Harnessing Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Opportunities and Barriers to Clinical Integration
Abstract
1. Introduction
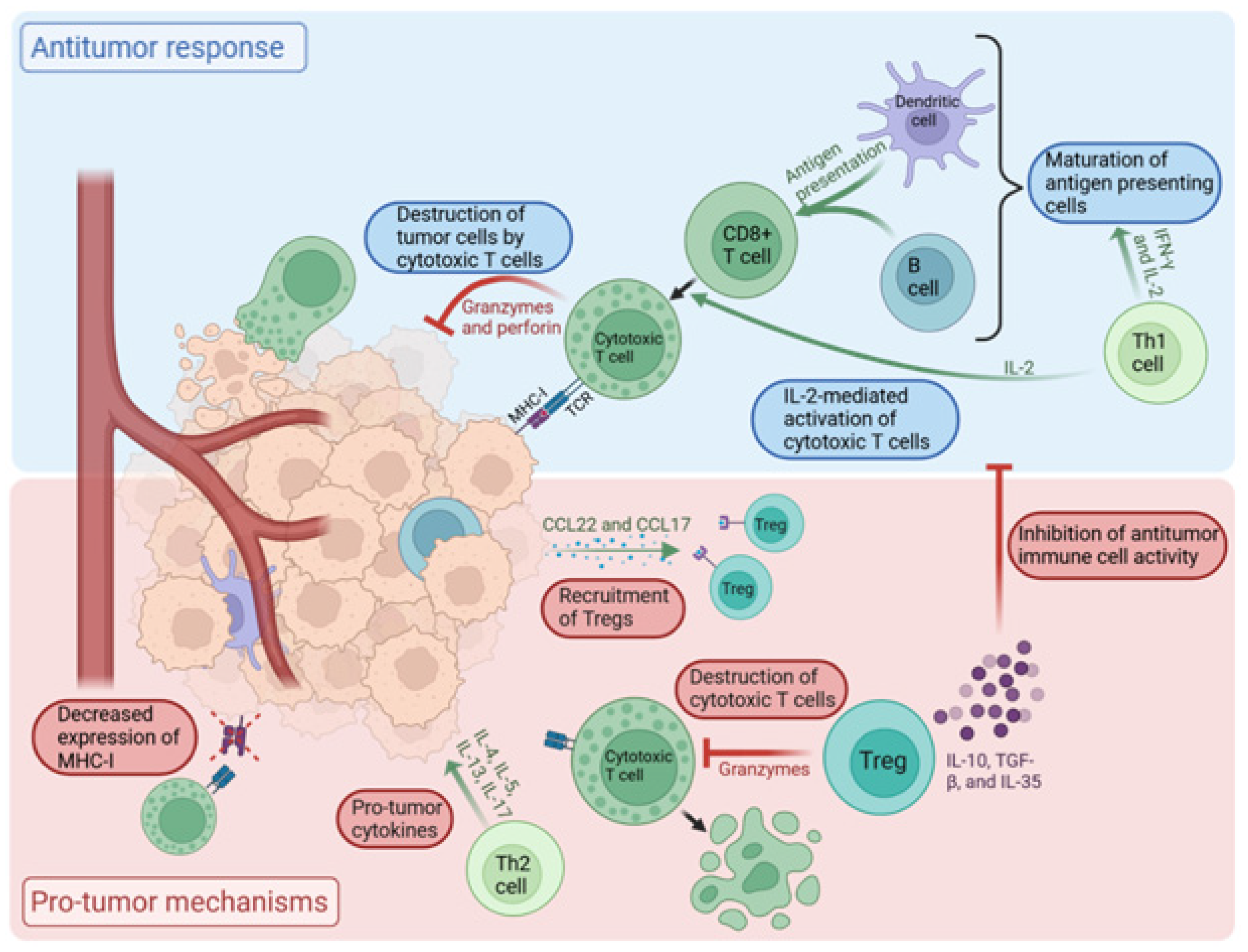
2. The Role of TILs in the Tumor Microenvironment
2.1. Tumor Microenvironment (TME)
2.2. TILs in Breast Cancer
2.3. TILs in TNBC
3. Prognostic Value of TILs in TNBC
3.1. Early-Stage TNBC
3.2. Metastatic TNBC
4. TILs as Predictive Biomarkers for Response to Immunotherapy
5. Challenges
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. Oncologist 2014, 19, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Bosselut, R. CD4–CD8 differentiation in the thymus: Connecting circuits and building memories. Curr. Opin. Immunol. 2012, 24, 139–145. [Google Scholar] [CrossRef]
- Piroozkhah, M.; Gholinezhad, Y.; Piroozkhah, M.; Shams, E.; Nazemalhosseini-Mojarad, E. The molecular mechanism of actions and clinical utilities of tumor infiltrating lymphocytes in gastrointestinal cancers: A comprehensive review and future prospects toward personalized medicine. Front. Immunol. 2023, 14, 1298891. [Google Scholar] [CrossRef]
- Kim, H.; Cantor, H. CD4 T-cell subsets and tumor immunity: The helpful and the not-so-helpful. Cancer Immunol. Res. 2014, 2, 91–98. [Google Scholar] [CrossRef]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Hay, Z.L.Z.; Slansky, J.E. Granzymes: The Molecular Executors of Immune-Mediated Cytotoxicity. Int. J. Mol. Sci. 2022, 23, 1833. [Google Scholar] [CrossRef]
- Zeng, Z.; Chew, H.Y.; Cruz, J.G.; Leggatt, G.R.; Wells, J.W. Investigating T Cell Immunity in Cancer: Achievements and Prospects. Int. J. Mol. Sci. 2021, 22, 2907. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Dhar, S.; Sa, G. Tumor-infiltrating T-regulatory cells adapt to altered metabolism to promote tumor-immune escape. Curr. Res. Immunol. 2021, 2, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Ramamoorthi, G.; Albert, G.; Gallen, C.; Beyer, A.; Snyder, C.; Koski, G.; Disis, M.L.; Czerniecki, B.J.; Kodumudi, K. Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy. Front. Immunol. 2021, 12, 669474. [Google Scholar] [CrossRef]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef]
- Kasza, A. IL-1 and EGF regulate expression of genes important in inflammation and cancer. Cytokine 2013, 62, 22–33. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Coleman, C. Tumor Microenvironment. Available online: https://BioRender.com/m9tvzue (accessed on 20 April 2025).
- Shou, J.; Zhang, Z.; Lai, Y.; Chen, Z.; Huang, J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs: A systematic review and meta-analysis. BMC Cancer 2016, 16, 687. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast 2017, 35, 142–150. [Google Scholar] [CrossRef]
- Ciarka, A.; Piątek, M.; Pęksa, R.; Kunc, M.; Senkus, E. Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Prognostic and Predictive Significance Across Molecular Subtypes. Biomedicines 2024, 12, 763. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Dieci, M.V.; Mathieu, M.C.; Guarneri, V.; Conte, P.; Delaloge, S.; Andre, F.; Goubar, A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann. Oncol. 2015, 26, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Issa-Nummer, Y.; Darb-Esfahani, S.; Loibl, S.; Kunz, G.; Nekljudova, V.; Schrader, I.; Sinn, B.V.; Ulmer, H.; Kronenwett, R.; Just, M.; et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS ONE 2013, 8, e79775. [Google Scholar] [CrossRef] [PubMed]
- Pruneri, G.; Vingiani, A.; Bagnardi, V.; Rotmensz, N.; De Rose, A.; Palazzo, A.; Colleoni, A.M.; Goldhirsch, A.; Viale, G. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann. Oncol. 2016, 27, 249–256. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Dieci, M.V.; Frassoldati, A.; Generali, D.; Bisagni, G.; Piacentini, F.; Cavanna, L.; Cagossi, K.; Puglisi, F.; Michelotti, A.; Berardi, R.; et al. Tumor-infiltrating lymphocytes and molecular response after neoadjuvant therapy for HR+/HER2− breast cancer: Results from two prospective trials. Breast Cancer Res. Treat. 2017, 163, 295–302. [Google Scholar] [CrossRef]
- Dieci, M.V.; Prat, A.; Tagliafico, E.; Paré, L.; Ficarra, G.; Bisagni, G.; Piacentini, F.; Generali, D.G.; Conte, P.; Guarneri, V. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann. Oncol. 2016, 27, 1867–1873. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations with Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated with Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef]
- Perez, E.A.; Ballman, K.V.; Tenner, K.S.; Thompson, E.A.; Badve, S.S.; Bailey, H.; Baehner, F.L. Association of Stromal Tumor-Infiltrating Lymphocytes with Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients with Early-Stage HER2-Positive Breast Cancer. JAMA Oncol. 2016, 2, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef]
- Papaioannou, E.; Sakellakis, M.; Melachrinou, M.; Tzoracoleftherakis, E.; Kalofonos, H.; Kourea, E. A Standardized Evaluation Method for FOXP3+ Tregs and CD8+ T-cells in Breast Carcinoma: Association with Breast Carcinoma Subtypes, Stage and Prognosis. Anticancer. Res. 2019, 39, 1217–1232. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.W.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Xu, X.; Yu, K.; Jin, X.; Hu, X.; Zuo, W.; Hao, S.; Wu, J.; Liu, G.; et al. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res. 2016, 18, 33. [Google Scholar] [CrossRef]
- Bareche, Y.; Venet, D.; Ignatiadis, M.; Aftimos, P.; Piccart, M.; Rothe, F.; Sotiriou, C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 2018, 29, 895–902. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.; Wang, Z.; Wu, P.; Qiu, F.; Huang, J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: A systematic review and meta-analysis. Clin. Transl. Oncol. 2016, 18, 497–506. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.H.; Muñoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.-A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Caro, C.A.; Ramírez, M.A.; Rey-Vargas, L.; Bejarano-Rivera, L.M.; Ballen, D.F.; Nuñez, M.; Mejía, J.C.; Sua-Villegas, L.F.; Cock-Rada, A.; Zabaleta, J.; et al. Tumor infiltrating lymphocytes (TILs) are a prognosis biomarker in Colombian patients with triple negative breast cancer. Sci. Rep. 2023, 13, 21324. [Google Scholar] [CrossRef] [PubMed]
- Abdullaeva, S.R.; Semiglazova, T.Y.; Artemyeva, A.S.; Zagoruiko, V.A.; Kudriashova, T.I.; Filatova, L.V.; Semiglazov, V.V.; Krivorotko, P.V.; Semiglazov, V.F. Tumor infiltrating lymphocytes (TILs) in triple negative breast cancer. Arkhiv Patol. 2024, 86, 5. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef]
- Thomas, A.; Reis-Filho, J.S.; Geyer, C.E.; Wen, H.Y. Rare subtypes of triple negative breast cancer: Current understanding and future directions. NPJ Breast Cancer 2023, 9, 55. [Google Scholar] [CrossRef]
- Geurts, V.C.M.; Balduzzi, S.; Steenbruggen, T.G.; Linn, S.C.; Siesling, S.; Badve, S.S.; DeMichele, A.; Ignatiadis, M.; Leon-Ferre, R.A.; Goetz, M.P.; et al. Tumor-Infiltrating Lymphocytes in Patients with Stage I Triple-Negative Breast Cancer Untreated with Chemotherapy. JAMA Oncol. 2024, 10, 1077–1086. [Google Scholar] [CrossRef]
- Stover, D.G.; Salgado, R.; Savenkov, O.; Ballman, K.; Mayer, E.L.; Magbanua, M.J.M.; Loi, S.; Vater, M.; Glover, K.; Watson, M.; et al. Association between tumor-infiltrating lymphocytes and survival in patients with metastatic breast cancer receiving first-line chemotherapy: Analysis of CALGB 40502. NPJ Breast Cancer 2024, 10, 75. [Google Scholar] [CrossRef]
- Nederlof, I.; Isaeva, O.I.; de Graaf, M.; Gielen, R.C.A.M.; Bakker, N.A.M.; Rolfes, A.L.; Garner, H.; Boeckx, B.; Traets, J.J.H.; Mandjes, I.A.M.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in early-stage triple-negative breast cancer: A phase 2 adaptive trial. Nat. Med. 2024, 30, 3223–3235. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Loi, S.; Winer, E.; Lipatov, O.; Im, S.; Goncalves, A.; Cortes, J.; Lee, K.S.; Schmid, P.; Testa, L.; Witzel, I.; et al. Abstract PD5-03: Relationship between tumor-infiltrating lymphocytes (TILs) and outcomes in the KEYNOTE-119 study of pembrolizumab vs chemotherapy for previously treated metastatic triple-negative breast cancer (mTNBC). Cancer Res. 2020, 80, PD5-03. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-.; Grischke, E.-.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- Tower, H.; Ruppert, M.; Britt, K. The Immune Microenvironment of Breast Cancer Progression. Cancers 2019, 11, 1375. [Google Scholar] [CrossRef]
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef]
- Takenaka, M.; Seki, N.; Toh, U.; Hattori, S.; Kawahara, A.; Yamaguchi, T.; Koura, K.; Takahashi, R.; Otsuka, H.; Takahashi, H.; et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol. Clin. Oncol. 2013, 1, 625–632. [Google Scholar] [CrossRef]
- Blackley, E.F.; Loi, S. Targeting immune pathways in breast cancer: Review of the prognostic utility of TILs in early stage triple negative breast cancer (TNBC). Breast 2019, 48 (Suppl. S1), S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Onagi, H.; Horimoto, Y.; Sakaguchi, A.; Ikarashi, D.; Yanagisawa, N.; Nakayama, T.; Nakatsura, T.; Ishizuka, Y.; Sasaki, R.; Watanabe, J.; et al. High platelet-to-lymphocyte ratios in triple-negative breast cancer associates with immunosuppressive status of TILs. Breast Cancer Res. 2022, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- Baram, T.; Erlichman, N.; Dadiani, M.; Balint-Lahat, N.; Pavlovski, A.; Meshel, T.; Morzaev-Sulzbach, D.; Gal-Yam, E.N.; Barshack, I.; Ben-Baruch, A. Chemotherapy Shifts the Balance in Favor of CD8+ TNFR2+ TILs in Triple-Negative Breast Tumors. Cells 2021, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Yen, E.; Chang, J.T.; Bassett, R.L.; Alatrash, G.; Garber, H.; Huo, L.; Yang, F.; Philips, A.V.; Ding, Q.; et al. Immune Phenotype and Response to Neoadjuvant Therapy in Triple-Negative Breast Cancer. Clin. Cancer Res. 2021, 27, 5365–5375. [Google Scholar] [CrossRef]
- Cabioglu, N.; Onder, S.; Oner, G.; Karatay, H.; Tukenmez, M.; Muslumanoglu, M.; İgci, A.; Eralp, Y.; Aydiner, A.; Saip, P.; et al. TIM3 expression on TILs is associated with poor response to neoadjuvant chemotherapy in patients with locally advanced triple-negative breast cancer. BMC Cancer 2021, 21, 357. [Google Scholar] [CrossRef]
- O’Meara, T.; Safonov, A.; Casadevall, D.; Qing, T.; Silber, A.; Killelea, B.; Hatzis, C.; Pusztai, L. Immune microenvironment of triple-negative breast cancer in African-American and Caucasian women. Breast Cancer Res. Treat. 2019, 175, 247–259. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Miller, I.; Koo, J.; Joshi, V.; Kurian, A.W.; Allison, K.H.; John, E.M.; Telli, M.L. Tumor-infiltrating lymphocytes and breast cancer mortality in racially and ethnically diverse participants of the Northern California Breast Cancer Family Registry. JNCI Cancer. Spectr. 2024, 8, pkae023. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Cotogni, P.; Cereda, E.; Bossi, P.; Aprile, G.; Delrio, P.; Gnagnarella, P.; Mascheroni, A.; Monge, T.; Corradi, E.; et al. Nutritional Support in Cancer patients: Update of the Italian Intersociety Working Group practical recommendations. J. Cancer 2022, 13, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Tsvetkova, V.; Orvieto, E.; Piacentini, F.; Ficarra, G.; Griguolo, G.; Miglietta, F.; Giarratano, T.; Omarini, C.; Bonaguro, S.; et al. Immune characterization of breast cancer metastases: Prognostic implications. Breast Cancer Res. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Canc Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Sharma, P.; Stecklein, S.R.; Yoder, R.; Staley, J.M.; Schwensen, K.; O’Dea, A.; Nye, L.; Satelli, D.; Crane, G.; Madan, R.; et al. Clinical and Biomarker Findings of Neoadjuvant Pembrolizumab and Carboplatin Plus Docetaxel in Triple-Negative Breast Cancer: NeoPACT Phase 2 Clinical Trial. JAMA Oncol. 2024, 10, 227–235. [Google Scholar] [CrossRef]
- Karn, T.; Denkert, C.; Weber, K.E.; Holtrich, U.; Hanusch, C.; Sinn, B.V.; Higgs, B.W.; Jank, P.; Sinn, H.P.; Huober, J.; et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann. Oncol. 2020, 31, 1216–1222. [Google Scholar] [CrossRef]
- Loi, S.; Adams, S.; Schmid, P.; Cortés, J.; Cescon, D.W.; Winer, E.P.; Toppmeyer, D.L.; Rugo, H.S.; Laurentiis, M.D.; Nanda, R.; et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): Results from KEYNOTE-086. Ann. Oncol. 2017, 28, v608. [Google Scholar] [CrossRef]
- Massa, D.; Vernieri, C.; Nicolè, L.; Criscitiello, C.; Boissière-Michot, F.; Guiu, S.; Bobrie, A.; Griguolo, G.; Miglietta, F.; Vingiani, A.; et al. Immune and gene-expression profiling in estrogen receptor low and negative early breast cancer. J. Natl. Cancer Inst. 2024, 116, 1914–1927. [Google Scholar] [CrossRef]
- Loi, S.; Salgado, R.; Curigliano, G.; Romero Díaz, R.I.; Delaloge, S.; Rojas García, C.I.; Kok, M.; Saura, C.; Harbeck, N.; Mittendorf, E.A.; et al. Neoadjuvant nivolumab and chemotherapy in early estrogen receptor-positive breast cancer: A randomized phase 3 trial. Nat. Med. 2025, 31, 433–441. [Google Scholar] [CrossRef]
- Sompuram, S.R.; Torlakovic, E.E.; ‘t Hart, N.A.; Vani, K.; Bogen, S.A. Quantitative comparison of PD-L1 IHC assays against NIST standard reference material 1934. Mod. Pathol. 2022, 35, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Kos, Z.; Roblin, E.; Kim, R.S.; Michiels, S.; Gallas, B.D.; Chen, W.; van de Vijver, K.K.; Goel, S.; Adams, S.; Demaria, S.; et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 2020, 6, 17. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [PubMed]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from the International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Leon-Ferre, R.A.; Jonas, S.F.; Salgado, R.; Loi, S.; de Jong, V.; Carter, J.M.; Nielsen, T.O.; Leung, S.; Riaz, N.; Chia, S.; et al. Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. JAMA 2024, 331, 1135–1144. [Google Scholar] [CrossRef]
- Denkert, C.; Wienert, S.; Poterie, A.; Loibl, S.; Budczies, J.; Badve, S.; Bago-Horvath, Z.; Bane, A.; Bedri, S.; Brock, J.; et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: Results of the ring studies of the international immuno-oncology biomarker working group. Mod. Pathol. 2016, 29, 1155–1164. [Google Scholar] [CrossRef]
- Wu, R.; Horimoto, Y.; Oshi, M.; Benesch, M.G.K.; Khoury, T.; Takabe, K.; Ishikawa, T. Emerging measurements for tumor-infiltrating lymphocytes in breast cancer. Jpn. J. Clin. Oncol. 2024, 54, 620–629. [Google Scholar] [CrossRef]
- Presti, D.; Dall’Olio, F.G.; Besse, B.; Ribeiro, J.M.; Di Meglio, A.; Soldato, D. Tumor infiltrating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2022, 177, 103773. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Roque, T. Pembrolizumab and Chemotherapy Treatment or no Treatment Guided by the Level of TILs in Resected Early-stage TNBC (ETNA). In Clinical Trial NCT06078384; UNICANCER: Cali, Colombia, 2025. [Google Scholar]
- Netherlands Cancer Institute (NKI). OPTImaL: Optimisation of Treatment for Patients with Low Stage Triple-Negative Breast Cancer with High Stromal Tumor-infiltrating Lymphocytes; Netherlands Cancer Institute (NKI): Amsterdam, The Netherlands, 2024. [Google Scholar]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef]
- Wang, X.; Barrera, C.; Bera, K.; Viswanathan, V.S.; Azarianpour-Esfahani, S.; Koyuncu, C.; Velu, P.; Feldman, M.D.; Yang, M.; Fu, P.; et al. Spatial interplay patterns of cancer nuclei and tumor-infiltrating lymphocytes (TILs) predict clinical benefit for immune checkpoint inhibitors. Sci. Adv. 2022, 8, eabn3966. [Google Scholar] [CrossRef] [PubMed]
- Lopez Janeiro, A.; Miraval Wong, E.; Jiménez-Sánchez, D.; Ortiz de Solorzano, C.; Lozano, M.D.; Teijeira, A.; Schalper, K.A.; Melero, I.; De Andrea, C.E. Spatially resolved tissue imaging to analyze the tumor immune microenvironment: Beyond cell-type densities. J. Immunother. Cancer 2024, 12, e008589. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.M.; Chumsri, S.; Hinerfeld, D.A.; Ma, Y.; Wang, X.; Zahrieh, D.; Hillman, D.W.; Tenner, K.S.; Kachergus, J.M.; Brauer, H.A.; et al. Distinct spatial immune microlandscapes are independently associated with outcomes in triple-negative breast cancer. Nat. Commun. 2023, 14, 2215. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Bifulco, C.; Bindea, G.; Marliot, F.; Lugli, A.; Lee, J.J.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter International Society for Immunotherapy of Cancer Study of the Consensus Immunoscore for the Prediction of Survival and Response to Chemotherapy in Stage III Colon Cancer. J. Clin. Oncol. 2020, 38, 3638–3651. [Google Scholar] [CrossRef]
- Xu, J.; Yang, M.; Lan, H.; Jin, K. Could immunoscore improve the prognostic and therapeutic management in patients with solid tumors? Int. Immunopharmacol. 2023, 124, 110981. [Google Scholar] [CrossRef]
- Rapoport, B.L.; Nayler, S.; Mlecnik, B.; Smit, T.; Heyman, L.; Bouquet, I.; Martel, M.; Galon, J.; Benn, C.; Anderson, R. Tumor-Infiltrating Lymphocytes (TILs) in Early Breast Cancer Patients: High CD3+, CD8+, and Immunoscore Are Associated with a Pathological Complete Response. Cancers 2022, 14, 2525. [Google Scholar] [CrossRef]
- Nakane, H.; Sudo, T.; Kawahara, A.; Yomoda, T.; Shigaki, T.; Fujiyoshi, K.; Ohchi, T.; Koushi, K.; Yoshida, T.; Ogata, T.; et al. Simplified and Optimized Immune Score for Colorectal Cancer Microenvironment. Anticancer. Res. 2023, 43, 3793–3798. [Google Scholar] [CrossRef]
- Ward-Hartstonge, K.A.; McCall, J.L.; McCulloch, T.R.; Kamps, A.; Girardin, A.; Cretney, E.; Munro, F.M.; Kemp, R.A. Inclusion of BLIMP-1+ effector regulatory T cells improves the Immunoscore in a cohort of New Zealand colorectal cancer patients: A pilot study. Cancer Immunol. Immunother. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Fiorin, A.; López Pablo, C.; Lejeune, M.; Hamza Siraj, A.; Della Mea, V. Enhancing AI Research for Breast Cancer: A Comprehensive Review of Tumor-Infiltrating Lymphocyte Datasets. J. Imaging Inform. Med. 2024, 37, 2996–3008. [Google Scholar] [CrossRef] [PubMed]
- Kwong, M.L.M.; Yang, J.C. Lifileucel: FDA-approved T-cell therapy for melanoma. Oncologist 2024, 29, 648–650. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, G.; Wan, X. Challenges and new technologies in adoptive cell therapy. J. Hematol. Oncol. 2023, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Zacharakis, N.; Huq, L.M.; Seitter, S.J.; Kim, S.P.; Gartner, J.J.; Sindiri, S.; Hill, V.K.; Li, Y.F.; Paria, B.C.; Ray, S.; et al. Breast Cancers Are Immunogenic: Immunologic Analyses and a Phase II Pilot Clinical Trial Using Mutation-Reactive Autologous Lymphocytes. J. Clin. Oncol. 2022, 40, 1741–1754. [Google Scholar] [CrossRef]
- Chic, N.; Ciruelos, E.; Saura, C.; Gonzalez, E.; Álvarez-Vallina, L.; Lasarte, J.J.; Gros, A.; Villanueva, L.; Canes, J.; Angelats, L.; et al. Abstract OT2-10-04: Treatment of advanced or metastatic triple-negative breast cancer with adoptive therapy of PD1+ tumor-infiltrating lymphocytes (TILS001 trial). Cancer Res. 2023, 83, OT2-10-04. [Google Scholar] [CrossRef]
| Trial | Design | Population | Interventions | Results |
|---|---|---|---|---|
| ECOG 2197 & 1199 (2014) [46] | Two phase III, randomized trials. | TIL analysis included 481 patients with operable TNBC (from both trials). | E2197: adjuvant doxorubicin plus either cyclophosphamide or docetaxel. E1199: adjuvant doxorubicin plus cyclophosphamide followed by one of four taxane regimens. | Higher sTIL score was significantly correlated with improved DFS, distant recurrence-free survival, and OS. Results of iTIL analysis did not reach statistical significance. |
| KEYNOTE-173 (2020) [43] | Phase Ib trial. | 60 patients with early-stage TNBC. | Neoadjuvant pembrolizumab + chemotherapy. | Median pretreatment sTIL levels were higher in patients who achieved pCR than in those who did not (42% vs. 10%). |
| CALGB 40502 (2024) [49] | Phase III, randomized trial. | 799 patients with advanced (stage IIIC or IV) breast cancer. | First-line nab-paclitaxel, ixabepilone, or paclitaxel, with or without bevacizumab. | Low sTIL scores were significantly associated with worse PFS (HR 1.34) and OS (HR 1.32). When controlled for hormone receptor status, the trend was similar but did not reach statistical significance. |
| BELLINI (2024) [50] Nederlof, I.; Isaeva, O.I.; de Graaf, M.; Gielen, R.C.A.M.; Bakker, N.A.M.; Rolfes, A.L.; Garner, H.; Boeckx, B.; Traets, J.J.H.; Mandjes, I.A.M.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in early-stage triple-negative breast cancer: A phase 2 adaptive trial. Nat. Med. 2024, 30, 3223–3235. https://doi.org/10.1038/s41591-024-03249-3. Available online: https://pubmed.ncbi.nlm.nih.gov/39284953/ (accessed on 16 March 2025). | Phase II, adaptive trial. | 46 patients with early-stage TNBC. | Neoadjuvant nivolumab (with or without ipilimumab). | High pretreatment TILs were associated with improved response to immunotherapy. Responders had shorter CD8+ to tumor distance. |
| KEYNOTE-086 (2019) [51] | Phase II, single-arm trial. | 254 patients with metastatic TNBC. | Pembrolizumab monotherapy after progression on one or more systemic therapies. | Patients with high sTILs (>/= median amount within the sample of patients) had increased ORR (odds ratio 1.26). |
| KEYNOTE-119 (2020) [52] | Phase III, randomized trial. | 622 patients with previously treated metastatic TNBC. | Pembrolizumab monotherapy vs. single-agent chemotherapy. | High sTIL scores were significantly associated with improved OS, ORR, PFS, and duration of response in the pembrolizumab arm, but not the chemotherapy arm. |
| Gepar-Nuevo (2022) [53] 1. Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-.; Grischke, E.-.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. https://doi.org/10.1093/annonc/mdz158. Available online: https://pubmed.ncbi.nlm.nih.gov/31095287/ (accessed on 27 September 2024). | Phase II, randomized trial. | 117 patients with metastatic TNBC. | Nab-paclitaxel followed by epirubicin and cyclophosphamide, plus durvalumab or placebo given every 4 weeks. | sTILs as a continuous variable were significantly associated with improved response rates in both treatment arms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, C.; Selvakumar, T.; Thurlapati, A.; Graf, K.; Pavuluri, S.; Mehrotra, S.; Sahin, O.; Sivapiragasam, A. Harnessing Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Opportunities and Barriers to Clinical Integration. Int. J. Mol. Sci. 2025, 26, 4292. https://doi.org/10.3390/ijms26094292
Coleman C, Selvakumar T, Thurlapati A, Graf K, Pavuluri S, Mehrotra S, Sahin O, Sivapiragasam A. Harnessing Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Opportunities and Barriers to Clinical Integration. International Journal of Molecular Sciences. 2025; 26(9):4292. https://doi.org/10.3390/ijms26094292
Chicago/Turabian StyleColeman, Cara, Tharakeswari Selvakumar, Aswani Thurlapati, Kevin Graf, Sushma Pavuluri, Shikhar Mehrotra, Ozgur Sahin, and Abirami Sivapiragasam. 2025. "Harnessing Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Opportunities and Barriers to Clinical Integration" International Journal of Molecular Sciences 26, no. 9: 4292. https://doi.org/10.3390/ijms26094292
APA StyleColeman, C., Selvakumar, T., Thurlapati, A., Graf, K., Pavuluri, S., Mehrotra, S., Sahin, O., & Sivapiragasam, A. (2025). Harnessing Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Opportunities and Barriers to Clinical Integration. International Journal of Molecular Sciences, 26(9), 4292. https://doi.org/10.3390/ijms26094292







