In Vitro Immunomodulatory Effects of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Primed with a Cannabidiol-Rich Extract
Abstract
1. Introduction
2. Results
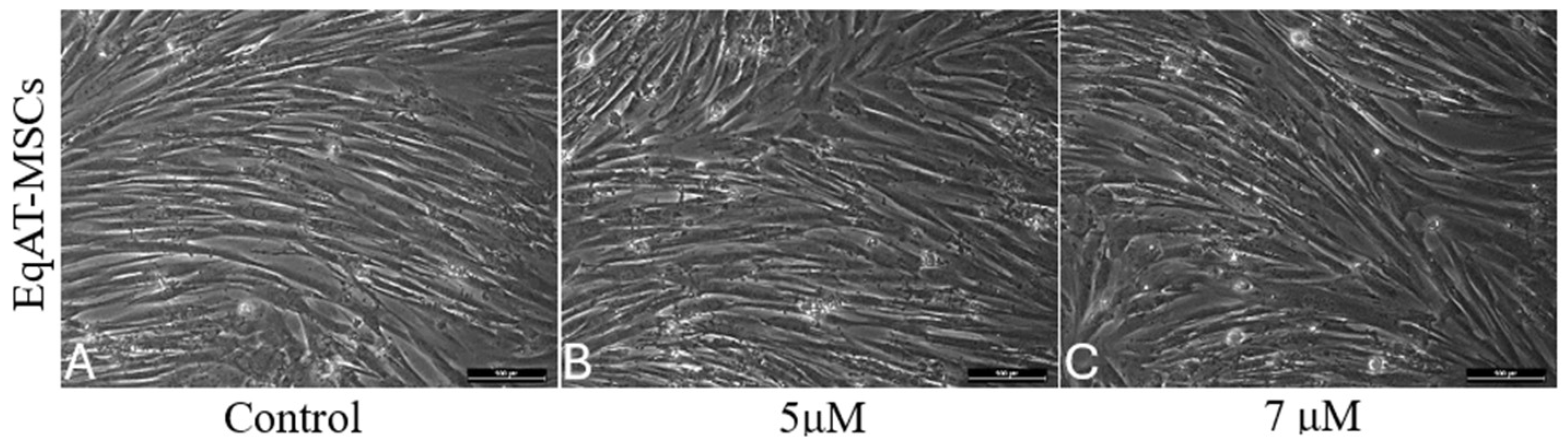
2.1. Morphological Changes
2.2. Cellular Metabolic Activity
2.3. β-Galactosidase Activity
2.4. Apoptosis Detection
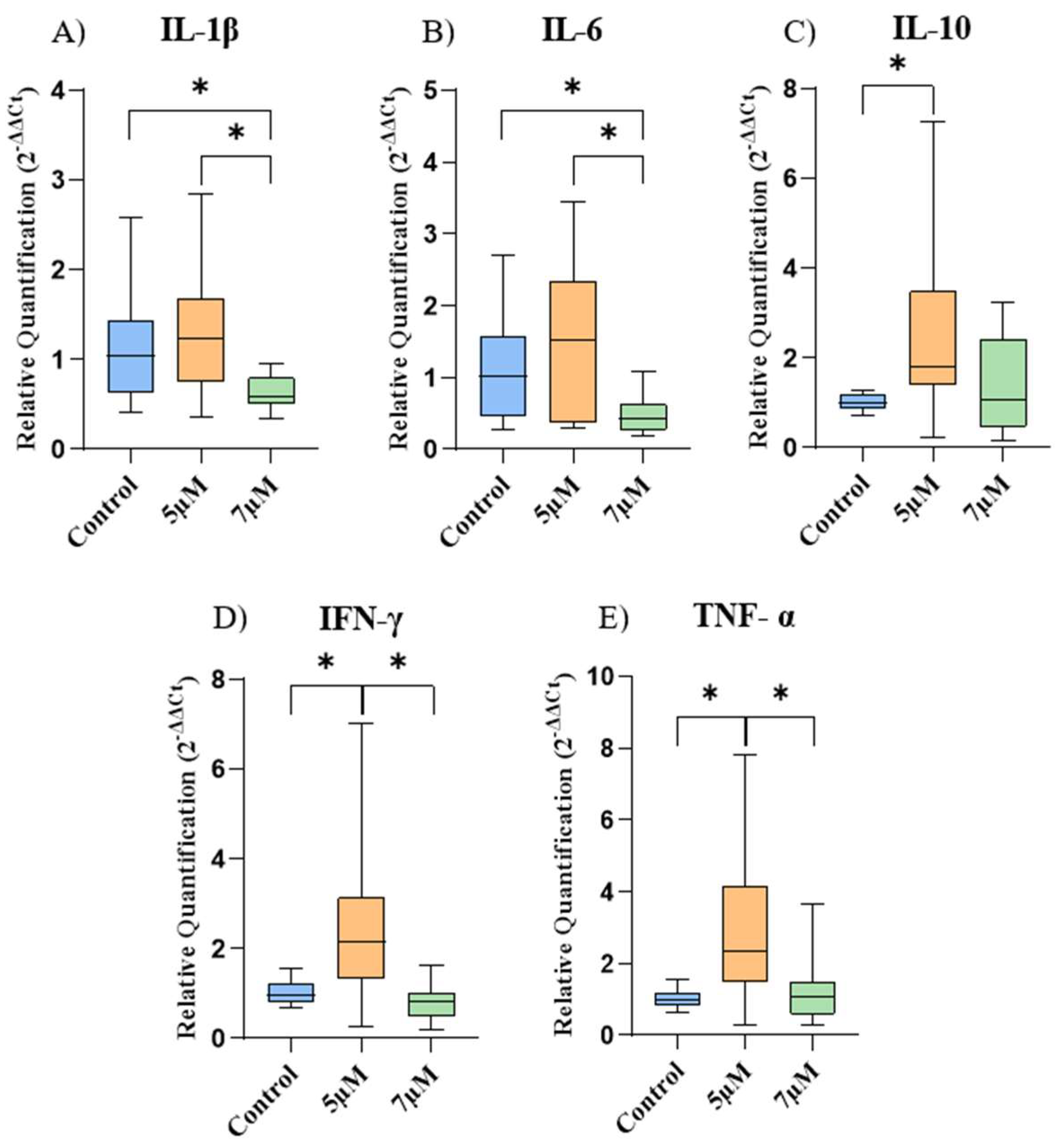
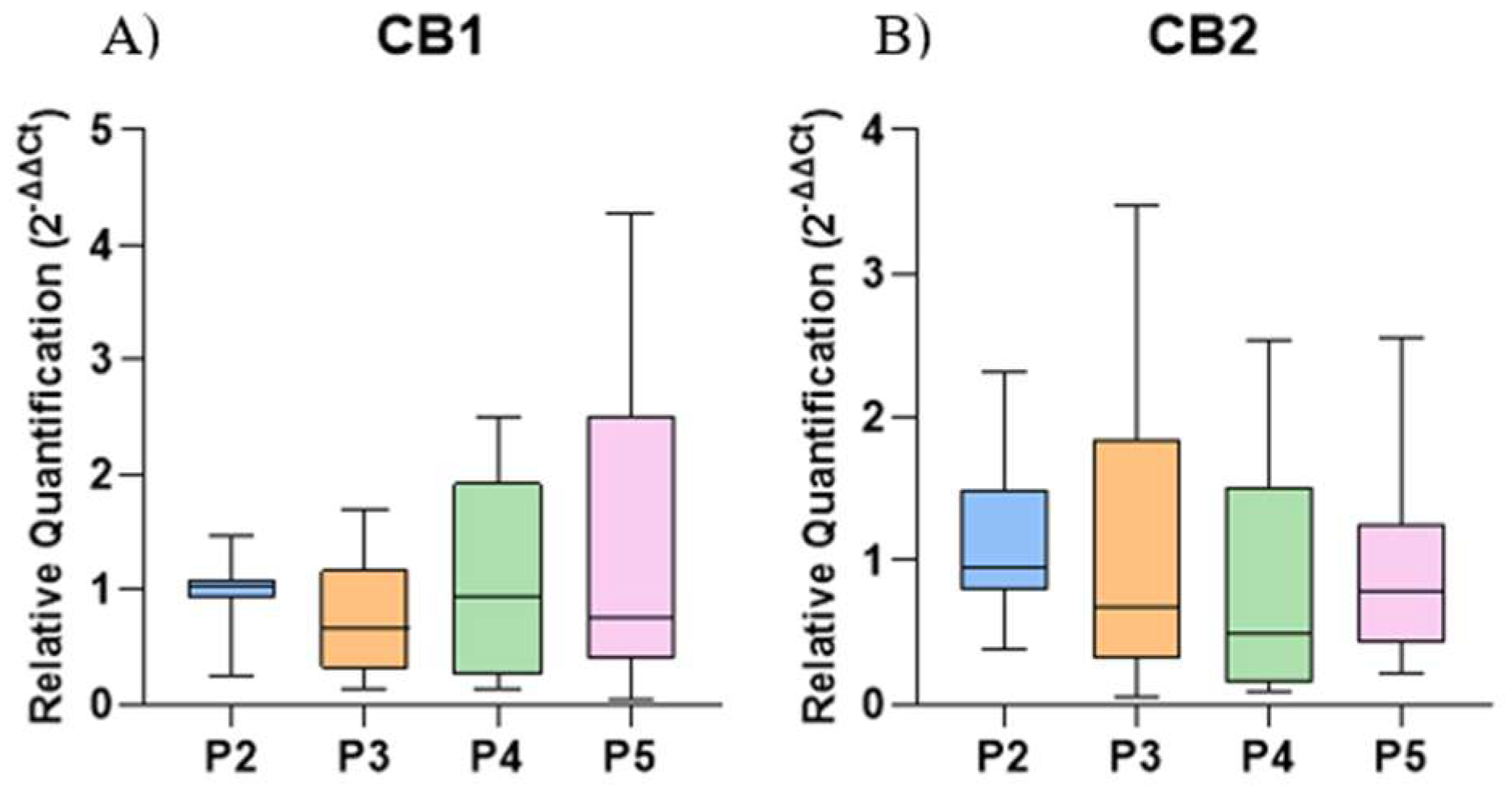
2.5. Gene Expression of Cytokines and Cannabinoid Receptors
3. Discussion
4. Materials and Methods
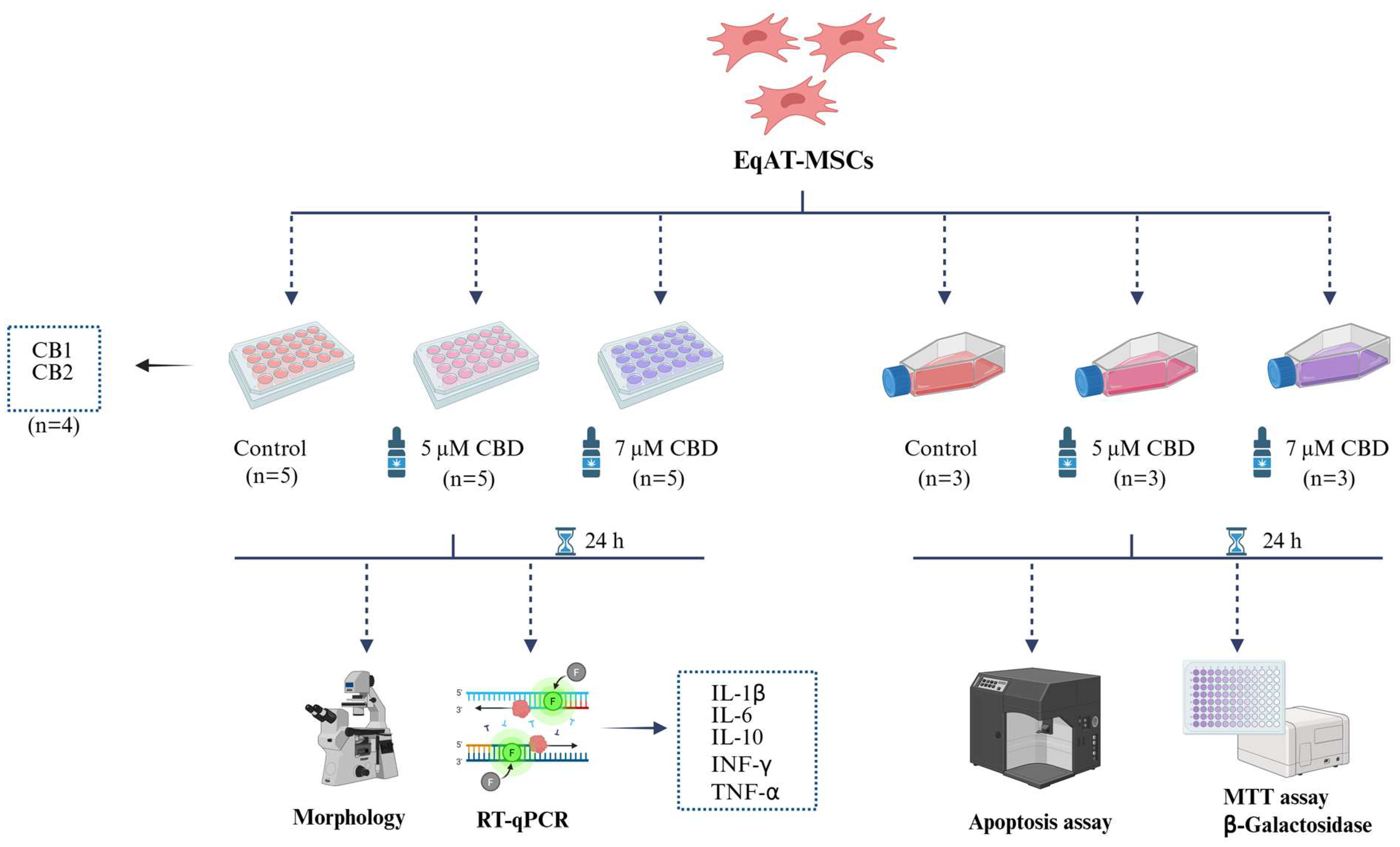
4.1. Experimental Design
4.2. EqAT-MSCs
4.3. CBD-Rich Cannabis Extract
4.4. EqAT-MSCs Primed with CBD-Rich Cannabis Extract
4.5. Morphological Evaluation
4.6. MTT Assay
4.7. Senescence-Associated β-Galactosidase Activity Assay
4.8. Apoptosis Assay
4.9. Gene Expression
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal stem cells for neurological disorders. Adv. Sci. 2021, 8, 2002944. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Alvites, R.; Branquinho, M.; Sousa, A.C.; Lopes, B.; Sousa, P.; Maurício, A.C. Mesenchymal stem/stromal cells and their paracrine activity—Immunomodulation mechanisms and how to influence the therapeutic potential. Pharmaceutics 2022, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.M.; Liu, K.J.; Hsu, P.J.; Wei, C.F.; Bai, C.H.; Ho, L.J.; Sytwu, H.K.; Yen, B.L. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J. Leukoc. Biol. 2014, 96, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Maličev, E.; Jazbec, K. An overview of mesenchymal stem cell heterogeneity and concentration. Pharmaceuticals 2024, 17, 350. [Google Scholar] [CrossRef]
- Smith, R.K.; Garvican, E.R.; Fortier, L.A. The current ‘state of play’ of regenerative medicine in horses: What the horse can tell the human. Regen. Med. 2014, 9, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Baptista, P.M.; Consiglio, A.L.; Melotti, L.; Patruno, M.; Jenner, F.; Feichter, E.S.; Dutton, L.C.; Connolly, D.J.; Steenbeek, F.G.V.; et al. Large animal models in regenerative medicine and tissue engineering: To do or not to do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef]
- Haider, K.H. Priming mesenchymal stem cells to develop “super stem cells”. World J. Stem Cells 2024, 16, 623. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Oliveira, C.C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Al-Mrahleh, M.; Matar, S.; Jafar, H.; Wehaibi, S.; Aslam, N.; Awidi, A. Human Wharton’s jelly-derived mesenchymal stromal cells primed by tumor necrosis factor-α and interferon-γ modulate the innate and adaptive immune cells of type 1 diabetic patients. Front. Immunol. 2021, 12, 732549. [Google Scholar] [CrossRef]
- Amorim, R.M.; Clark, K.C.; Walker, N.J.; Kumar, P.; Herout, K.; Borjesson, D.L.; Wang, A. Placenta-derived multipotent mesenchymal stromal cells: A promising potential cell-based therapy for canine inflammatory brain disease. Stem Cell Res. Ther. 2020, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Albareda, J.; Prades, M.; Gosálvez, J.; Roy, R.; Zaragoza, P.; Martín-Burriel, I.; et al. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: Implications in immunomodulation–immunogenicity balance, cell viability, and differentiation potential. Stem Cells Dev. 2017, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, H.; Pourfathollah, A.A.; Zarif, M.N.; Hashemi, S.M. Increasing proliferation of murine adipose tissue-derived mesenchymal stem cells by TNF-α plus IFN-γ. Immunopharmacol. Immunotoxicol. 2016, 38, 68–76. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current strategies to enhance adipose stem cell function: An update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.V.O.; Kamura, B.C.; Oliveira, J.P.M.; Chimenes, N.D.; Carvalho, M.; Santos, L.A.; Dias-Melicio, L.A.; Amorim, R.L.; Amorim, R.M. In vitro transdifferentiation potential of equine mesenchymal stem cells into Schwann-like cells. Stem Cells Dev. 2023, 32, 422–432. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng. Part B Rev. 2022, 28, 966–977. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Martínez, V.; De-Hond, A.I.; Borrelli, F.; Capasso, R.; Castillo, M.D.; Abalo, R. Cannabidiol and other non-psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: Useful nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Gemma, A.; Ferrari, M.; Cosentino, M.; Marino, F. Effects of cannabidiol on innate immunity: Experimental evidence and clinical relevance. Int. J. Mol. Sci. 2023, 24, 3125. [Google Scholar] [CrossRef]
- Fellous, T.; Maio, F.; Kalkan, H.; Carannante, B.; Boccella, S.; Petrosino, S.; Maione, S.; Marzo, V.; Iannotti, F.A. Phytocannabinoids promote viability and functional adipogenesis of bone marrow-derived mesenchymal stem cells through different molecular targets. Biochem. Pharmacol. 2020, 175, 113859. [Google Scholar] [CrossRef]
- Li, L.; Feng, J.; Sun, L.; Xuan, Y.W.; Wen, L.; Li, Y.X.; Yang, S.; Zhu, B.; Tian, X.Y.; Li, S.; et al. Cannabidiol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in the inflammatory microenvironment via the CB2-dependent p38 MAPK signaling pathway. Int. J. Stem Cells 2022, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, A.; Marycz, K.; Garbowska, K.K.; Kornicka, J.; Zadrozny, M.B.; Groborz, S. Cannabidiol (CBD) protects adipose-derived mesenchymal stem cells (ASCs) against endoplasmic reticulum stress development and its complications. Int. J. Environ. Res. Public Health 2022, 19, 10864. [Google Scholar] [CrossRef]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front. Physiol. 2016, 7, 559. [Google Scholar] [CrossRef]
- Yu, L.; Zeng, L.; Zhang, Z.; Zhu, G.; Xu, Z.; Xia, J.; Weng, J.; Li, J.; Pathak, J.L. Cannabidiol rescues TNF-α-inhibited proliferation, migration, and osteogenic/odontogenic differentiation of dental pulp stem cells. Biomolecules 2023, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xiao, D.; Xu, Y.; Zhao, J.; Jiang, L.; Hu, X.; Zhang, Y.; Yu, L. Up-regulation of immunomodulatory effects of mouse bone-marrow derived mesenchymal stem cells by tetrahydrocannabinol pre-treatment involving cannabinoid receptor CB2. Oncotarget 2016, 7, 6436–6447. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Vitetta, L.; Quezada, M.; Hall, S. Enhancing endocannabinoid control of stress with cannabidiol. J. Clin. Med. 2021, 10, 5852. [Google Scholar] [CrossRef]
- Stasiulewicz, A.; Znajdek, K.; Grudzién, M.; Pawinski, T.; Sulkowska, J.I. A guide to targeting the endocannabinoid system in drug design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef]
- Lee, B.C.; Kang, K.S. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res. Ther. 2020, 11, 397. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: Current status and future prospects. Stem Cell Res. Ther. 2022, 13, 146. [Google Scholar] [CrossRef]
- Naya, N.M.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and cellular mechanisms of action of cannabidiol. Molecules 2023, 28, 5980. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Scionti, D.; Diomede, F.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol activates neuronal precursor genes in human gingival mesenchymal stromal cells. J. Cell. Biochem. 2017, 118, 1531–1546. [Google Scholar] [CrossRef]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Tedesco, M.; Battista, N.; Pasquariello, N.; Pucci, M.; Gasperi, V.; Scaldaferri, M.L.; Farini, D.; Felici, M.; Maccarrone, M. Characterization of the endocannabinoid system in mouse embryonic stem cells. Stem Cells Dev. 2011, 20, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, T.; Karthaus, N.; Kim, B.S.; Beier, J.P. The endocannabinoid receptors CB1 and CB2 affect the regenerative potential of adipose tissue MSCs. Exp. Cell Res. 2020, 389, 111881. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Libro, R.; Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the expression of Alzheimer’s disease-related genes in mesenchymal stem cells. Int. J. Mol. Sci. 2016, 18, 26. [Google Scholar] [CrossRef]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Ma, H.; Xu, F.; Liu, C.; Seeram, N.P. A network pharmacology approach to identify potential molecular targets for cannabidiol’s anti-inflammatory activity. Cannabis Cannabinoid Res. 2021, 6, 288–299. [Google Scholar] [CrossRef]
- Liu, C.; Ma, H.; Slitt, A.L.; Seeram, N.P. Inhibitory effect of cannabidiol on the activation of NLRP3 inflammasome is associated with its modulation of the P2X7 receptor in human monocytes. J. Nat. Prod. 2020, 83, 2025–2029. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids as Key Regulators of Inflammasome Signaling: A Current Perspective. Front. Immunol. 2021, 11, 613613. [Google Scholar] [CrossRef] [PubMed]
- Minshawi, F.; Lanvermann, S.; McKenzie, E.; Jeffery, R.; Couper, K.; Papoutsopoulou, S.; Roers, A.; Muller, W. The generation of an engineered interleukin-10 protein with improved stability and biological function. Front. Immunol. 2020, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Chang, D.; Liu, Y.; Liu, T.; Zhou, X. Neuroprotection of cannabidiol, its synthetic derivatives and combination preparations against microglia-mediated neuroinflammation in neurological disorders. Molecules 2022, 27, 4961. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Roche, P.A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22–27. [Google Scholar] [CrossRef]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 2019, 44, 101344. [Google Scholar] [CrossRef]
- Turner, S.; Barker, V.D.; Adams, A.A. Effects of cannabidiol on the in vitro lymphocyte pro-inflammatory cytokine production of senior horses. J. Equine Vet. Sci. 2021, 103, 103668. [Google Scholar] [CrossRef]
- Turner, S.; Knych, H.K.; Adams, A.A. The effects of cannabidiol on immune function and health parameters in senior horses. Vet. Immunol. Immunopathol. 2023, 257, 110549. [Google Scholar] [CrossRef]
- Li, X.; Köner, H.; Liu, X. Susceptibility to intracellular infections: Contributions of TNF to immune defense. Front. Microbiol. 2020, 11, 1643. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Barberini, D.J.; Freitas, N.P.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; Landim-Alvarenga, F.C.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: Immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 25. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward | Reverse |
|---|---|---|
| CB1 | GAGCAAGGACCTGAGACATGCT | TGCGCAGTGCCTTCACAT |
| CB2 | AAAGAAGAGGCCCCGAAGTC | CCACTGGGTGATTTTCACATCA |
| IL-1β | GCAGCCATGGCAGCAGTA | ATTGCCGCTGCAGTAAGTCA |
| IL-6 | AACAACTCACCTCATCCTTCGAA | CGAACAGCTCTCAGGCTGAAC |
| IL-10 | CGGCCCAGACATCAAGGA | TCGGAGGGTCTTCAGCTTTTC |
| IFN-γ | CTGTCGCCCAAAGCTAACCT | GGCCTCGAAATGGATTCTGA |
| TNF-α | TTGGATGGGCTGTACCTCATC | GGGCAGCCTTGGCCTTT |
| ACTB | CGGCGGCTCCATTCTG | CTGCTTGCTGATCCACATCTG |
| B2M | CACCCAGCAGAGAATGGAAAG | CGGATGGAACCCAGAGACA |
| GAPDH | GGCAAGTTCCATGGCACAGT | GGGCTTTCCGTTGATGACAA |
| HPRT | GCTCGAGATGTGATGAAGGAGAT | CCCCCTTGAGCACACAGAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battistin, L.; Moya, L.F.A.; Ferreira, L.V.d.O.; Braz, A.M.M.; Carvalho, M.d.; Golim, M.d.A.; Amorim, R.M. In Vitro Immunomodulatory Effects of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Primed with a Cannabidiol-Rich Extract. Int. J. Mol. Sci. 2025, 26, 4208. https://doi.org/10.3390/ijms26094208
Battistin L, Moya LFA, Ferreira LVdO, Braz AMM, Carvalho Md, Golim MdA, Amorim RM. In Vitro Immunomodulatory Effects of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Primed with a Cannabidiol-Rich Extract. International Journal of Molecular Sciences. 2025; 26(9):4208. https://doi.org/10.3390/ijms26094208
Chicago/Turabian StyleBattistin, Lorena, Luís Felipe Arantes Moya, Lucas Vinícius de Oliveira Ferreira, Aline Márcia Marques Braz, Márcio de Carvalho, Marjorie de Assis Golim, and Rogério Martins Amorim. 2025. "In Vitro Immunomodulatory Effects of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Primed with a Cannabidiol-Rich Extract" International Journal of Molecular Sciences 26, no. 9: 4208. https://doi.org/10.3390/ijms26094208
APA StyleBattistin, L., Moya, L. F. A., Ferreira, L. V. d. O., Braz, A. M. M., Carvalho, M. d., Golim, M. d. A., & Amorim, R. M. (2025). In Vitro Immunomodulatory Effects of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Primed with a Cannabidiol-Rich Extract. International Journal of Molecular Sciences, 26(9), 4208. https://doi.org/10.3390/ijms26094208







