Abnormally Increased Prolactin Levels in Women with Polycystic Ovarian Syndrome Are Associated with Risk of Obesity, Insulin Resistance and Prediabetes
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Study Population
4.3. Laboratory Analysis
4.4. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic Dysfunction in Polycystic Ovary Syndrome: Pathogenic Role of Androgen Excess and Potential Therapeutic Strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Yildirim, B.; Sabir, N.; Kaleli, B. Relationship of Intra-Abdominal Fat Distribution to Metabolic Disorders in Nonobese Patients with Polycystic Ovary Syndrome. Fertil. Steril. 2003, 79, 1358–1364. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Atkin, S.L. The Link Between Polycystic Ovary Syndrome and Type 2 Diabetes: What Do We Know Today? Diabetes Manag. 2011, 1, 641. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; Zhang, F.; Zhang, S.; Chen, X.; Liang, W.; Xie, Q. Resistance to Insulin and Elevated Levels of Androgen: A Major Cause of Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 741764. [Google Scholar] [CrossRef]
- Aydın, G.A.; Özsoy, H.G.T. The Relationship between Prolactin and Adipose Tissue and Metabolic Parameters in Patients with Polycystic Ovary Syndrome. Eur. Res. J. 2020, 6, 517–526. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hugo, E.R.; Brandebourg, T.D.; LaPensee, C.R. Focus on Prolactin as a Metabolic Hormone. Trends Endocrinol. Metab. 2006, 17, 110–116. [Google Scholar] [CrossRef]
- Mahboobifard, F.; Rahmati, M.; Amiri, M.; Azizi, F.; Ramezani Tehrani, F. To What Extent Does Polycystic Ovary Syndrome Influence the Cut-Off Value of Prolactin? Findings of a Community-Based Study. Adv. Med. Sci. 2022, 67, 79–86. [Google Scholar] [CrossRef]
- Daimon, M.; Kamba, A.; Murakami, H.; Mizushiri, S.; Osonoi, S.; Yamaichi, M.; Matsuki, K.; Sato, E.; Tanabe, J.; Takayasu, S.; et al. Association Between Serum Prolactin Levels and Insulin Resistance in Non-Diabetic Men. PLoS ONE 2017, 12, e0175204. [Google Scholar] [CrossRef] [PubMed]
- Berinder, K.; Nyström, T.; Höybye, C.; Hall, K.; Hulting, A.L. Insulin Sensitivity and Lipid Profile in Prolactinoma Patients Before and After Normalization of Prolactin by Dopamine Agonist Therapy. Pituitary 2011, 14, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Cintia, M. BMI and Metabolic Profile in Patients with Prolactinoma before and after Treatment with Dopamine Agonists. Obesity 2011, 19, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Bruska-Sikorska, M.; Rojek, M.; Junik, R. Hyperprolactinemia and Insulin Resistance. Endokrynol. Pol. 2022, 73, 959–967. [Google Scholar] [CrossRef]
- Yang, H.; Lin, J.; Li, H.; Liu, Z.; Chen, X.; Chen, Q. Prolactin Is Associated with Insulin Resistance and Beta-Cell Dysfunction in Infertile Women with Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 571229. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S14–S31. [Google Scholar] [CrossRef]
- Boudreaux, M.Y.; Talbott, E.O.; Kip, K.E.; Brooks, M.M.; Witchel, S.F. Risk of T2DM and impaired fasting glucose among PCOS subjects: Results of an 8-year follow-up. Curr. Diabetes Rep. 2006, 6, 77–83. [Google Scholar] [CrossRef]
- Gambineri, A.; Patton, L.; Altieri, P.; Pagotto, U.; Pizzi, C.; Manzoli, L.; Pasquali, R. Polycystic ovary syndrome is a risk factor for type 2 diabetes: Results from a long-term prospective study. Diabetes 2012, 61, 2369–2374. [Google Scholar] [CrossRef]
- Yang, H.; Di, J.; Pan, J.; Yu, R.; Teng, Y.; Cai, Z.; Deng, X. The association between prolactin and metabolic parameters in PCOS women: A retrospective analysis. Front. Endocrinol. 2020, 11, 263. [Google Scholar] [CrossRef]
- Macotela, Y.; Triebel, J.; Clapp, C. Time for a new perspective on prolactin in metabolism. Trends Endocrinol. Metab. 2020, 31, 276–286. [Google Scholar] [CrossRef]
- Davoudi, Z.; Araghi, F.; Vahedi, M.; Mokhtari, N.; Gheisari, M. Prolactin level in polycystic ovary syndrome (PCOS): An approach to the diagnosis and management. Acta Biomed. 2021, 92, e2021291. [Google Scholar] [CrossRef]
- Kamrul-Hasan, A.B.M.; Aalpona, F.T.Z. Metabolic association of serum prolactin in polycystic ovary syndrome: A retrospective analysis of 840 patients in Bangladesh. Endocr. Metab. Sci. 2023, 14, 100153. [Google Scholar] [CrossRef]
- Delcour, C.; Robin, G.; Young, J.; Dewailly, D. PCOS and hyperprolactinemia: What do we know in 2019? Clin. Med. Insights Reprod. Health 2019, 13, 1179558119871921. [Google Scholar] [CrossRef] [PubMed]
- Saei Ghare Naz, M.; Mousavi, M.; Mahboobifard, F.; Niknam, A.; Ramezani, T.F. A Meta-Analysis of Observational Studies on Prolactin Levels in Women with Polycystic Ovary Syndrome. Diagnostics 2022, 12, 2924. [Google Scholar] [CrossRef]
- Szosland, K.; Pawlowicz, P.; Lewinski, A. Prolactin secretion in polycystic ovary syndrome (PCOS). Neuroendocrinol. Lett. 2015, 36, 53–58. [Google Scholar]
- Momani, M.S.; Al Tarawni, A.; Momani, Y.M.; Rahhal, S.; Elhaj, I.; Al-Halhouli, D.; Alhawari, H. Effect of age, gender, food intake, obesity, and smoking on serum levels of prolactin in healthy adults. J. Pers. Med. 2024, 14, 905. [Google Scholar] [CrossRef]
- Al Sabie, F.; Tariq, Z.; Erickson, D.; Donegan, D. Association between prolactinoma and body mass index. Endocr. Pract. 2021, 27, 312–317. [Google Scholar] [CrossRef]
- Ponce, A.J.; Galván-Salas, T.; Lerma-Alvarado, R.M.; Ruiz-Herrera, X.; Hernández-Cortés, T.; Valencia-Jiménez, R.; Cárdenas-Rodríguez, L.E.; de la Escalera, G.M.; Clapp, C.; Macotela, Y. Low prolactin levels are associated with visceral adipocyte hypertrophy and insulin resistance in humans. Endocrine 2020, 67, 331–343. [Google Scholar] [CrossRef]
- Auriemma, R.S.; De Alcubierre, D.; Pirchio, R.; Pivonello, R.; Colao, A. Glucose abnormalities associated with prolactin-secreting pituitary adenomas. Front. Endocrinol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Tuzcu, A.; Bahceci, M.; Dursun, M.; Turgut, C.; Bahceci, S. Insulin sensitivity and hyperprolactinemia. J. Endocrinol. Investig. 2003, 26, 341–346. [Google Scholar] [CrossRef]
- Goyal, A.; Ganie, M.A. Idiopathic hyperprolactinemia presenting as polycystic ovary syndrome in identical twin sisters: A case report and literature review. Cureus 2018, 10, e3004. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, J.; Xu, Y.; Li, M.; Sun, J.; Zhang, J.; Xu, B.; Xu, M.; Chen, Y.; Bi, Y.; et al. Circulating Prolactin Associates with Diabetes and Impaired Glucose Regulation: A Population-Based Study. Diabetes Care 2013, 36, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Impaired Prolactin-Lowering Effects of Metformin in Women with Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 5474. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; De Tullio, A.; Iovino, M.; Disoteo, O.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes. Biomedicines 2023, 11, 2993. [Google Scholar] [CrossRef]
- Simonds, S.E.; Cowley, M.A. Speed-dieting: Dopamine agonists promote weight loss. Nat. Metab. 2019, 1, 851–852. [Google Scholar] [CrossRef]
- Byberg, S.; Futtrup, J.; Andreassen, M.; Krogh, J. Metabolic effects of dopamine agonists in patients with prolactinomas: A systematic review and meta-analysis. Endocr. Connect. 2019, 8, 1395–1404. [Google Scholar] [CrossRef]
- World Health Organization Obesity and Overweight 2024. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 19 February 2025).

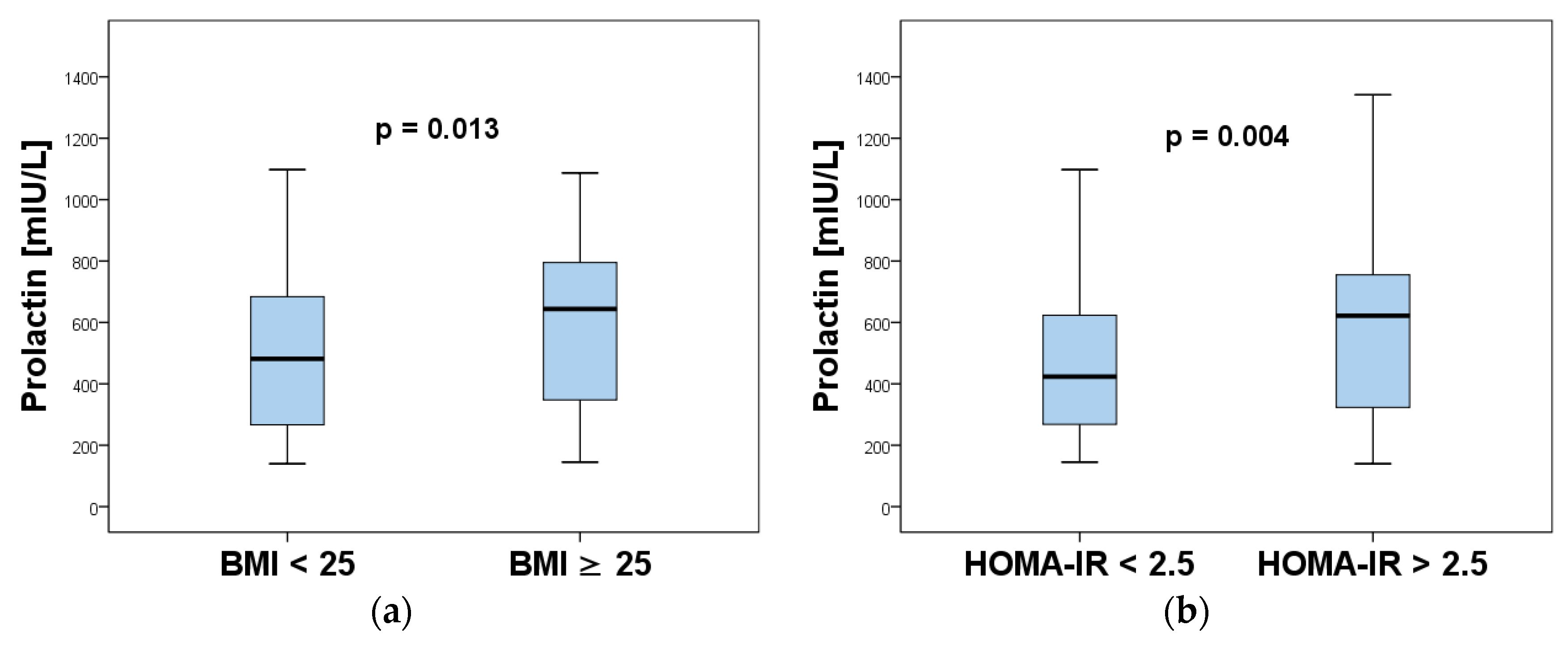
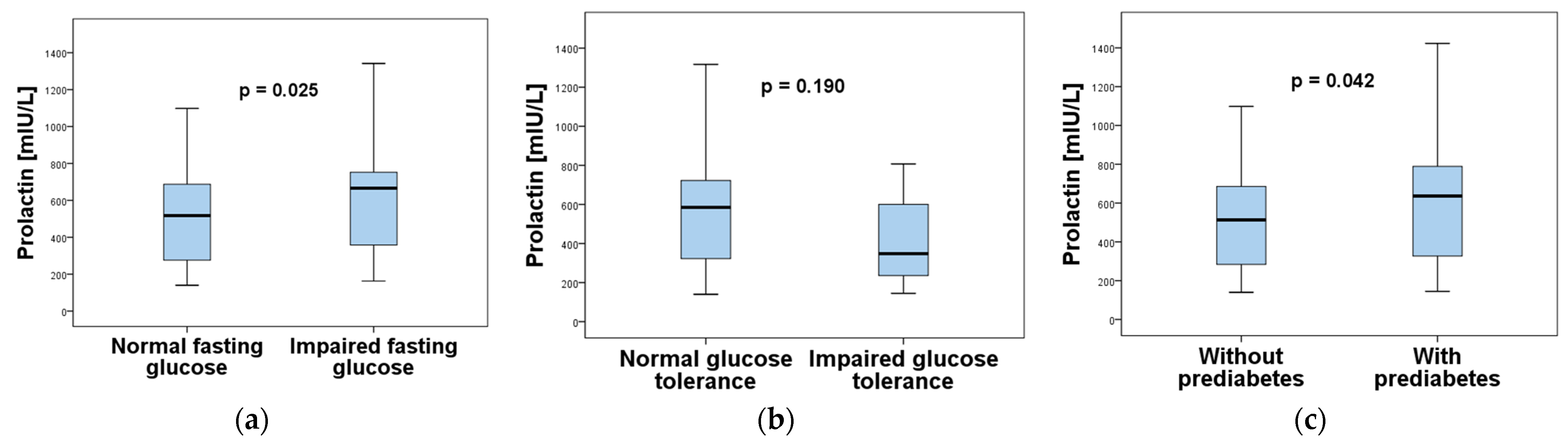
| Characteristics | Normoprolactinemia (n = 93) | Hyperprolactinemia (n = 64) | p-Value |
|---|---|---|---|
| Age (years) | 28.9 ± 5.0 | 28.7 ± 5.4 | 0.699 |
| Body mass index (kg/m2) | 24.0 (21.0–30.0) | 28.0 (23.5–33.8) | 0.007 * |
| FSH (mIU/L) | 5.78 (4.90–6.68) | 5.32 (4.6–6.49) | 0.222 |
| LH (mIU/L) | 9.90 (8.42–13.40) | 9.38 (7.19–11.01) | 0.104 |
| TSH (mIU/L) | 2.50 (1.42–3.41) | 2.39 (1.69–3.40) | 0.933 |
| AMH (ng/mL) | 7.90 (6.12–10.30) | 7.23(6.18–9.01) | 0.358 |
| Estradiol (pmol/L) | 142.0 (93.0–171.0) | 137.0 (97.3–204.5 | 0.571 |
| Testosterone (nmol/L) | 1.60 (1.12–2.10) | 1.57 (1.18–1.98) | 0.513 |
| DHEAS (nmol/L) | 8.81 (6.87–11.20) | 9.50 (6.74–12.30) | 0.342 |
| Androstenedione (nmol/L) | 5.87 (3.65–8.56) | 6.17 (4.21–8.16) | 0.751 |
| 17-OH Progesterone (nmol/L) | 2.12 (1.25–3.24) | 2.33 (1.64–3.70) | 0.138 |
| Fasting glucose (mmol/L) | 5.23 ± 0.55 | 5.48 ± 0.47 | 0.003 * |
| 1-h plasma glucose post-OGTT (mmol/L) | 7.25 (5.90–8.78) | 7.44 (5.47–8.65) | 0.686 |
| 2-h plasma glucose post-OGTT (mmol/L) | 5.98 (5.12–6.84) | 6.11 (5.10–7.01) | 0.939 |
| IRI (mIU/L) | 13.40 (8.51–17.98) | 18.48 (13.25–26.35) | <0.001 * |
| HOMA-IR | 2.97 (1.84–4.11) | 4.40 (3.02–6.29) | <0.001 * |
| HOMA-B | 154.6 (104.5–232.3) | 172.4 (133.9–263.3) | 0.083 |
| Prolactin | ||
|---|---|---|
| Characteristics | Correlation Coefficient (r) | p-Value |
| Age | −0.033 | 0.680 |
| BMI | 0.180 | 0.024 * |
| FSH | −0.087 | 0.280 |
| LH | −0.140 | 0.081 |
| TSH | −0.003 | 0.966 |
| AMH | −0.004 | 0.964 |
| Estradiol | 0.050 | 0.536 |
| Testosterone | 0.006 | 0.938 |
| DHEAS | 0.053 | 0.510 |
| Androstenedione | −0.033 | 0.685 |
| 17-OH Progesterone | 0.067 | 0.402 |
| Hyperprolactinemia | |||
|---|---|---|---|
| Characteristics | Unadjusted Odds Ratio (95% CI) | Chi-Square | p-Value |
| Overweight/Obesity | 2.59 (1.34–4.97) | 8.26 | 0.004 |
| Insulin resistance | 3.33 (1.54–7.19) | 9.90 | 0.002 |
| Prediabetes | 1.98 (1.02–3.85) | 4.11 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanachkova, V.; Stankova, T. Abnormally Increased Prolactin Levels in Women with Polycystic Ovarian Syndrome Are Associated with Risk of Obesity, Insulin Resistance and Prediabetes. Int. J. Mol. Sci. 2025, 26, 4239. https://doi.org/10.3390/ijms26094239
Yanachkova V, Stankova T. Abnormally Increased Prolactin Levels in Women with Polycystic Ovarian Syndrome Are Associated with Risk of Obesity, Insulin Resistance and Prediabetes. International Journal of Molecular Sciences. 2025; 26(9):4239. https://doi.org/10.3390/ijms26094239
Chicago/Turabian StyleYanachkova, Vesselina, and Teodora Stankova. 2025. "Abnormally Increased Prolactin Levels in Women with Polycystic Ovarian Syndrome Are Associated with Risk of Obesity, Insulin Resistance and Prediabetes" International Journal of Molecular Sciences 26, no. 9: 4239. https://doi.org/10.3390/ijms26094239
APA StyleYanachkova, V., & Stankova, T. (2025). Abnormally Increased Prolactin Levels in Women with Polycystic Ovarian Syndrome Are Associated with Risk of Obesity, Insulin Resistance and Prediabetes. International Journal of Molecular Sciences, 26(9), 4239. https://doi.org/10.3390/ijms26094239






