Structure and Inhibition of the Human Na+/H+ Exchanger SLC9B2
Abstract
1. Introduction
2. Results
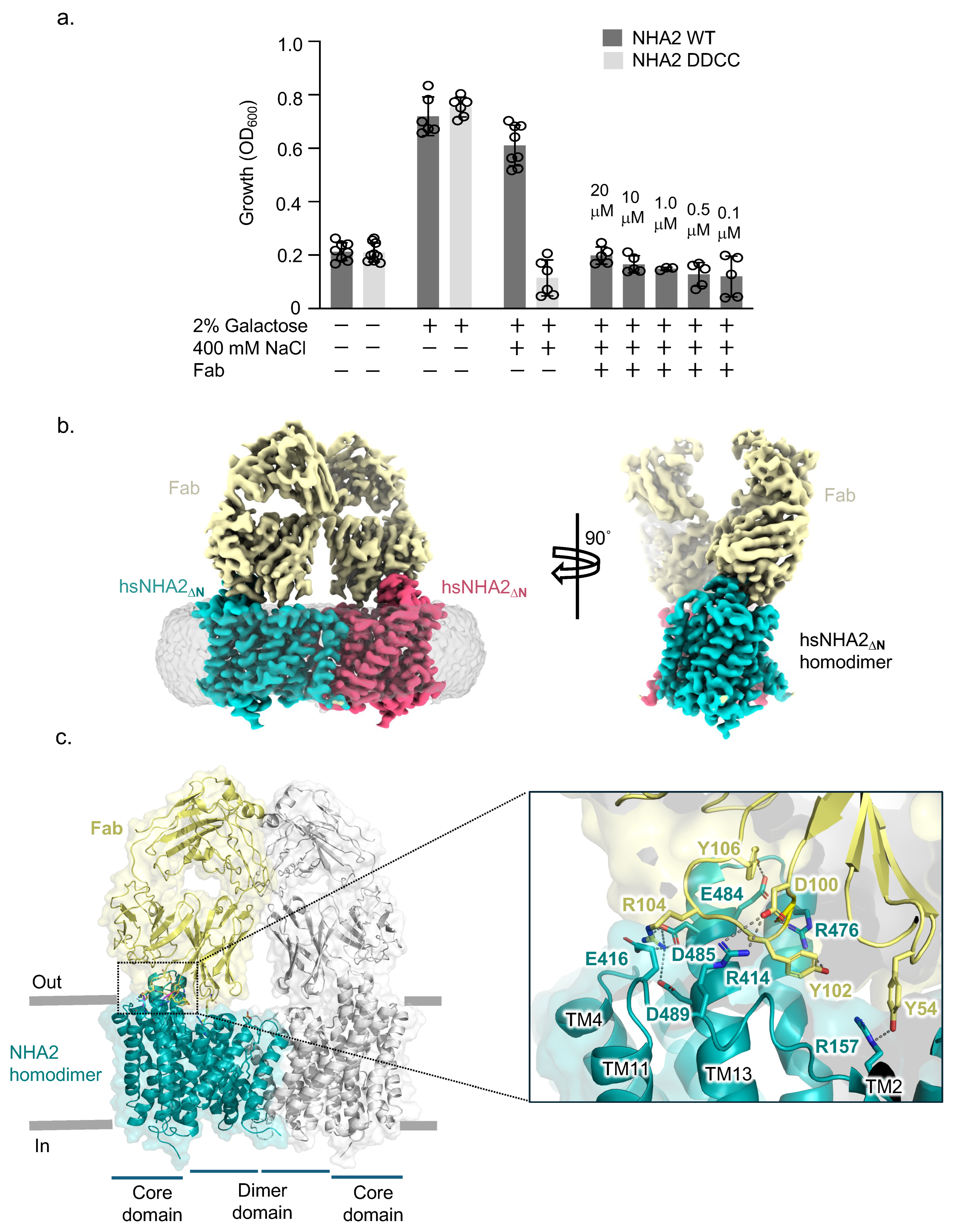
2.1. Single Particle Cryogenic Electron Microscopy Structure of Human NHA2-Fab Complex
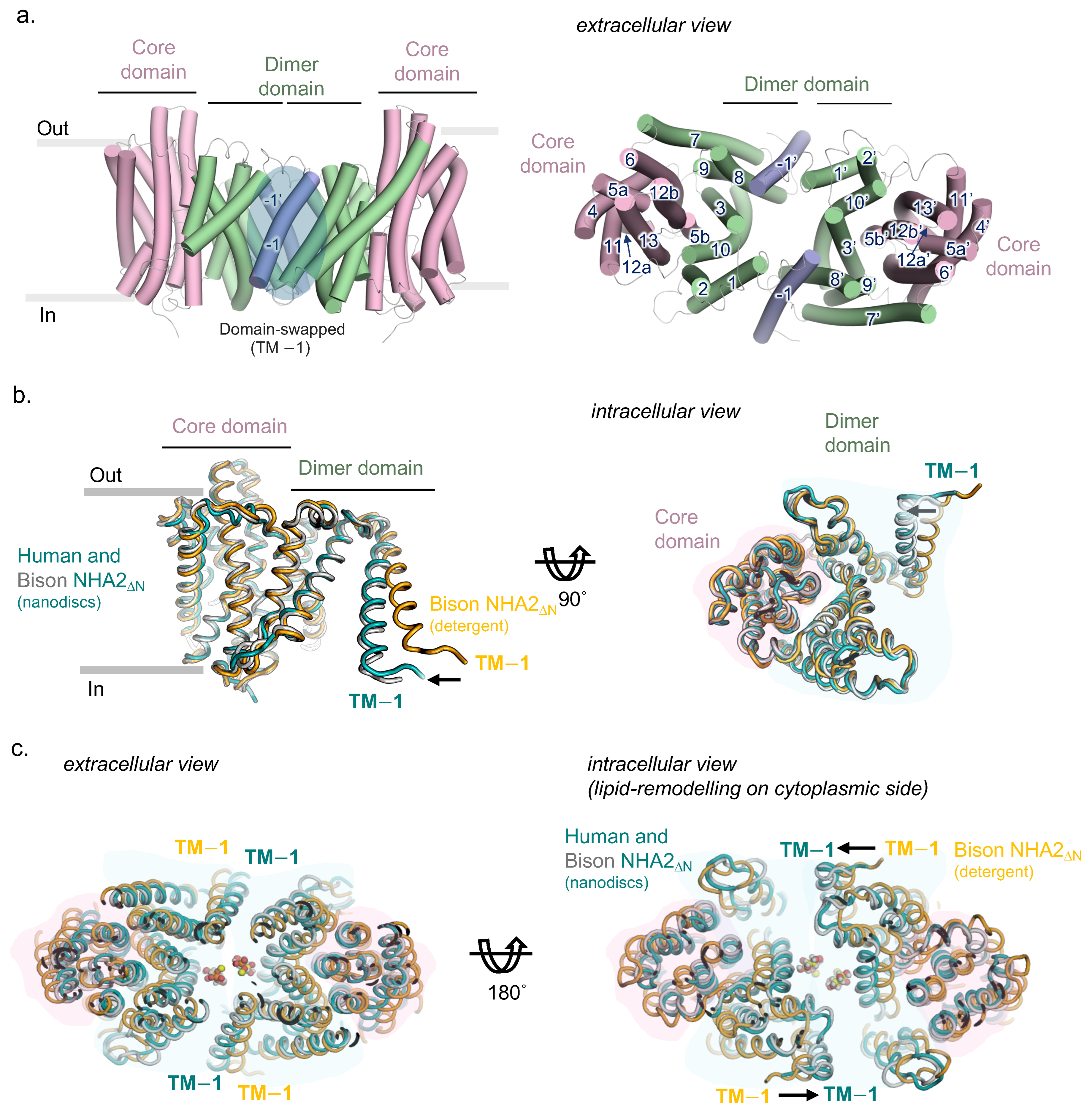
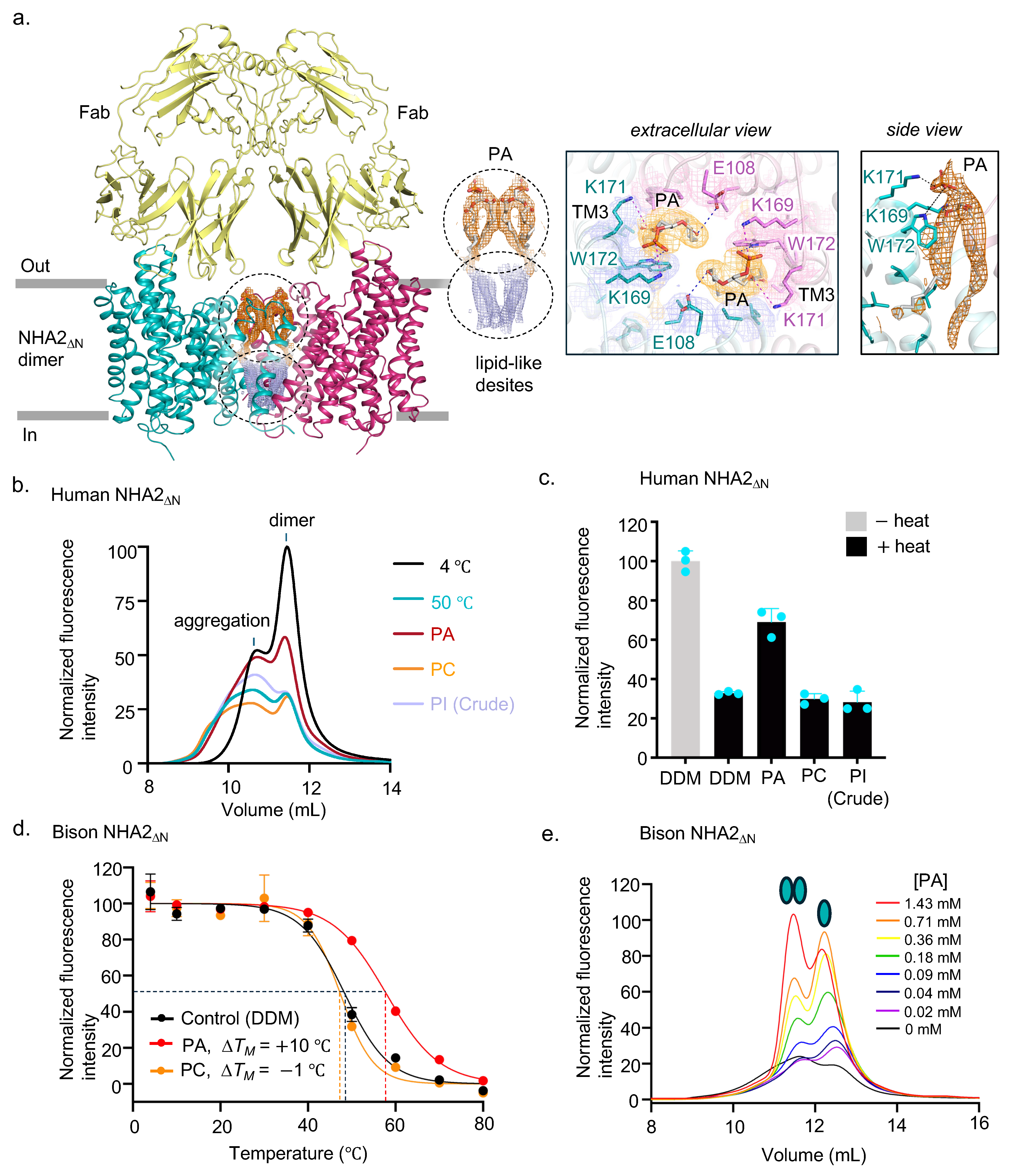
2.2. Homodimer Positioning and Lipid-Mediated Dimerization
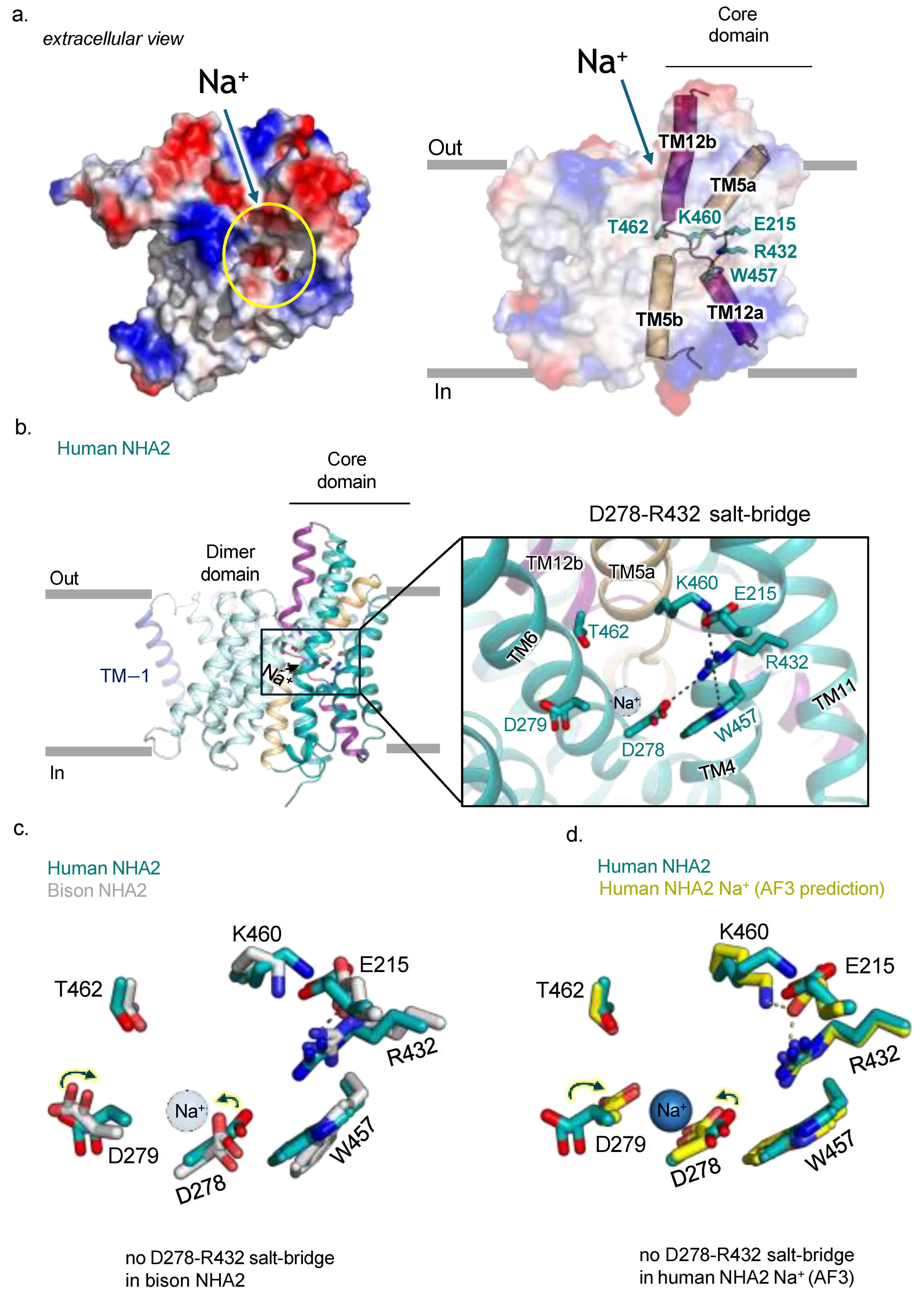
2.3. Ion Binding Site in Human NHA2 Reveals New Salt Bridge Interactions
2.4. Human NHA2∆N in Complex with the Inhibitor Phloretin
3. Discussion
4. Materials and Methods
4.1. Human NHA2∆N and Variants Expression and Purification
4.2. Cryo-EM Sample Preparation
4.3. Determination of Lipid Preferences of NHA2ΔΝ by GFP-Based Thermal Shift Assay
4.4. Generation of the Fab
4.5. Cryo-EM Grid Sample Preparation and Data Acquisition
4.6. Cryo-EM Data Processing
4.7. Model Building and Refinement
4.8. Complementation Assay for Human NHA2 Against Salt Stress in Yeast
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NHA | Na+/H+ antiporter |
| SLC | Solute carrier |
| TM | Transmembrane |
| PA | Phosphatidic acid |
| PI | Phosphatidylinositol |
| WNK4-NCC | With-no-lysine kinase 4-sodium-chloride cotransporter |
| MSP1E2 | Membrane scaffold protein 1E2 |
| Cryo-EM | Cryogenic electron microscopy |
| FSC | Fourier shell correlation |
| DDM | n-dodecyl-ß-D-maltoside |
| TEV | Tobacco etch virus |
| GFP | Green fluorescent protein |
| CHS | Cholesteryl hemisuccinate |
| β-OG | n-Octyl β-D-glucopyranoside |
| FSEC | Fluorescence-detection size-exclusion chromatography |
| FSEC-TS | FSEC-based thermostability assay |
| CTF | Contrast transfer function |
| RBMC | Reference-based motion correction |
| AF3 | Alphafold3 |
References
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Counillon, L. The SLC9A-C Mammalian Na+/H+ Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol. Rev. 2019, 99, 2015–2113. [Google Scholar] [CrossRef]
- Fuster, D.G.; Alexander, R.T. Traditional and Emerging Roles for the SLC9 Na+/H+ Exchangers. Pflugers Arch. 2014, 466, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.L.; Donowitz, M.; Rao, R. Evolutionary Origins of Eukaryotic Sodium/Proton Exchangers. Am. J. Physiol. Cell Physiol. 2005, 288, C223–C239. [Google Scholar] [CrossRef]
- Slepkov, E.R.; Rainey, J.K.; Sykes, B.D.; Fliegel, L. Structural and Functional Analysis of the Na+/H+ Exchanger. Biochem. J. 2007, 401, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zachos, N.C.; Tse, M.; Donowitz, M. Molecular Physiology of Intestinal Na+/H+ Exchange. Annu. Rev. Physiol. 2005, 67, 411–443. [Google Scholar] [CrossRef]
- Donowitz, M.; Mohan, S.; Zhu, C.X.; Chen, T.E.; Lin, R.; Cha, B.; Zachos, N.C.; Murtazina, R.; Sarker, R.; Li, X. NHE3 Regulatory Complexes. J. Exp. Biol. 2009, 212, 1638–1646. [Google Scholar] [CrossRef]
- Matsuoka, R.; Fudim, R.; Jung, S.; Zhang, C.; Bazzone, A.; Chatzikyriakidou, Y.; Robinson, C.V.; Nomura, N.; Iwata, S.; Landreh, M.; et al. Structure, Mechanism and Lipid-Mediated Remodeling of the Mammalian Na+/H+ Exchanger NHA2. Nat. Struct. Mol. Biol. 2022, 29, 108–120. [Google Scholar] [CrossRef]
- Lee, C.; Kang, H.J.; von Ballmoos, C.; Newstead, S.; Uzdavinys, P.; Dotson, D.L.; Iwata, S.; Beckstein, O.; Cameron, A.D.; Drew, D. A Two-Domain Elevator Mechanism for Sodium/Proton Antiport. Nature 2013, 501, 573–577. [Google Scholar] [CrossRef]
- Fuster, D.G.; Zhang, J.; Shi, M.; Bobulescu, I.A.; Andersson, S.; Moe, O.W. Characterization of the Sodium/Hydrogen Exchanger NHA2. J. Am. Soc. Nephrol. 2008, 19, 1547–1556. [Google Scholar] [CrossRef]
- Xiang, M.; Feng, M.; Muend, S.; Rao, R. A Human Na+/H+ Antiporter Sharing Evolutionary Origins with Bacterial NhaA May Be a Candidate Gene for Essential Hypertension. Proc. Natl. Acad. Sci. USA 2007, 104, 18677–18681. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, M.A.; Gyimesi, G.; Ho, T.M.; Hediger, M.A.; Fuster, D.G. The Less Well-Known Little Brothers: The SLC9B/NHA Sodium Proton Exchanger Subfamily-Structure, Function, Regulation and Potential Drug-Target Approaches. Front. Physiol. 2022, 13, 898508. [Google Scholar] [CrossRef]
- Mangili, R.; Bending, J.J.; Scott, G.; Li, L.K.; Gupta, A.; Viberti, G. Increased Sodium-Lithium Countertransport Activity in Red Cells of Patients with Insulin-Dependent Diabetes and Nephropathy. N. Engl. J. Med. 1988, 318, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Canessa, M.; Adragna, N.; Solomon, H.S.; Connolly, T.M.; Tosteson, D.C. Increased Sodium-Lithium Countertransport in Red Cells of Patients with Essential Hypertension. N. Engl. J. Med. 1980, 302, 772–776. [Google Scholar] [CrossRef]
- Vaccaro, O.; Cuomo, V.; Trevisan, M.; Cirillo, M.; Panarelli, W.; Laurenzi, M.; Mancini, M.; Riccardi, G.; Group Gubbio Study Research. Enhanced Na-Li Countertransport: A Marker of Inherited Susceptibility to Type 2 Diabetes. Int. J. Epidemiol. 2005, 34, 1123–1128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kondapalli, K.C.; Kallay, L.M.; Muszelik, M.; Rao, R. Unconventional Chemiosmotic Coupling of NHA2, a Mammalian Na+/H+ Antiporter, to a Plasma Membrane H+ Gradient. J. Biol. Chem. 2012, 287, 36239–36250. [Google Scholar] [CrossRef]
- Kondapalli, K.C.; Alexander, R.T.; Pluznick, J.L.; Rao, R. NHA2 Is Expressed in Distal Nephron and Regulated by Dietary Sodium. J. Physiol. Biochem. 2017, 73, 199–205. [Google Scholar] [CrossRef]
- Anderegg, M.A.; Albano, G.; Hanke, D.; Deisl, C.; Uehlinger, D.E.; Brandt, S.; Bhardwaj, R.; Hediger, M.A.; Fuster, D.G. The Sodium/Proton Exchanger NHA2 Regulates Blood Pressure through a WNK4-NCC Dependent Pathway in the Kidney. Kidney Int. 2021, 99, 350–363. [Google Scholar] [CrossRef]
- Deisl, C.; Simonin, A.; Anderegg, M.; Albano, G.; Kovacs, G.; Ackermann, D.; Moch, H.; Dolci, W.; Thorens, B.; Hediger, M.A.; et al. Sodium/Hydrogen Exchanger NHA2 Is Critical for Insulin Secretion in Beta-Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10004–10009. [Google Scholar] [CrossRef]
- Fafournoux, P.; Noel, J.; Pouyssegur, J. Evidence That Na+/H+ Exchanger Isoforms NHE1 and NHE3 Exist as Stable Dimers in Membranes with a High Degree of Specificity for Homodimers. J. Biol. Chem. 1994, 269, 2589–2596. [Google Scholar] [CrossRef]
- Winklemann, I.; Matsuoka, R.; Meier, P.F.; Shutin, D.; Zhang, C.; Orellana, L.; Sexton, R.; Landreh, M.; Robinson, C.V.; Beckstein, O.; et al. Structure and Elevator Mechanism of the Mammalian Sodium/Proton Exchanger NHE9. EMBO J. 2020, 39, e105908. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, Y.; Ilie, A.; Kim, D.; Boucher, A.; Li, B.; Zhang, X.C.; Orlowski, J.; Zhao, Y. Structure and Mechanism of the Human NHE1-CHP1 Complex. Nat. Commun. 2021, 12, 3474. [Google Scholar] [CrossRef]
- Coincon, M.; Uzdavinys, P.; Nji, E.; Dotson, D.L.; Winkelmann, I.; Abdul-Hussein, S.; Cameron, A.D.; Beckstein, O.; Drew, D. Crystal Structures Reveal the Molecular Basis of Ion Translocation in Sodium/Proton Antiporters. Nat. Struct. Mol. Biol. 2016, 23, 248–255. [Google Scholar] [CrossRef]
- Drew, D.; Boudker, O. Shared Molecular Mechanisms of Membrane Transporters. Annu. Rev. Biochem. 2016, 85, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Paulino, C.; Hizlan, D.; Vonck, J.; Yildiz, O.; Kuhlbrandt, W. Structure of the Archaeal Na+/H+ Antiporter NhaP1 and Functional Role of Transmembrane Helix 1. EMBO J. 2011, 30, 439–449. [Google Scholar] [CrossRef]
- Paulino, C.; Wohlert, D.; Kapotova, E.; Yildiz, O.; Kuhlbrandt, W. Structure and Transport Mechanism of the Sodium/Proton Antiporter MjNhaP1. eLife 2014, 3, e03583. [Google Scholar] [CrossRef] [PubMed]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ Antiporter and Insights into Mechanism of Action and Regulation by pH. Nature 2005, 435, 1197–1202. [Google Scholar] [CrossRef]
- Kokane, S.; Gulati, A.; Meier, P.F.; Matsuoka, R.; Pipatpolkai, T.; Albano, G.; Ho, T.M.; Delemotte, L.; Fuster, D.; Drew, D. PIP2-Mediated Oligomerization of the Endosomal Sodium/Proton Exchanger NHE9. Nat. Commun. 2025, 16, 3055. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of Cell Volume Regulation in Vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef]
- Kariuki, S.N.; Marin-Menendez, A.; Introini, V.; Ravenhill, B.J.; Lin, Y.C.; Macharia, A.; Makale, J.; Tendwa, M.; Nyamu, W.; Kotar, J.; et al. Red Blood Cell Tension Protects against Severe Malaria in the Dantu Blood Group. Nature 2020, 585, 579–583. [Google Scholar] [CrossRef]
- Prasad, H.; Dang, D.K.; Kondapalli, K.C.; Natarajan, N.; Cebotaru, V.; Rao, R. NHA2 Promotes Cyst Development in an in vitro Model of Polycystic Kidney Disease. J. Physiol. 2019, 597, 499–519. [Google Scholar] [CrossRef]
- Maresova, L.; Sychrova, H. Physiological Characterization of Saccharomyces Cerevisiae Kha1 Deletion Mutants. Mol. Microbiol. 2005, 55, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Mehta, V.; Gulati, A.; Drew, D. Structure and Electromechanical Coupling of a Voltage-Gated Na+/H+ Exchanger. Nature 2023, 623, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.; Moe, O.W.; Hilgemann, D.W. Steady-State Function of the Ubiquitous Mammalian Na/H Exchanger (NHE1) in Relation to Dimer Coupling Models with 2Na/2H Stoichiometry. J. Gen. Physiol. 2008, 132, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Kokane, S.; Perez-Boerema, A.; Alleva, C.; Meier, P.F.; Matsuoka, R.; Drew, D. Structure and Mechanism of the K+/H+ Exchanger KefC. Nat. Commun. 2024, 15, 4751. [Google Scholar] [CrossRef]
- Masrati, G.; Dwivedi, M.; Rimon, A.; Gluck-Margolin, Y.; Kessel, A.; Ashkenazy, H.; Mayrose, I.; Padan, E.; Ben-Tal, N. Broad Phylogenetic Analysis of Cation/Proton Antiporters Reveals Transport Determinants. Nat. Commun. 2018, 9, 4205. [Google Scholar] [CrossRef]
- Lee, C.; Yashiro, S.; Dotson, D.L.; Uzdavinys, P.; Iwata, S.; Sansom, M.S.; von Ballmoos, C.; Beckstein, O.; Drew, D.; Cameron, A.D. Crystal Structure of the Sodium-Proton Antiporter NhaA Dimer and New Mechanistic Insights. J. Gen. Physiol. 2014, 144, 529–544. [Google Scholar] [CrossRef]
- Winkelmann, I.; Uzdavinys, P.; Kenney, I.M.; Brock, J.; Meier, P.F.; Wagner, L.M.; Gabriel, F.; Jung, S.; Matsuoka, R.; von Ballmoos, C.; et al. Crystal Structure of the Na+/H+ Antiporter NhaA at Active pH Reveals the Mechanistic Basis for pH Sensing. Nat. Commun. 2022, 13, 6383. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Zimmermannova, O.; Kubes, M.; Przeczkova, T.; Masrati, G. Residues R177 and S178 of the Human Na+/H+ Antiporter NHA2 Are Involved in Its Inhibition by the Flavonoid Phloretin. FEBS Lett. 2024, 599, 901–911. [Google Scholar] [CrossRef]
- Bolla, J.R.; Corey, R.A.; Sahin, C.; Gault, J.; Hummer, A.; Hopper, J.T.S.; Lane, D.P.; Drew, D.; Allison, T.M.; Stansfeld, P.J.; et al. A Mass-Spectrometry-Based Approach to Distinguish Annular and Specific Lipid Binding to Membrane Proteins. Angew. Chem. Int. Ed. Engl. 2020, 59, 3523–3528. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma Membranes Are Asymmetric in Lipid Unsaturation, Packing and Protein Shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; Boudker, O. Ion and Lipid Orchestration of Secondary Active Transport. Nature 2024, 626, 963–974. [Google Scholar] [CrossRef]
- Shin, J.J.; Loewen, C.J. Putting the pH into Phosphatidic Acid Signaling. BMC Biol. 2011, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Doktorova, M.; Symons, J.L.; Levental, I. Structural and Functional Consequences of Reversible Lipid Asymmetry in Living Membranes. Nat. Chem. Biol. 2020, 16, 1321–1330. [Google Scholar] [CrossRef]
- Masrati, G.; Kessel, A.; Ben-Tal, N. Cation/Proton Antiporters: Novel Structure-Driven Pharmaceutical Opportunities. Trends Pharmacol. Sci. 2023, 44, 258–262. [Google Scholar] [CrossRef]
- Karmazyn, M. NHE-1: Still a Viable Therapeutic Target. J. Mol. Cell Cardiol. 2013, 61, 77–82. [Google Scholar] [CrossRef]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. Disrupting Proton Dynamics and Energy Metabolism for Cancer Therapy. Nat. Rev. Cancer 2013, 13, 611–623. [Google Scholar] [CrossRef]
- Li, Y.; Song, J.; Mikusevic, V.; Marden, J.J.; Becerril, A.; Kuang, H.; Wang, B.; Rice, W.J.; Mindell, J.A.; Wang, D.N. Substrate Translocation and Inhibition in Human Dicarboxylate Transporter NaDC3. Nat. Struct. Mol. Biol. 2025, 32, 502–512. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, R.; Guan, C.; Zhang, Y.; Chen, Z.; Hoerer, S.; Nar, H.; Chen, L. Structural Basis of Inhibition of the Human SGLT2-MAP17 Glucose Transporter. Nature 2022, 601, 280–284. [Google Scholar] [CrossRef]
- Drew, D.; Newstead, S.; Sonoda, Y.; Kim, H.; von Heijne, G.; Iwata, S. GFP-Based Optimization Scheme for the Overexpression and Purification of Eukaryotic Membrane Proteins in Saccharomyces Cerevisiae. Nat. Protoc. 2008, 3, 784–798. [Google Scholar] [CrossRef]
- Newstead, S.; Kim, H.; von Heijne, G.; Iwata, S.; Drew, D. High-Throughput Fluorescent-Based Optimization of Eukaryotic Membrane Protein Overexpression and Purification in Saccharomyces Cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 13936–13941. [Google Scholar] [CrossRef]
- Chatzikyriakidou, Y.; Ahn, D.H.; Nji, E.; Drew, D. The GFP Thermal Shift Assay for Screening Ligand and Lipid Interactions to Solute Carrier Transporters. Nat. Protoc. 2021, 16, 5357–5376. [Google Scholar] [CrossRef] [PubMed]
- Nji, E.; Chatzikyriakidou, Y.; Landreh, M.; Drew, D. An Engineered Thermal-Shift Screen Reveals Specific Lipid Preferences of Eukaryotic and Prokaryotic Membrane Proteins. Nat. Commun. 2018, 9, 4253. [Google Scholar] [CrossRef] [PubMed]
- Punjani, A.; Rubinstein, J.L.; Fleet, D.J.; Brubaker, M.A. CryoSPARC: Algorithms for Rapid Unsupervised Cryo-EM Structure Determination. Nat. Methods 2017, 14, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and Development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Afonine, P.V.; Poon, B.K.; Read, R.J.; Sobolev, O.V.; Terwilliger, T.C.; Urzhumtsev, A.; Adams, P.D. Real-Space Refinement in PHENIX for Cryo-EM and Crystallography. Acta Crystallogr. D Struct. Biol. 2018, 74, 531–544. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Kokane, S.; Li, H.; Iwata, S.; Nomura, N.; Drew, D. Structure and Inhibition of the Human Na+/H+ Exchanger SLC9B2. Int. J. Mol. Sci. 2025, 26, 4221. https://doi.org/10.3390/ijms26094221
Jung S, Kokane S, Li H, Iwata S, Nomura N, Drew D. Structure and Inhibition of the Human Na+/H+ Exchanger SLC9B2. International Journal of Molecular Sciences. 2025; 26(9):4221. https://doi.org/10.3390/ijms26094221
Chicago/Turabian StyleJung, Sukkyeong, Surabhi Kokane, Hang Li, So Iwata, Norimichi Nomura, and David Drew. 2025. "Structure and Inhibition of the Human Na+/H+ Exchanger SLC9B2" International Journal of Molecular Sciences 26, no. 9: 4221. https://doi.org/10.3390/ijms26094221
APA StyleJung, S., Kokane, S., Li, H., Iwata, S., Nomura, N., & Drew, D. (2025). Structure and Inhibition of the Human Na+/H+ Exchanger SLC9B2. International Journal of Molecular Sciences, 26(9), 4221. https://doi.org/10.3390/ijms26094221




