Based on the TLR4/NLRP3 Pathway and Its Impact on the Formation of NETs to Explore the Mechanism of Ginsenoside Rg1 on Acute Gouty Arthritis
Abstract
1. Introduction
2. Results
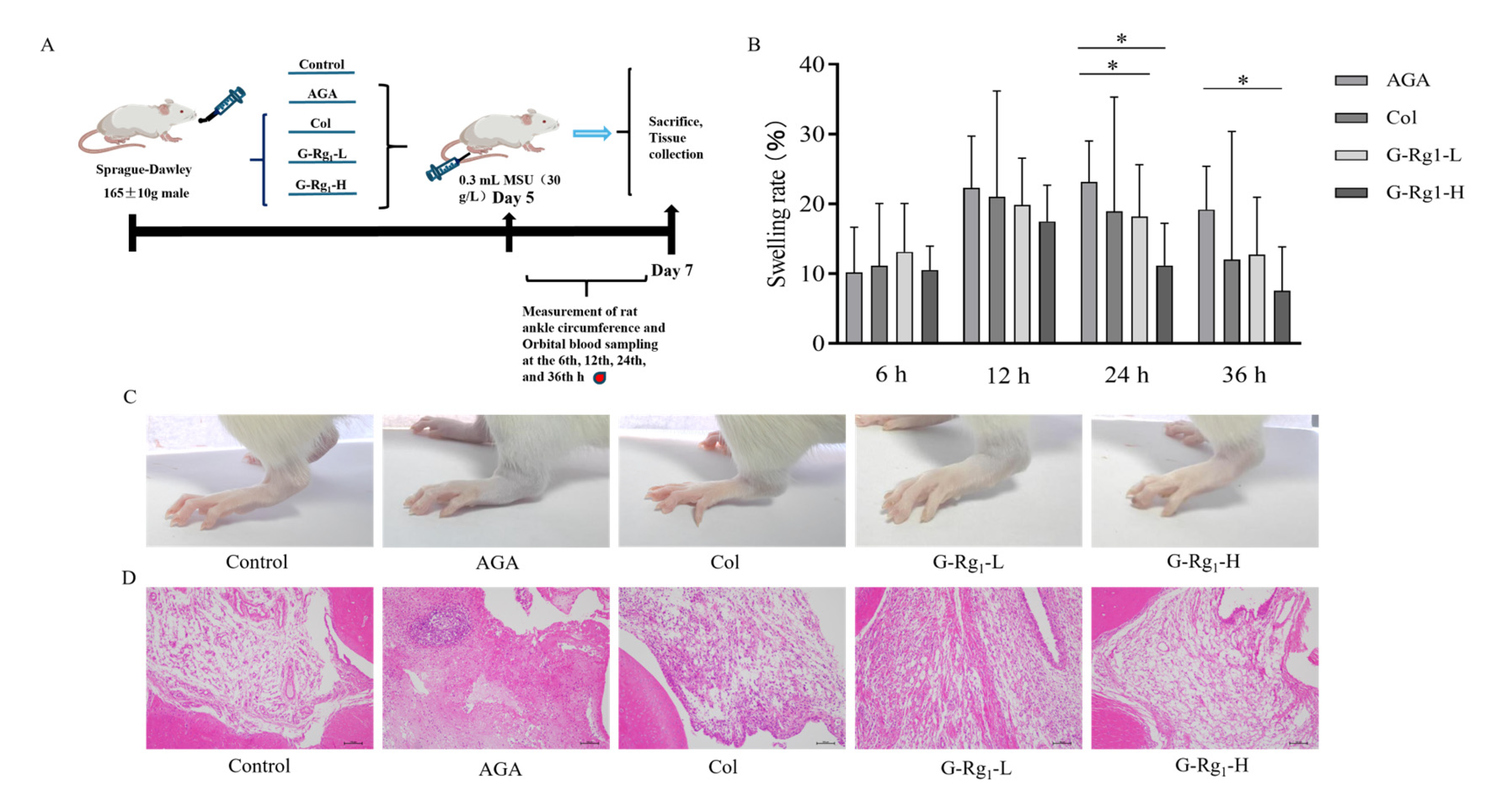
2.1. Effects of G-Rg1 on Ankle Joint Pathology in AGA Rats
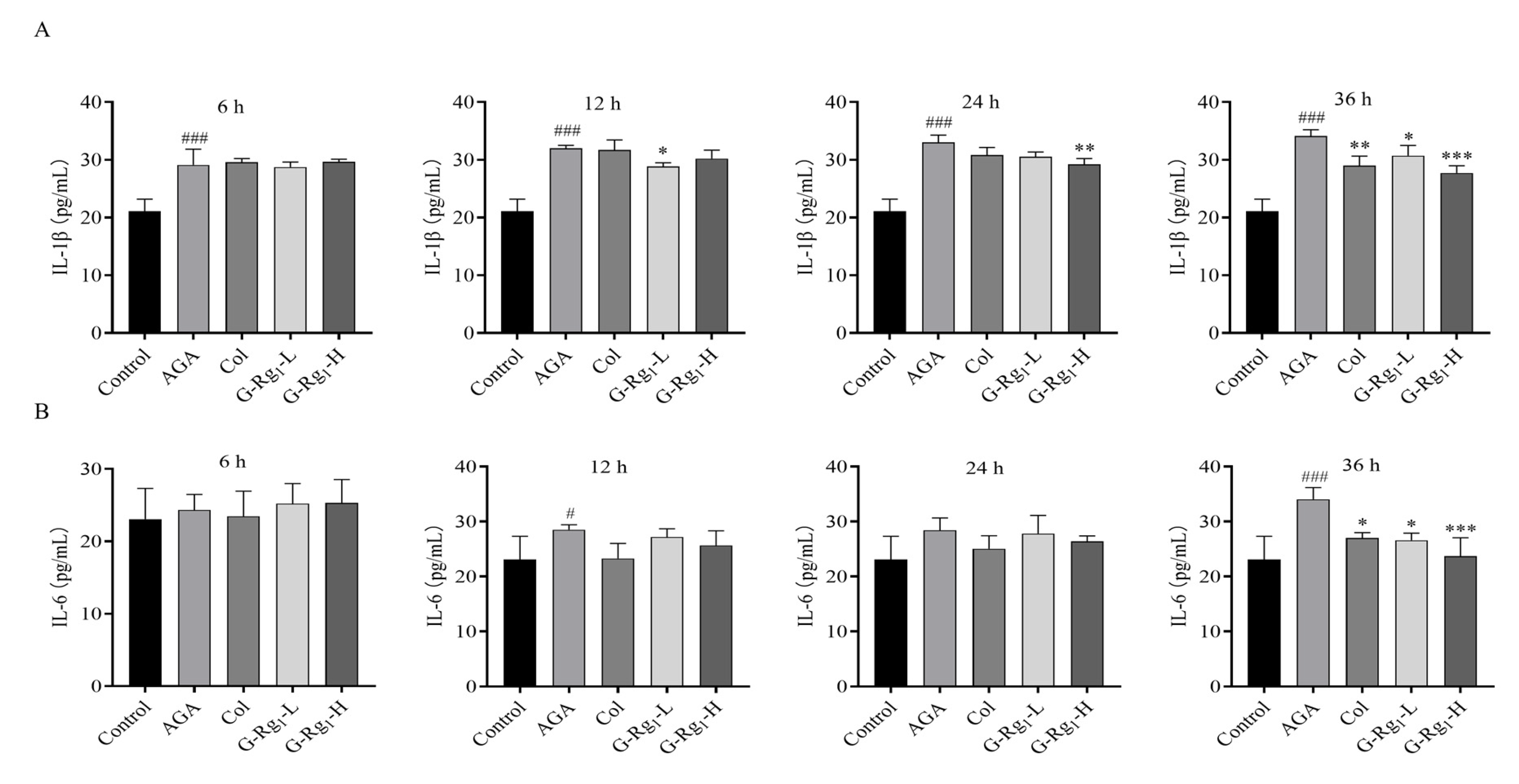
2.2. Effects of G-Rg1 on Serum Inflammatory Factors in AGA Rats
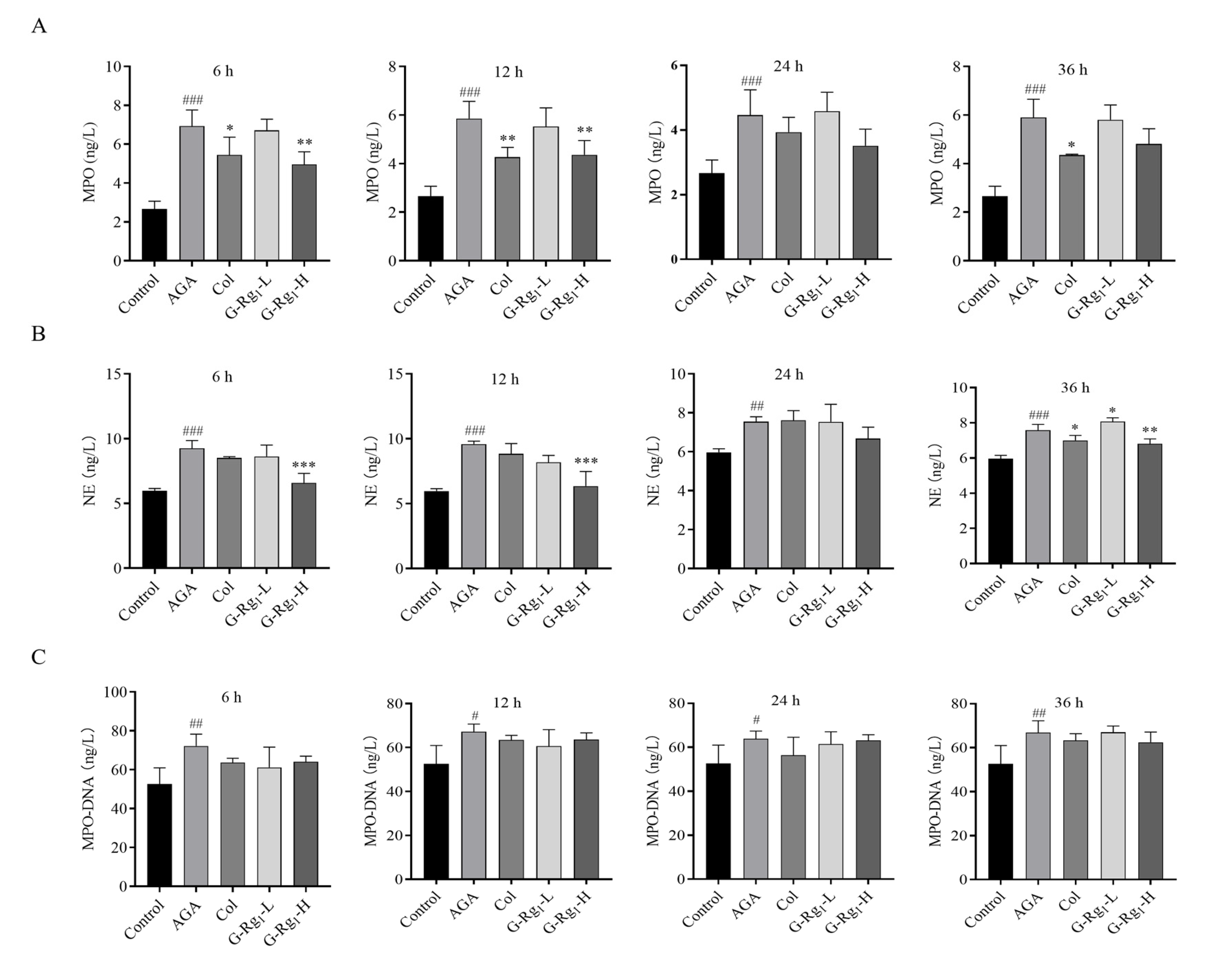
2.3. Effects of G-Rg1 on NETs Markers in the Serum of AGA Rats
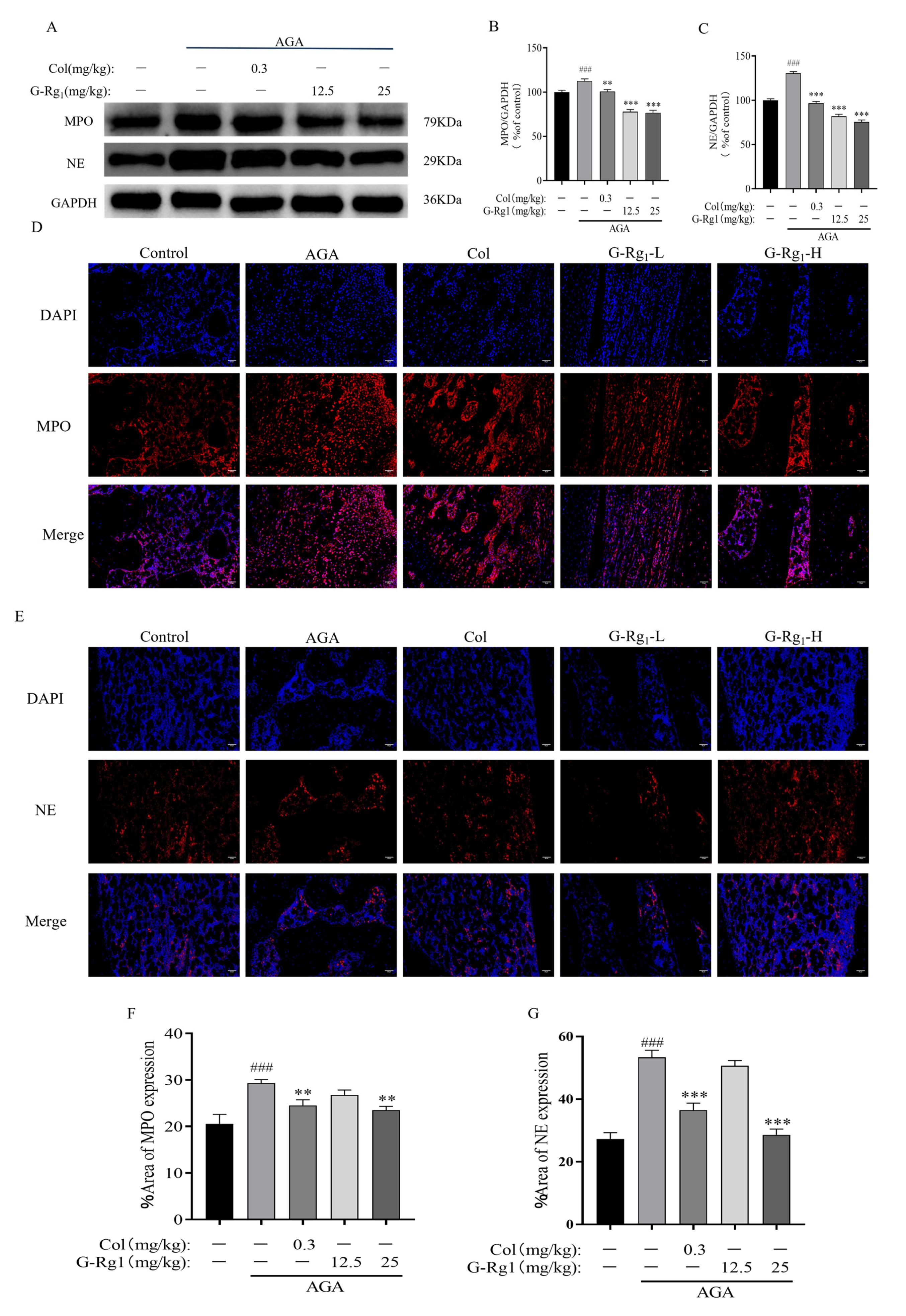
2.4. Effects of G-Rg1 on the Expression of NETs-Related Proteins in Synovial Tissues of AGA Rats
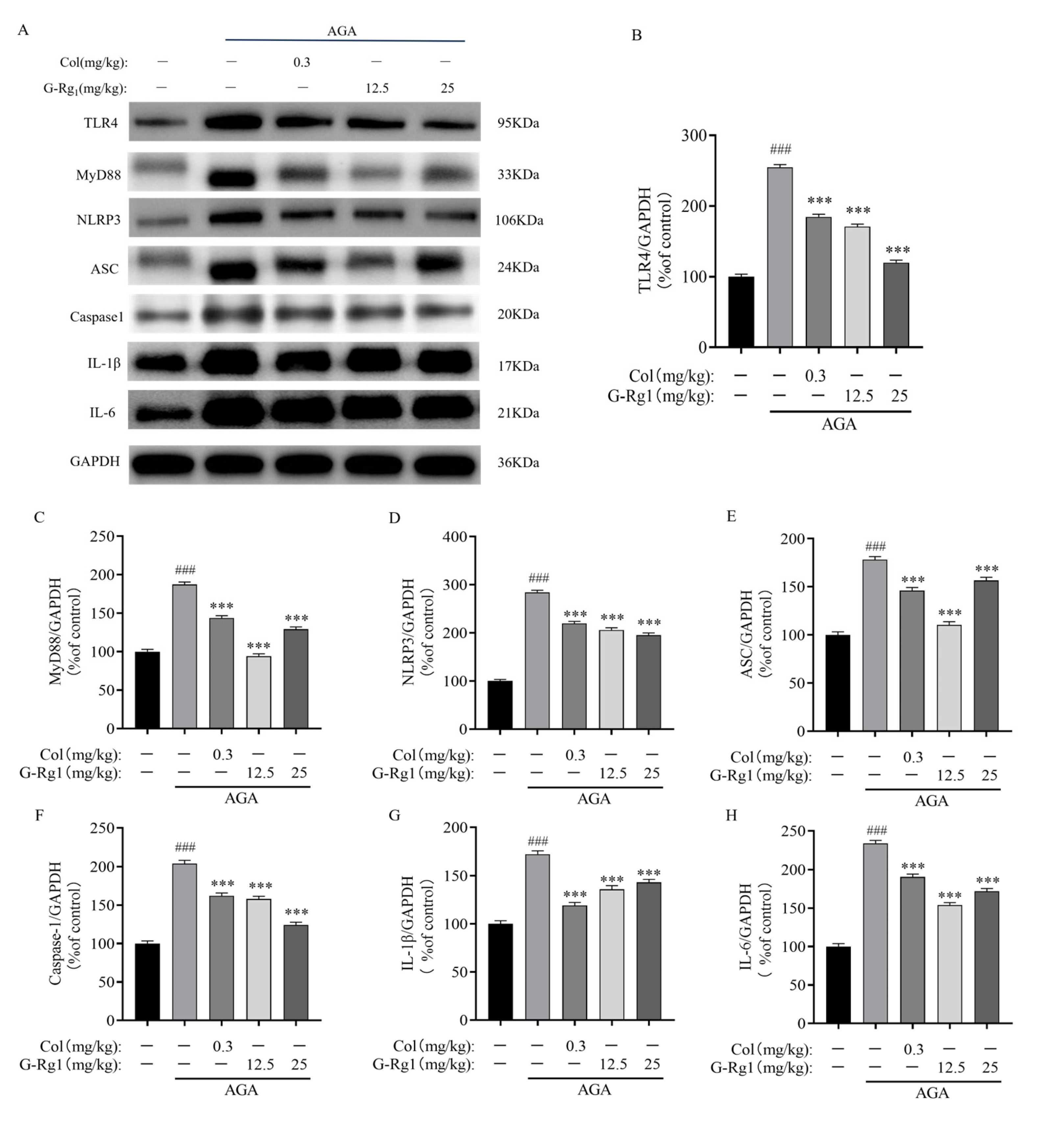
2.5. Effects of G-Rg1 on Protein Expression of the TLR4/NLRP3 Pathway in Synovial Tissues of AGA Rats
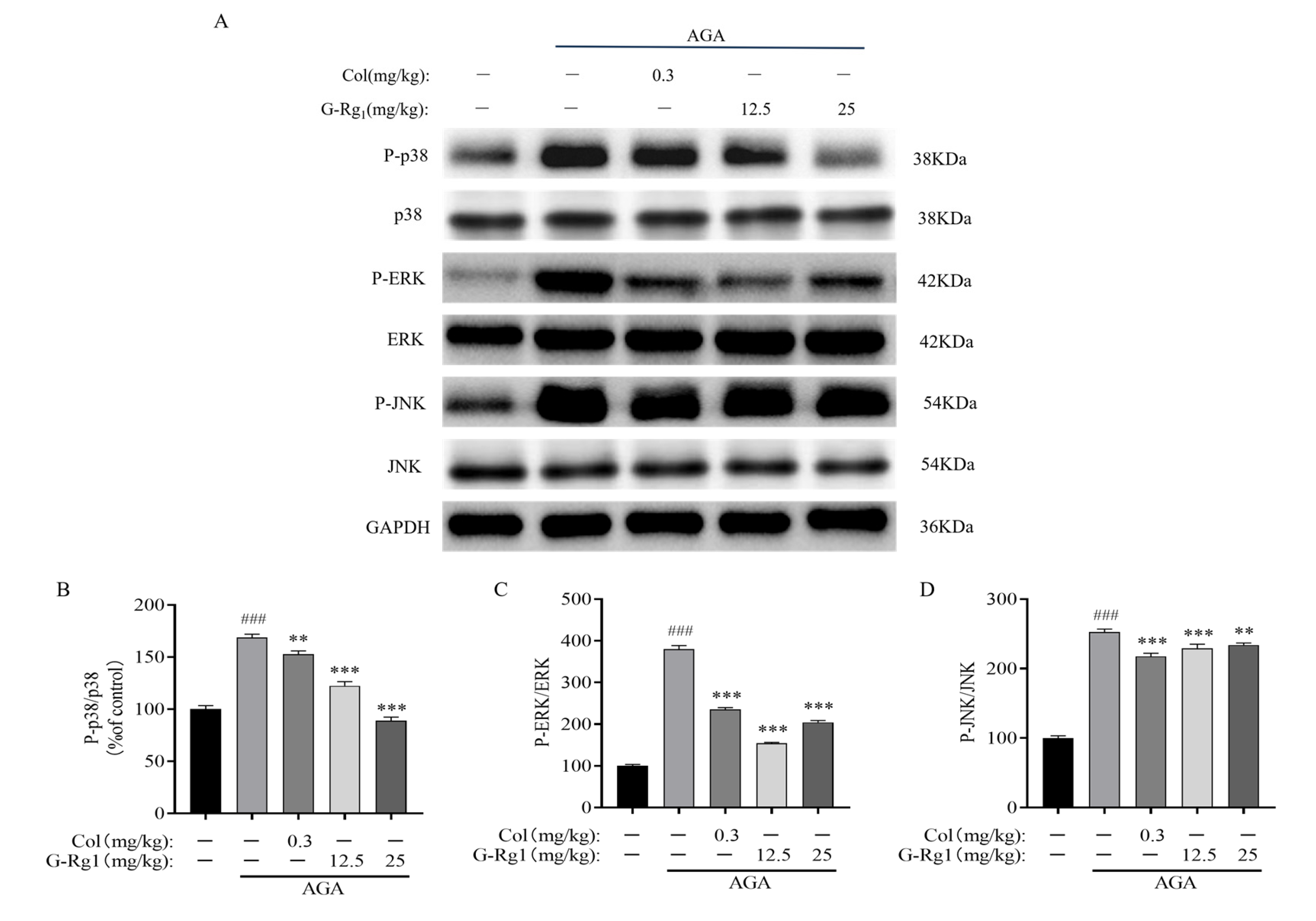
2.6. Effects of G-Rg1 on MAPK Pathway Protein Expression in Synovial Tissues of AGA Rats
3. Discussion
4. Materials and Methods
4.1. Instruments and Reagents
4.2. Experimental Animals
4.3. Methods
4.3.1. Animal Grouping and Establishment of the AGA Rat Model
4.3.2. Measurement of Rat Ankle Joint Swelling Rate
4.3.3. Histopathological and Morphological Analysis of Rat Ankle Synovial Tissue
4.3.4. Determination of NETs and Inflammatory Factors in Rat Serum
4.3.5. Analysis of NETs-Related Proteins and TLR4/NLRP3 Pathway Protein Expression in Rat Ankle Synovial Tissues
4.3.6. Detection of NETs in Rat Ankle Joint Synovial Tissue
4.3.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- So, A. Developments in the scientific and clinical understanding of gout. Arthritis Res. Ther. 2008, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Gaffo, A.L.; Schumacher, H.R.; Saag, K.G.; Taylor, W.J.; Dinnella, J.; Outman, R.; Chen, L.; Dalbeth, N.; Sivera, F.; Vázquez-Mellado, J.; et al. Developing a provisional definition of flare in patients with established gout. Arthritis Rheum. 2012, 64, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Mbuyi, N.; Hood, C. An update on gout diagnosis and management for the primary care provider. Nurse Pract. 2020, 45, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Scott, P.; Sydlaske, A.; Rose, D.M.; Terkeltaub, R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005, 52, 2936–2946. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Lin, X.; Wang, H.; An, X.; Zhang, J.; Kuang, J.; Hou, J.; Yan, M. Baeckein E suppressed NLRP3 inflammasome activation through inhibiting both the priming and assembly procedure: Implications for gout therapy. Phytomedicine 2021, 84, 153521. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Li, L.; Chen, S.; Quan, Y.; Yi, J.; Zeng, J.; Li, Y.; Zhao, J.; Yin, Z. Mechanism Investigation of Wuwei Shexiang Pills on Gouty Arthritis via Network Pharmacology, Molecule Docking, and Pharmacological Verification. Evid. Based Complement. Alternat. Med. 2022, 2022, 2377692. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Ye, X.J.; He, X.H.; Ouyang, D.Y. The Signaling Pathways Regulating NLRP3 Inflammasome Activation. Inflammation 2021, 44, 1229–1245. [Google Scholar] [CrossRef]
- de Jong, M.M.E.; Fokkema, C.; Papazian, N.; Czeti, Á.; Appelman, M.K.; Vermeulen, M.; van Heusden, T.; Hoogenboezem, R.M.; van Beek, G.; Tahri, S.; et al. An IL-1β-driven neutrophil-stromal cell axis fosters a BAFF-rich protumor microenvironment in individuals with multiple myeloma. Nat. Immunol. 2024, 25, 820–833. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, K.; Mao, R.; Zhong, D.; Xu, Z.; Xu, J.; Xiong, M. Ginsenoside Rg1 inhibits oxidative stress and inflammation in rats with spinal cord injury via Nrf2/HO-1 signaling pathway. Neuroreport 2022, 33, 81–89. [Google Scholar] [CrossRef]
- Luo, M.; Yan, D.; Sun, Q.; Tao, J.; Xu, L.; Sun, H.; Zhao, H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J. Cell. Biochem. 2020, 121, 2994–3004. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, Y.; Zhu, C.; Ren, T.; Zhang, Y.; Xiao, L.; Fang, B. Ginsenoside Rg1 Mitigates Porcine Intestinal Tight Junction Disruptions Induced by LPS through the p38 MAPK/NLRP3 Inflammasome Pathway. Toxics 2022, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Stoimenou, M.; Tzoros, G.; Skendros, P.; Chrysanthopoulou, A. Methods for the Assessment of NET Formation: From Neutrophil Biology to Translational Research. Int. J. Mol. Sci. 2022, 23, 15823. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Liu, Y.; Zhao, Y.; Herrmann, M. Neutrophil Extracellular Traps Formation and Aggregation Orchestrate Induction and Resolution of Sterile Crystal-Mediated Inflammation. Front. Immunol. 2018, 9, 1559. [Google Scholar] [CrossRef]
- Zhang, B.; Lian, W.; Zhao, J.; Wang, Z.; Liu, A.; Du, G. DL0410 Alleviates Memory Impairment in D-Galactose-Induced Aging Rats by Suppressing Neuroinflammation via the TLR4/MyD88/NF-κB Pathway. Oxid. Med. Cell. Longev. 2021, 4, 6521146. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kambas, K.; Chrysanthopoulou, A.; Skendros, P.; Apostolidou, E.; Kourtzelis, I.; Drosos, G.I.; Boumpas, D.T.; Ritis, K. Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS ONE 2011, 6, e29318. [Google Scholar] [CrossRef]
- Jeong, J.H.; Choi, S.J.; Ahn, S.M.; Oh, J.S.; Kim, Y.G.; Lee, C.K.; Yoo, B.; Hong, S. Neutrophil extracellular trap clearance by synovial macrophages in gout. Arthritis Res. Ther. 2021, 23, 88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yu, Y.; Sun, Q.; Li, Z.; Huo, X.; Sha, J.; Qu, D.; Sun, Y. Based on the TLR4/NLRP3 Pathway and Its Impact on the Formation of NETs to Explore the Mechanism of Ginsenoside Rg1 on Acute Gouty Arthritis. Int. J. Mol. Sci. 2025, 26, 4233. https://doi.org/10.3390/ijms26094233
Li Z, Yu Y, Sun Q, Li Z, Huo X, Sha J, Qu D, Sun Y. Based on the TLR4/NLRP3 Pathway and Its Impact on the Formation of NETs to Explore the Mechanism of Ginsenoside Rg1 on Acute Gouty Arthritis. International Journal of Molecular Sciences. 2025; 26(9):4233. https://doi.org/10.3390/ijms26094233
Chicago/Turabian StyleLi, Zhiman, Yang Yu, Qiang Sun, Zhilong Li, Xiaohui Huo, Jiyue Sha, Di Qu, and Yinshi Sun. 2025. "Based on the TLR4/NLRP3 Pathway and Its Impact on the Formation of NETs to Explore the Mechanism of Ginsenoside Rg1 on Acute Gouty Arthritis" International Journal of Molecular Sciences 26, no. 9: 4233. https://doi.org/10.3390/ijms26094233
APA StyleLi, Z., Yu, Y., Sun, Q., Li, Z., Huo, X., Sha, J., Qu, D., & Sun, Y. (2025). Based on the TLR4/NLRP3 Pathway and Its Impact on the Formation of NETs to Explore the Mechanism of Ginsenoside Rg1 on Acute Gouty Arthritis. International Journal of Molecular Sciences, 26(9), 4233. https://doi.org/10.3390/ijms26094233







