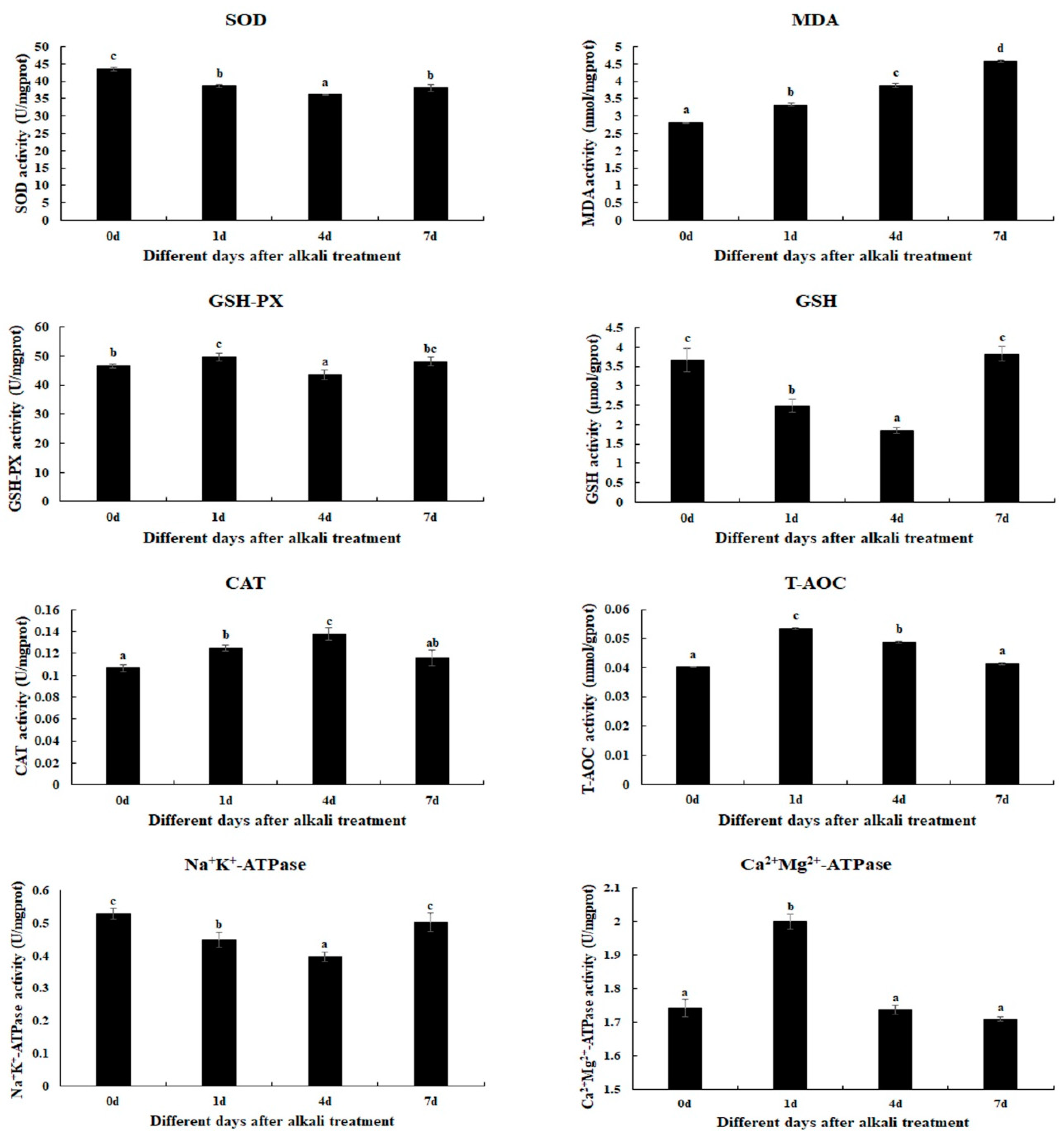
2.1. Changes in Antioxidant Enzymes Caused by Alkali Treatment
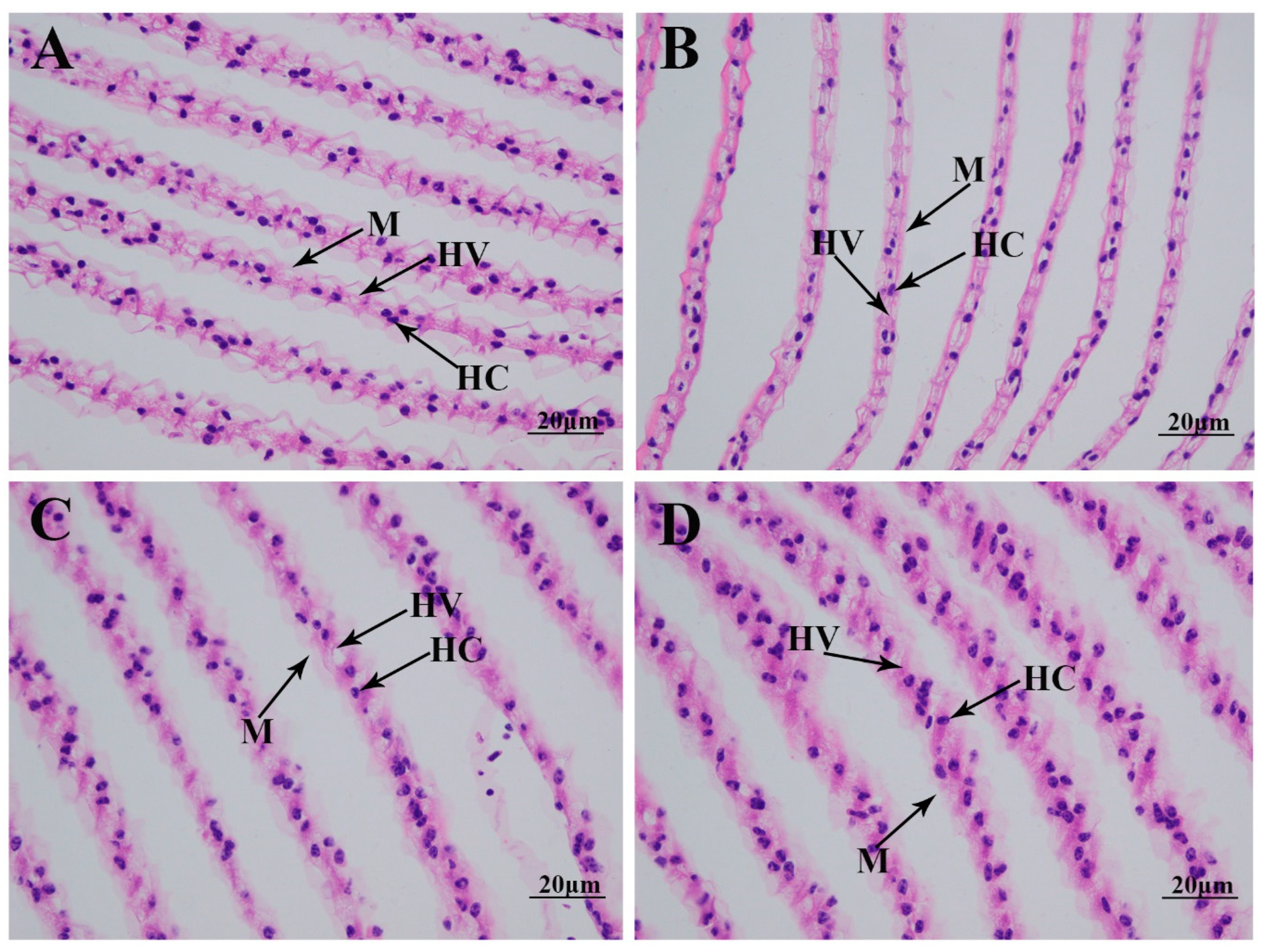
2.2. Morphological Changes in Gills Caused by Alkali Treatment
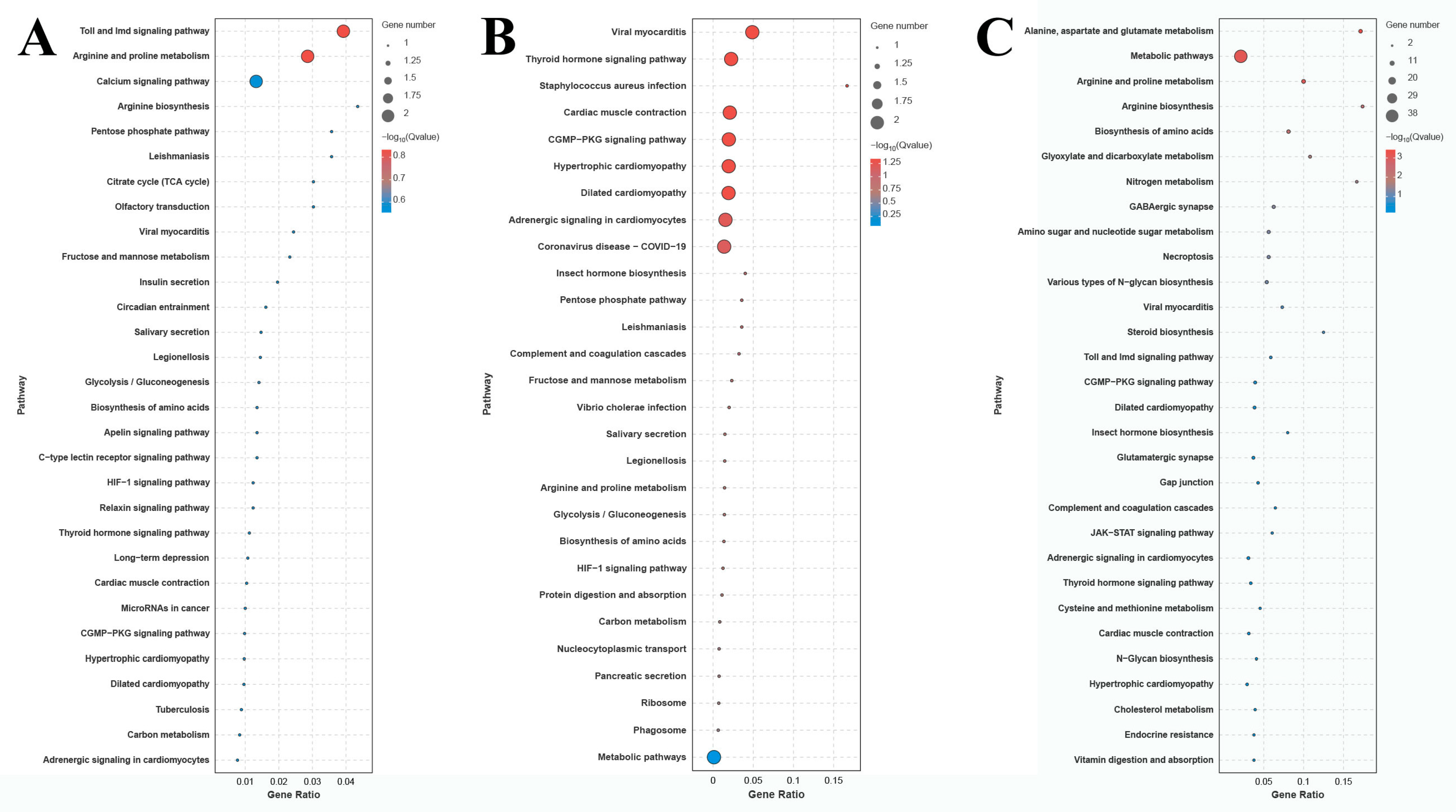
2.3. Changes in Gene Expressions in Gills Caused by Alkali Treatment
2.4. Identification of Candidate Genes Involved in the Alkaline Acclimation of M. nipponense
2.5. qPCR Verification
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Measurement of the Activities of Antioxidant Enzymes
4.3. Histological Observation
4.4. Transcriptome Profiling Analysis
4.5. qPCR Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, L.Q.; Ren, B.; Chang, Y.M.; Tang, R.; Zhang, L.M. Inland brackish (alkaline-saline) water resources and fisheries utilization in China. Chin. Fish. Econ. 2013, 31, 138–145. [Google Scholar]
- Liu, Y.X.; Fang, H.; Lai, Q.F.; Liang, L.Q. The current state and development strategy for China’s saline-alkaline fisheries. Strateg. Stud. CAE 2016, 18, 74–78. [Google Scholar]
- Wang, J.L.; Huang, X.J.; Zhong, T.Y.; Chen, Z.G. Review on sustainable utilization of salt-affected land. Acta Geogr. Sin. 2011, 66, 673–684. [Google Scholar]
- Xu, W.; Geng, L.W.; Jiang, H.F.; Tong, G.X. A review of development and utilization of fish culture in saline-alkaline water. Chin. J. Fish. 2015, 28, 44–47. [Google Scholar]
- Chi, B.J.; Liang, L.Q.; Liu, C.L.; Chang, Y.M.; Wang, S.; Han, Q.X.; Gao, G.Q. Adaptability of Tribolodon brandti (Dybowski) to NaCI concentration and alkalinity. J. Fish. China 2011, 18, 689–694. [Google Scholar]
- Wu, P.F.; Geng, L.W.; Jiang, H.F.; Tong, G.X.; Li, C.Y.; Xu, W. Tolerance of three Cobitidae fish species to high salinity and alkalinity. J. Fish. China 2017, 24, 248–257. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, Z.Q.; Dong, S.L. Preliminary studies on the tolerance of Colossoma brachypomum fingerlings to salinity and alkalinity. Period. Ocean Univ. China 1998, 3, 54–59. [Google Scholar]
- Yang, Y.; Li, M.; Luo, L.; Wang, S.; Zhang, R.; Guo, K.; Liu, J.; Li, H.; Zhao, Z. Study on toxicity of salinity and alkalinity on Eriocheir sinensis. J. Northeast Agric. Univ. 2022, 53, 36–41. [Google Scholar]
- Wang, G.; Zhang, Z.; Dong, S.; Chen, Z.; Zhang, M. Studies of toxicity of NaCl and alkalinity to post-larval Macrobrachium rosenbergii. J. Ocean Univ. 2001, 4, 523–528. [Google Scholar]
- Yang, Y.F.; Li, X.J.; Yang, X.Q.; Sun, L.M. Adapt ability of Litopenaeus vannamei to carbonate saline-alkaline waters in north east China. Mar. Sci. 2008, 1, 41–44. [Google Scholar]
- Zhang, X.L.; Cui, L.F.; Li, S.M.; Liu, X.Z.; Han, X.; Jiang, K.Y. Bureau of Fisheries, Ministry of Agriculture, P.R.C. Fisheries Economic Statistics. In China Fishery Yearbook; Beijing China Agricultural Press: Beijing, China, 2020. [Google Scholar]
- Ren, S.S.; Sun, B.; Luo, L.; Zhang, L.M.; Chang, Y.M.; Liang, L.Q. Tolerance of freshwater shrimp (Macrobrachium nipponense) to alkalinity and low temperature in Northeast China. Chin. J. Fish. 2020, 33, 24–28. [Google Scholar]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Dymowska, A.K.; Hwang, P.P.; Goss, G.G. Structure and function of ionocytes in the freshwater fish gill. Resp. Physiol. Neurobi. 2012, 184, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Fu, H.T.; Sun, S.M.; Qiao, H.; Zhang, W.Y.; Jin, S.B.; Jiang, S.F.; Xiong, Y.W.; Gong, Y.S. Experimental inoculation of oriental river prawn Macrobrachium nipponense with white spot syndrome virus (WSSV). Dis. Aquat. Organ. 2017, 126, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, M.; Fu, H.; Sun, S.; Qiao, H.; Zhang, W.; Gong, Y.; Jiang, S.; Xiong, Y.; Jin, S.; et al. Molecular cloning, expression, and in situ hybridization analysis of MnGPx-3 and MnGPx-4 from oriental river prawn, Macrobrachium nipponense, in response to hypoxia and reoxygenation. PLoS ONE 2020, 15, e0229171. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Kumai, Y.; Perry, S.F. The physiology of fish at low pH: The zebrafish as a model system. J. Exp. Biol. 2014, 217, 651–662. [Google Scholar] [CrossRef]
- Cagol, L.; Baldisserotto, B.; Becker, A.G.; Souza, C.D.F.; Ballester, E.L.C. Essential oil of Lippia alba in the diet of Macrobrachium rosenbergii: Effects on antioxidant enzymes and growth parameters. Aquac. Res. 2020, 51, 2243–2251. [Google Scholar] [CrossRef]
- Kong, Y.Q.; Ding, Z.L.; Zhang, Y.X.; Ye, J.Y.; Du, Z.Y. Dietary selenium requirement of juvenile oriental river prawn Macrobrachium nipponense. Aquaculture 2017, 476, 72–78. [Google Scholar] [CrossRef]
- Wang, W.N.; Zhou, J.; Wang, P.; Tian, T.T.; Zheng, Y.; Liu, Y.; Mai, W.J.; Wang, A.L. Oxidative stress, DNA samage and antioxidant enzyme gene expression in the pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Physiol. C 2009, 150, 428–435. [Google Scholar]
- Wang, T.; Shan, H.W.; Geng, Z.X.; Yu, P.; Ma, S. Dietary Supplementation with freeze-dried Ampithoe Sp. enhances the ammonian tolerance of Litopenaeus vannamei by reducing oxidative stress and endoplasmic reticulum stress and regulating lipid metabolism. Aquac. Rep. 2020, 16, 100264. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. C 2010, 151, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wu, D.; Zhang, Y.; Li, J.; Wang, L. Carbonate alkalinity and dietary protein levels affected growth performance, intestinal immune responses and intestinal microflora in Songpu mirror carp (Cyprinus carpio Songpu). Aquaculture 2021, 545, 737135. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.L.; Lin, T.T.; Shi, J.Q.; Zhou, K.; Wang, H.; Qi, H.F.; Lai, Q. Effects of carbonate alkalinity stress on SOD, ACP, and AKP activities in the liver and kidney of juvenile Gymnocypris przewalskii. J. Fish. China 2013, 20, 1212–1218. [Google Scholar] [CrossRef]
- Wu, P.F. Study on Saline-Alkali Adaptability of Loach in the Dali Lake Plateau. Master’s Thesis, Dalian Ocean University, Dalian, China, 2017. [Google Scholar]
- Jin, S.B.; Zhou, R.; Gao, X.B.; Xiong, Y.W.; Zhang, W.Y.; Qiao, H.; Wu, Y.; Jiang, S.F.; Fu, H.T. Identification of the effects of alkalinity exposure on the gills of oriental river prawns, Macrobrachium nipponense. BMC Genom. 2024, 25, 765. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chang, Y.M.; Zhao, X.F.; Sun, B.; Zhang, L.M.; Liang, L.Q.; Dong, Z.G. The effect of different bicarbonate alkalinity of the gill structure of Amur Ide (Leuciscus waleckii). Acta Hydrobiol. Si. 2020, 44, 736–743. [Google Scholar]
- Matey, V.; Richards, J.; Wang, Y.; Wood, C.M.; Rogers, J.; Davies, R.; Murray, B.W.; Chen, X.Q.; Du, J.; Brauner, C.J. The effect of hypoxia on gill morphology and ionoregulatory status in the Lake Qinghai scaleless carp, Gymnocypris przewalskii. J. Exp. Biol. 2008, 211, 1063–1074. [Google Scholar] [CrossRef]
- Qin, G.X.; Wei, Q.; Yu, J.Q. Histological characterization muscular and gill of Gymnocypris przewalskii. J. Qinghai Univ. 2010, 28, 4–7. [Google Scholar]
- Zhang, R.Y.; Li, G.G.; Zhang, C.F.; Tang, Y.T.; Zhao, K. Morphological differentiations of the gills of two Gymnocypris przewalskii subspecies in different habitats and their functional adaptations. Zool. Res. 2013, 34, 387–391. [Google Scholar]
- Zhang, J.B.; Cui, G.T.; Cai, C.F.; Ren, S.J.; Ni, Q.; Wang, C.R.; Li, W.J.; Ge, Y.Y.; Ding, H.M.; Zhang, C. Effects of short-term extreme pH stress on physiology and growth performance of Eriocheir sinensis. Freshw. Fish. 2020, 50, 99–106. [Google Scholar]
- Wang, L.B.; Pan, M.J.; Wang, M.Y.; Wang, R.Z.; Li, L.; Dong, S.L.; Li, W.D.; Tian, X.L. Kidney transcriptomic response of Lateolabrax maculatus to long-term alkalinity stressing. Period. Ocean Univ. China 2023, 2, 32–43. [Google Scholar]
- Shang, X.C.; Geng, L.W.; Yang, J.; Zhang, Y.T.; Xu, W. Transcriptome analysis reveals the mechanism of alkalinity exposure on spleen oxidative stress, inflammation and immune function of Luciobarbus capito. Ecotox. Environ. Saf. 2021, 225, 112748. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Xu, L.M.; Wang, S.L.; Jiang, Y.L.; Zhao, Z.X.; Zhang, Y.; Li, J.T.; Dong, C.J.; Xu, P.; et al. Gene expression changes leading extreme alkaline tolerance in Amur Ide (Leuciscus waleckii) inhabiting soda lake. BMC Genom. 2013, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Tang, R.; Dou, X.J.; Tao, R.; Sun, X.W.; Liang, L.Q. Transcriptome and expression profiling analysis of Leuciscus waleckii: An exploration of the alkali-adapted mechanisms of a freshwater teleost. Mol. Biosyst. 2014, 10, 491–504. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Athenstaedt, K.; Daum, G. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 23317–23323. [Google Scholar] [CrossRef]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef]
- Khara, F.; Shafiei, M.; Galehdari, H. A novel acidic and SDS tolerant halophilic lipase from moderate halophile Nesterenkonia sp. strain F: Molecular cloning, structure analysis and biochemical characterization. Biologia 2022, 77, 1135–1150. [Google Scholar] [CrossRef]
- Roussel, A.; Canaan, S.; Egloff, M.P.; Rivière, M.; Dupuis, L.; Verger, R.; Cambillau, C. Crystal structure of human gastric lipase and model of lysosomal acid lipase, two lipolytic enzymes of medical interest. J. Biol. Chem. 1999, 274, 16995–17002. [Google Scholar] [CrossRef]
- Carey, M.C.; Small, D.M.; Bliss, C.M. Lipid digestion and absorption. Annu. Rev. Physiol. 1983, 45, 651–677. [Google Scholar] [CrossRef]
- Dressler, K.A.; Mathias, S.; Kolesnick, R.N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science 1992, 255, 1715–1718. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Ion, G.; Fajka-Boja, R.; Kovács, F.; Szebeni, G.; Gombos, I.; Czibula, A.; Matkó, J.; Monostori, E. Acid sphingomyelinase mediated release of ceramide is essential to trigger the mitochondrial pathway of apoptosis by galectin-1. Cell Signal. 2006, 18, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Tamura, T.; Rabah, N.; Lee, D.Y.D.; Ruel, I.; Hafiane, A.; Iatan, I.; Nyholt, D.; Laporte, F.; Lazure, C. Carboxyl-terminal disulfide bond of acid sphingomyelinase is critical for its secretion and enzymatic function. Biochemistry 2007, 46, 14969–14978. [Google Scholar] [CrossRef]
- Vergarajauregui, S.; Martina, J.A.; Puertollano, R. LAPTMs regulate lysosomal function and interact with mucolipin 1: New clues for understanding mucolipidosis type IV. J. Cell Sci. 2011, 124, 459–468. [Google Scholar] [CrossRef][Green Version]
- Meng, Y.; Wang, L.; Chen, D.; Chang, Y.; Zhang, M.; Xu, J.J.; Zhou, R.; Zhang, Q.Y. LAPTM4B: An oncogene in various solid tumors and its functions. Oncogene 2016, 35, 6359–6365. [Google Scholar] [CrossRef]
- Bachère, E.; Rosa, R.D.; Schmitt, P.; Poirier, A.C.; Merou, N.; Charrière, G.M.; Destoumieux-Garzón, D. The new insights into the oyster antimicrobial defense: Cellular, molecular and genetic view. Fish Shellfish Immunol. 2015, 46, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.G.; Muroga, K. Cellular defense mechanisms in bivalve molluscs. Fish Pathol. 2008, 43, 1–17. [Google Scholar] [CrossRef]
- Lopes, D.; Maiato, H. The tubulin code in mitosis and cancer. Cells 2020, 9, 2356. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, Q.; Wu, X.; Liu, W.; Li, D.F.; Li, C.; Zhao, W.X.; Chen, L.L.; Zheng, Z.; Li, G.M. Tension of plus-end tracking protein Clip170 confers directionality and aggressiveness during breast cancer migration. Cell Death Dis. 2022, 13, 856. [Google Scholar] [CrossRef]
- Martínez-Hernández, J.; Parato, J.; Sharma, A.; Soleilhac, J.M.; Qu, X.; Tein, E.; Sproul, A.; Andrieux, A.; Goldberg, Y.; Moutin, M.J.; et al. Crosstalk between acetylation and the tyrosination/detyrosination cycle of α-tubulin in Alzheimer’s disease. Front. Cell Dev. Biol. 2022, 10, 926914. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Amrute-Nayak, M.; Allgeyer, E.; Li, X. MARK4 controls ischaemic heart failure through microtubule detyrosination. Nature 2021, 594, 560–565. [Google Scholar] [CrossRef]
- Margulies, K.B.; Prosser, B.L. Tubulin detyrosination: An emerging therapeutic target in hypertrophic cardiomyopathy. Circ. Heart Fail. 2021, 14, e008006. [Google Scholar] [CrossRef] [PubMed]
- Preetham, E.; Abdul Salam, R.; Jesu, A.; Ratree, W.; Matteo, C.; Einar, R.; Baskaralingam, V. The role of lectins in finfish: A review. Rev. Fish. Sci. Aquac. 2019, 27, 152–169. [Google Scholar]
- Lv, C.H.; Zhang, D.L.; Wang, Z.Y. A novel C-type lectin, nattectin-like protein, with a wide range of bacterial agglutination activity in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 2016, 50, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Cieśla, M.; Mierzejewska, J.; Adamczyk, M.; Farrants, A.K.Ö.; Boguta, M. Fructose bisphosphate aldolase is involved in the control of RNA polymerase III-directed transcription. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 1103–1110. [Google Scholar] [CrossRef]
- Pirovich, D.B.; Da’dara, A.A.; Skelly, P.J. Multifunctional fructose 1,6-bisphosphate aldolase as a therapeutic target. Front. Mol. Biosci. 2021, 8, 719678. [Google Scholar] [CrossRef]
- Zhang, C.S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.W.; Zhu, M.; et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef]
- Metón, I.; Fernández, F.; Baanante, I.V. Short- and long-term effects of refeeding on key enzyme activities in glycolysis–gluconeogenesis in the liver of gilthead seabream (Sparus aurata). Aquaculture 2003, 225, 99–107. [Google Scholar] [CrossRef]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef]
- Sagar, V.; Sahu, N.P.; Pal, A.K.; Hassaan, M.; Jain, K.K.; Salim, H.S.; El-Haroun, E.R. Effect of different stock types and dietary protein levels on key enzyme activities of glycolysis-gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in Macrobrachium rosenbergii. J. Appl. Ichthyol. 2019, 35, 1016–1024. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Comerford, S.A.; Huang, Z.; Du, X.; Wang, Y.; Cai, L.; Witkiewicz, A.K.; Walters, H.; Tantawy, M.N.; Fu, A.; Manning, H.C.; et al. Acetate dependence of tumors. Cell 2014, 159, 1591–1602. [Google Scholar] [CrossRef]
- Li, X.; Yu, W.; Qian, X.; Xia, Y.; Zheng, Y.; Lee, J.H.; Li, W.; Lyu, J.; Rao, G.; Zhang, X.; et al. Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Mol. Cell 2017, 66, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Schug, Z.T.; Peck, B.; Jones, D.T.; Zhang, Q.; Grosskurth, S.; Alam, I.S.; Goodwin, L.M.; Smethurst, E.; Mason, S.; Blyth, K.; et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 2015, 27, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Kumar, V.; Bhatt, D.N.; Irfan, M.; Datta, A. N-acetylglucosamine sensing and metabolic engineering for attenuating human and plant pathogens. Bioengineering 2022, 9, 64. [Google Scholar] [CrossRef]
- Ghosh, S.; Rao, K.H.; Sengupta, M.; Bhattacharya, S.K.; Datta, A. Two gene clusters co-ordinate for a functional N-acetylglucosamine catabolic pathway in Vibrio cholerae. Mol. Microbiol. 2011, 80, 1549–1560. [Google Scholar] [CrossRef]
- Corfield, A.P.; Berry, M. Glycan variation and evolution in the eukaryotes. Trends Biochem. Sci. 2015, 40, 351–359. [Google Scholar] [CrossRef]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Mun, S.; Noh, M.Y.; Geisbrecht, E.R.; Arakane, Y. Insect cuticular chitin contributes to form and function. Curr. Pharm. Des. 2020, 26, 3530–3545. [Google Scholar] [CrossRef]
- Shakeel, M.; Khan, A.; Du, J.; Basit, A.; Yang, G.M.; Haddi, K.; Abbas, S.; Alam, A.; Li, S.W. Knockdown of the glucosamine-6-phosphate N-acetyltransferase gene by RNA interference enhances the virulence of entomopathogenic fungi against rice leaffolder Cnaphalocrocis medinalis. Pestic. Biochem. Phys. 2024, 205, 106119. [Google Scholar] [CrossRef] [PubMed]
- Orlean, P. The Molecular Biology of the Yeast Saccharomyces, 3; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1997; pp. 229–262. [Google Scholar]
- SC/T 9406-2012; Water Quality for Aquaculture in Saline-Alkali Land, Ministry of Agriculture. China Agricultural Press: Beijing, China, 2012.
- Ma, X.K.; Liu, X.Z.; Wen, H.S.; Xu, Y.J.; Zhang, L.J. Histological observation on gonadal sex differentiation in Cynoglossus semilaevis Günther. Mar. Fish. Res. 2006, 27, 55–61. [Google Scholar]
- ShangGuan, B.M.; Liu, Z.Z.; Li, S.Q. Histological studies on ovarian development in Scylla serrata. J. Fish. China 1991, 15, 96–103. [Google Scholar]
- Jin, S.B.; Fu, H.T.; Zhou, Q.; Sun, S.M.; Jiang, S.F.; Xiong, Y.W.; Gong, Y.S.; Qiao, H.; Zhang, W.Y. Transcriptome analysis of androgenic gland for discovery of novel genes from the oriental river prawn, Macrobrachium nipponense, using Illumina Hiseq 2000. PLoS ONE 2013, 8, e76840. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Fu, Y.; Hu, Y.N.; Fu, H.T.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S. Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation. Sci. Rep. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Itoh, M. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Zwinderman, A.H. On the benjamini-hochberg method. Ann. Statist. 2006, 34, 1827–1849. [Google Scholar] [CrossRef]
- Jin, S.B.; Hu, Y.N.; Fu, H.T.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S.; Wu, Y. Potential functions of gem-associated protein 2-like isoform X1 in the oriental river prawn Macrobrachium nipponense: Cloning, qPCR, in situ hybridization, and RNAi analysis. Int. J. Mol. Sci. 2019, 20, 3995. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Hu, Y.N.; Fu, H.T.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S.; Wu, Y. Identification and characterization of the succinate dehydrogenase complex iron sulfur subunit B gene in the oriental river prawn Macrobrachium nipponense. Front. Genet. 2021, 12, 698318. [Google Scholar] [CrossRef]
- Hu, Y.N.; Fu, H.T.; Qiao, H.; Sun, S.M.; Zhang, W.Y.; Jin, S.B.; Jiang, S.F.; Gong, Y.S.; Xiong, Y.W.; Wu, Y. Validation and evaluation of reference genes for quantitative real-time PCR in Macrobrachium nipponense. Int. J. Mol. Sci. 2018, 19, 2258. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

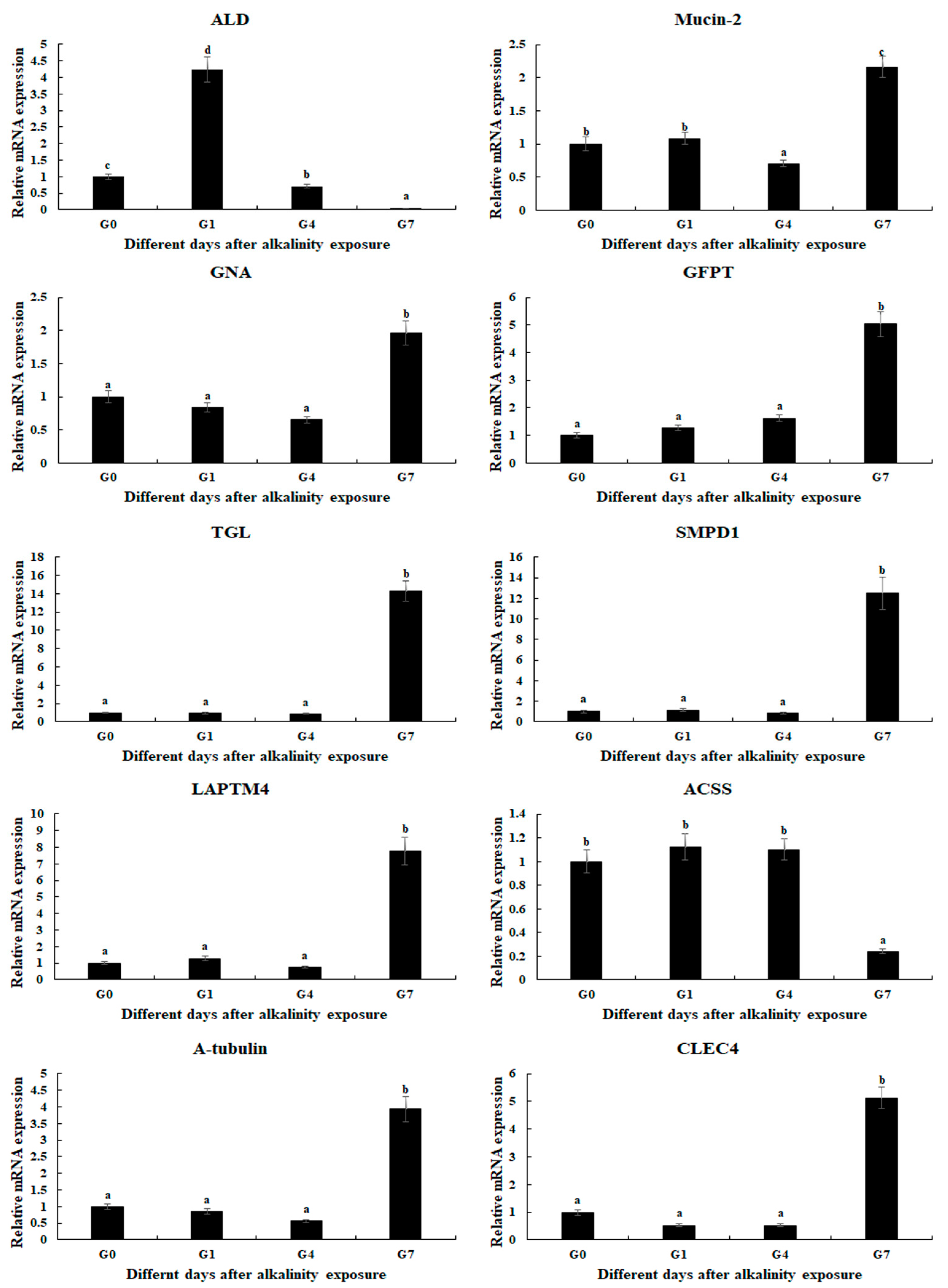
| Gene | Accession Number | Metabolic Pathway | Log2 (Fold Change) | ||
|---|---|---|---|---|---|
| S0 vs. S1 | S1 vs. S4 | S4 vs. S7 | |||
| Fructose-bisphosphate aldolase-like (ALD) | ncbi_135219851 | Biosynthesis of amino acids, glycolysis/gluconeogenesis | 5.4 | −6.9 | −5.3 |
| Mucin-2 | ncbi_135210831 | Amino sugar and nucleotide sugar metabolism | 2.7 | ||
| Glucosamine 6-phosphate N-acetyltransferase (GNA) | ncbi_135213629 | Amino sugar and nucleotide sugar metabolism | 1.7 | ||
| Glutamine—fructose-6-phosphate transaminase (GFPT) | ncbi_135226562 | Amino sugar and nucleotide sugar metabolism | 1.4 | ||
| Gastric triacylglycerol lipase (TGL) | ncbi_135218763 | Lysosome | 4.0 | ||
| Sphingomyelin phosphodiesterase 1 (SMPD1) | ncbi_135213205 | Lysosome | 3.2 | ||
| Lysosomal-associated transmembrane protein 4 (LAPTM4) | ncbi_135223659 | Lysosome | 2.0 | ||
| Acetyl-CoA synthetase 2 (ACSS2) | ncbi_135226390 | Glycolysis/gluconeogenesis | −1.3 | ||
| A-tubulin | ncbi_135198818 | Phagosome | 1.5 | ||
| C-type lectin domain family 4 (CLEC4) | ncbi_135199509 | Phagosome | 2.7 | ||
| Gene | Primer |
|---|---|
| ALD | F: TACCCACGACTTGGAACGTG |
| R: CTTTGCAACCTGTGCAGCAT | |
| Mucin-2 | F: AGCAAGGTCTCGTGTTGCTT |
| R: TTACTCTTGTTCTGCGCCGT | |
| GNA | F: AAAGCTGGACTGGTCGAAGG |
| R: TGTCTCCCACTTTGGTCAGC | |
| GFPT | F: AGTTGGAAGGTGCCTTTGCT |
| R: ATCGTGCAAGACCGTGTGAT | |
| TGL | F: CTAATCGGCAGAAACGCAGC |
| R: CGCTCTCTTAGGCTGACTCG | |
| SMPD | F: GCCAGTGTGTCGAGGAATCA |
| R: AGGGATGTTCACGCTCCAAG | |
| LAPTM4 | F: TGGGACTGGGAACCGATACT |
| R: CCATCACGTTTGGTCCTTGC | |
| ACSS2 | F: TTCCCCCATTTGCGCACTTA |
| R: CCGTCTGGAAACCACCTGAA | |
| A-tubulin | F: AACACGTCCCAAGAGCTGTC |
| R: AGTGTCCACGGGCATAGTTG | |
| CLEC4 | F: ATGGGAGTCACGTCAGAGGA |
| R: TTCCCGTGGTAAAGGACTGC | |
| EIF | F: CATGGATGTACCTGTGGTGAAAC |
| R: CATGGATGTACCTGTGGTGAAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Zhang, Y.; Fu, H.; Zhang, W.; Qiao, H.; Xiong, Y.; Jiang, S. Transcriptome Profiling Analysis Reveals Changes in the Antioxidant Defense System, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense Caused by Alkalinity Exposure. Int. J. Mol. Sci. 2025, 26, 4321. https://doi.org/10.3390/ijms26094321
Jin S, Zhang Y, Fu H, Zhang W, Qiao H, Xiong Y, Jiang S. Transcriptome Profiling Analysis Reveals Changes in the Antioxidant Defense System, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense Caused by Alkalinity Exposure. International Journal of Molecular Sciences. 2025; 26(9):4321. https://doi.org/10.3390/ijms26094321
Chicago/Turabian StyleJin, Shubo, Yuefan Zhang, Hongtuo Fu, Wenyi Zhang, Hui Qiao, Yiwei Xiong, and Sufei Jiang. 2025. "Transcriptome Profiling Analysis Reveals Changes in the Antioxidant Defense System, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense Caused by Alkalinity Exposure" International Journal of Molecular Sciences 26, no. 9: 4321. https://doi.org/10.3390/ijms26094321
APA StyleJin, S., Zhang, Y., Fu, H., Zhang, W., Qiao, H., Xiong, Y., & Jiang, S. (2025). Transcriptome Profiling Analysis Reveals Changes in the Antioxidant Defense System, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense Caused by Alkalinity Exposure. International Journal of Molecular Sciences, 26(9), 4321. https://doi.org/10.3390/ijms26094321








