Analysis of Mechanisms for Electron Uptake by Methanothrix harundinacea 6Ac During Direct Interspecies Electron Transfer
Abstract
1. Introduction
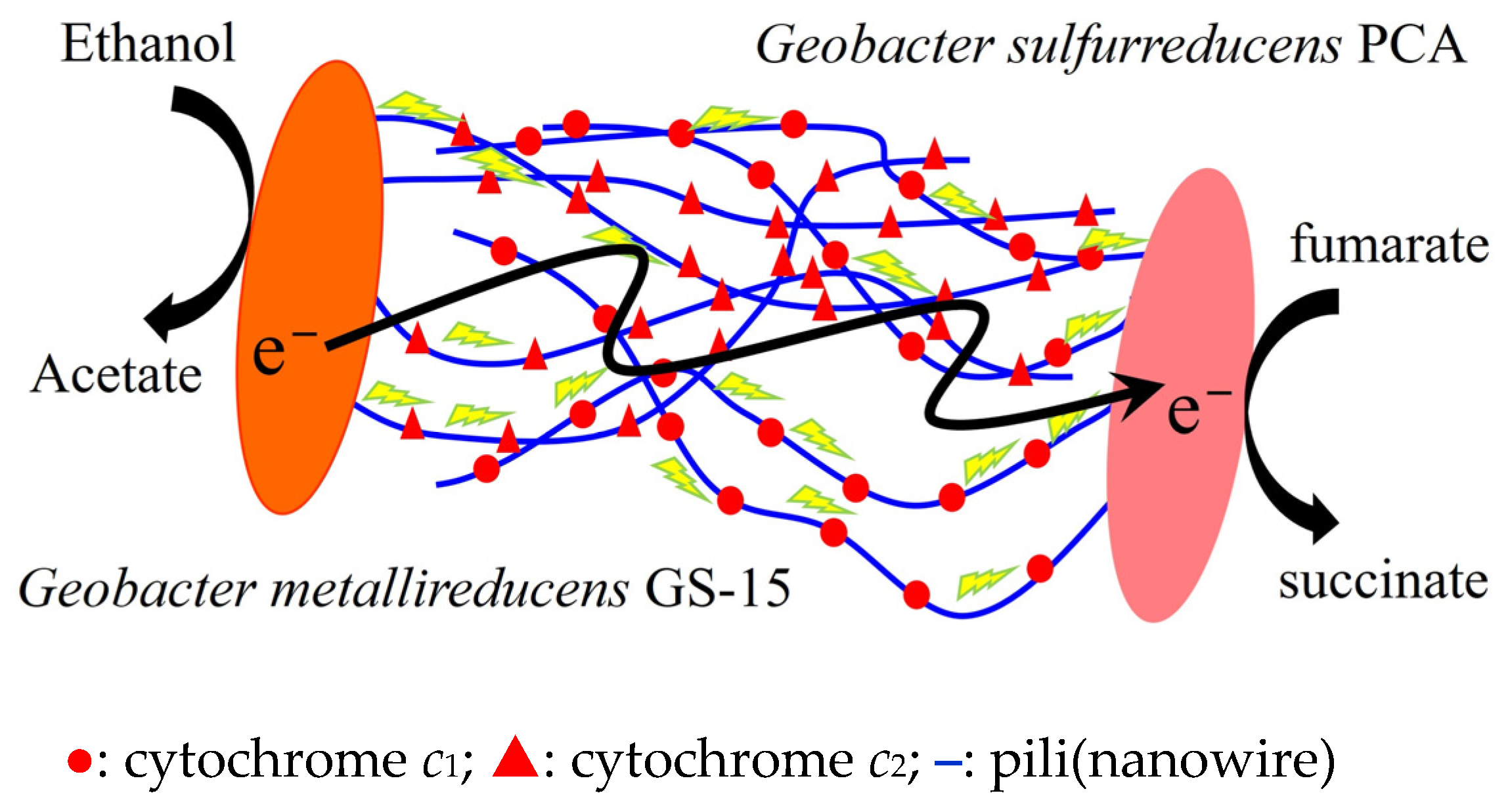
2. Possible Mechanisms for Extracellular Electron Uptake by M. harundinacea 6Ac
3. Possible Mechanisms for Generation of Fdred2− in M. harundinacea 6Ac During DIET
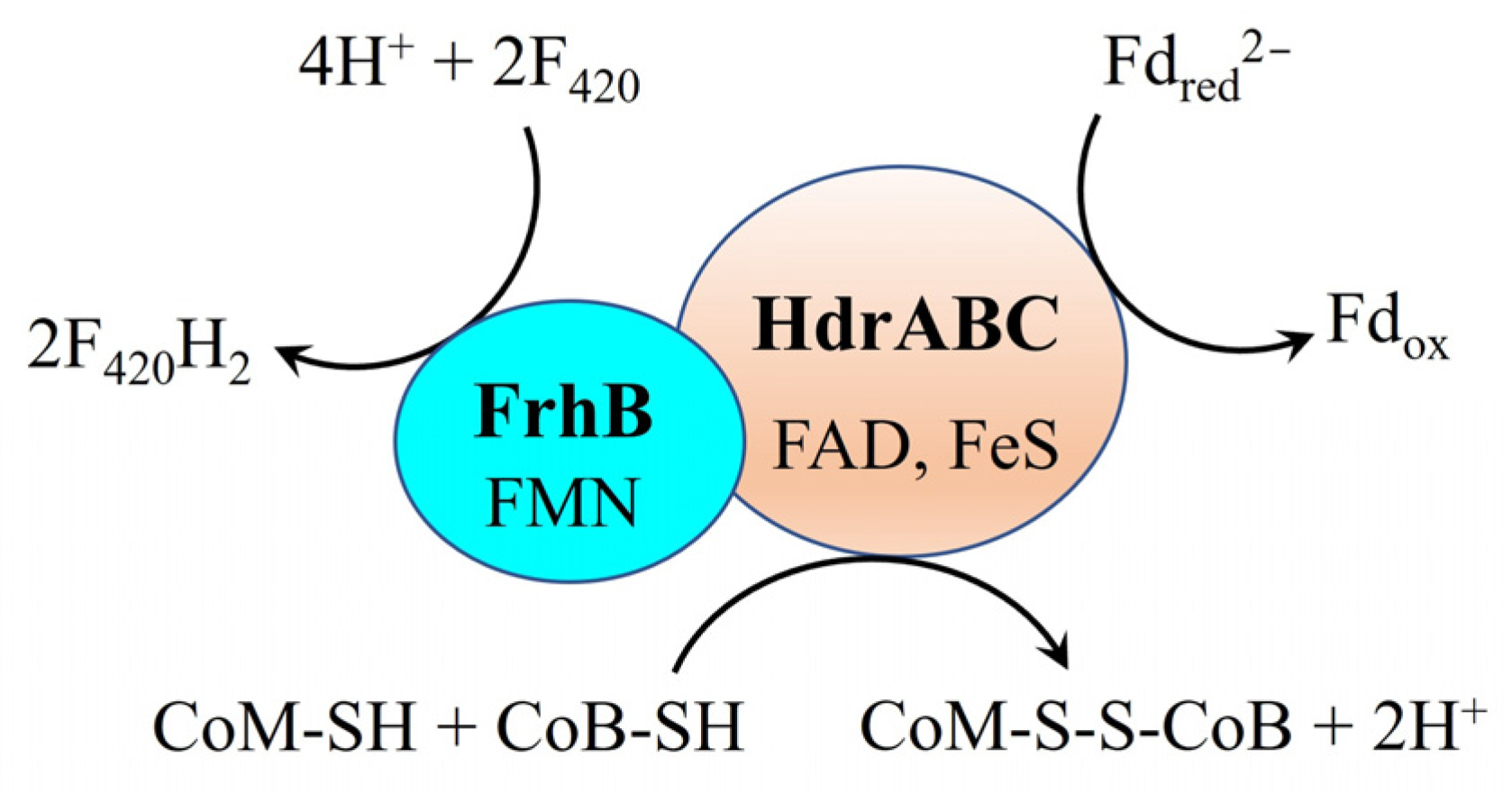
4. Possible Mechanisms Underlying the Generation of CoM-SH and CoB-SH in M. harundinacea 6Ac During DIET
5. Possible Mechanisms for F420H2 Generation in M. harundinacea 6Ac During DIET
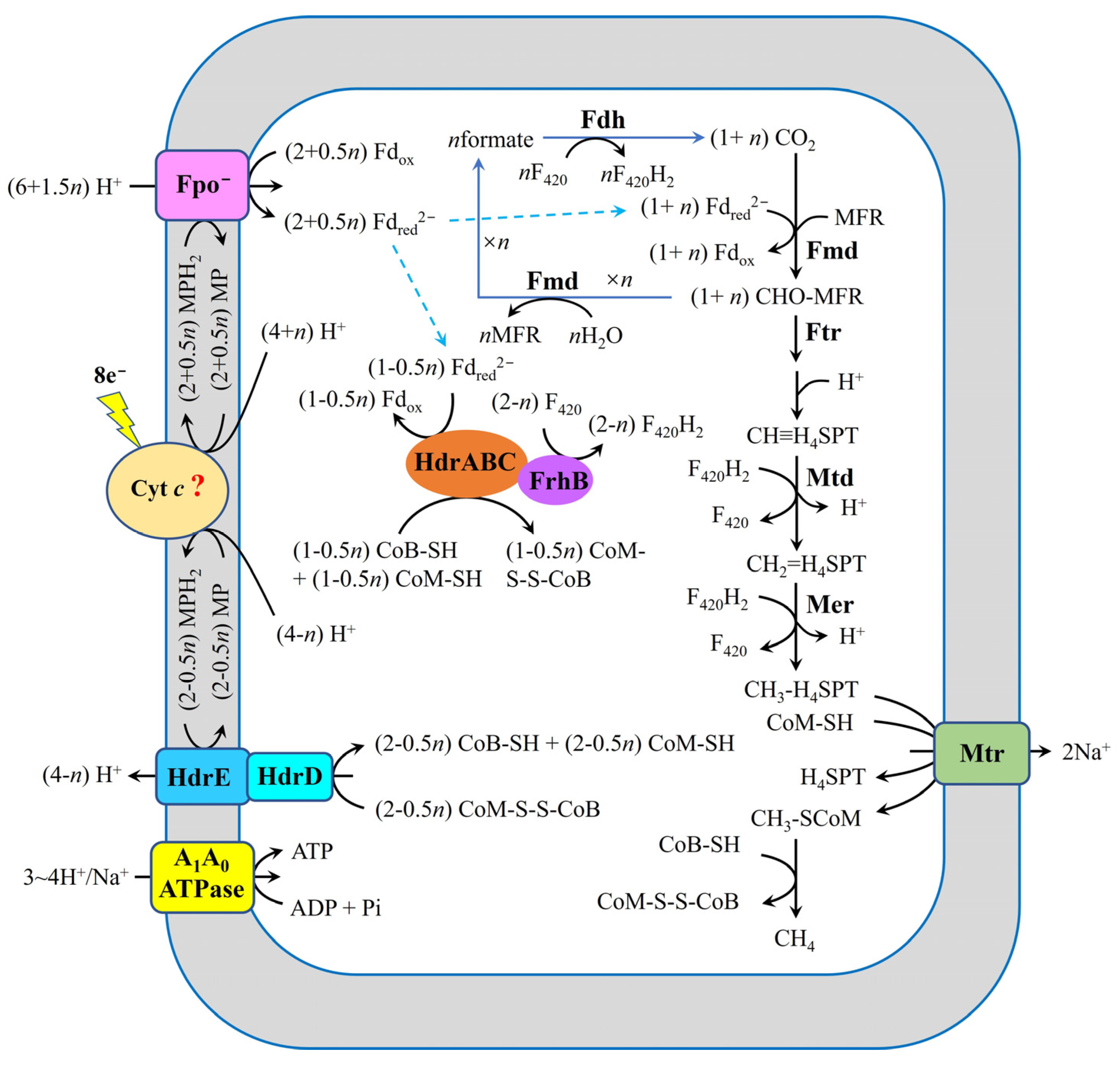
6. Pathway Proposed for Reduction of Carbon Dioxide in M. harundinacea 6Ac During DIET
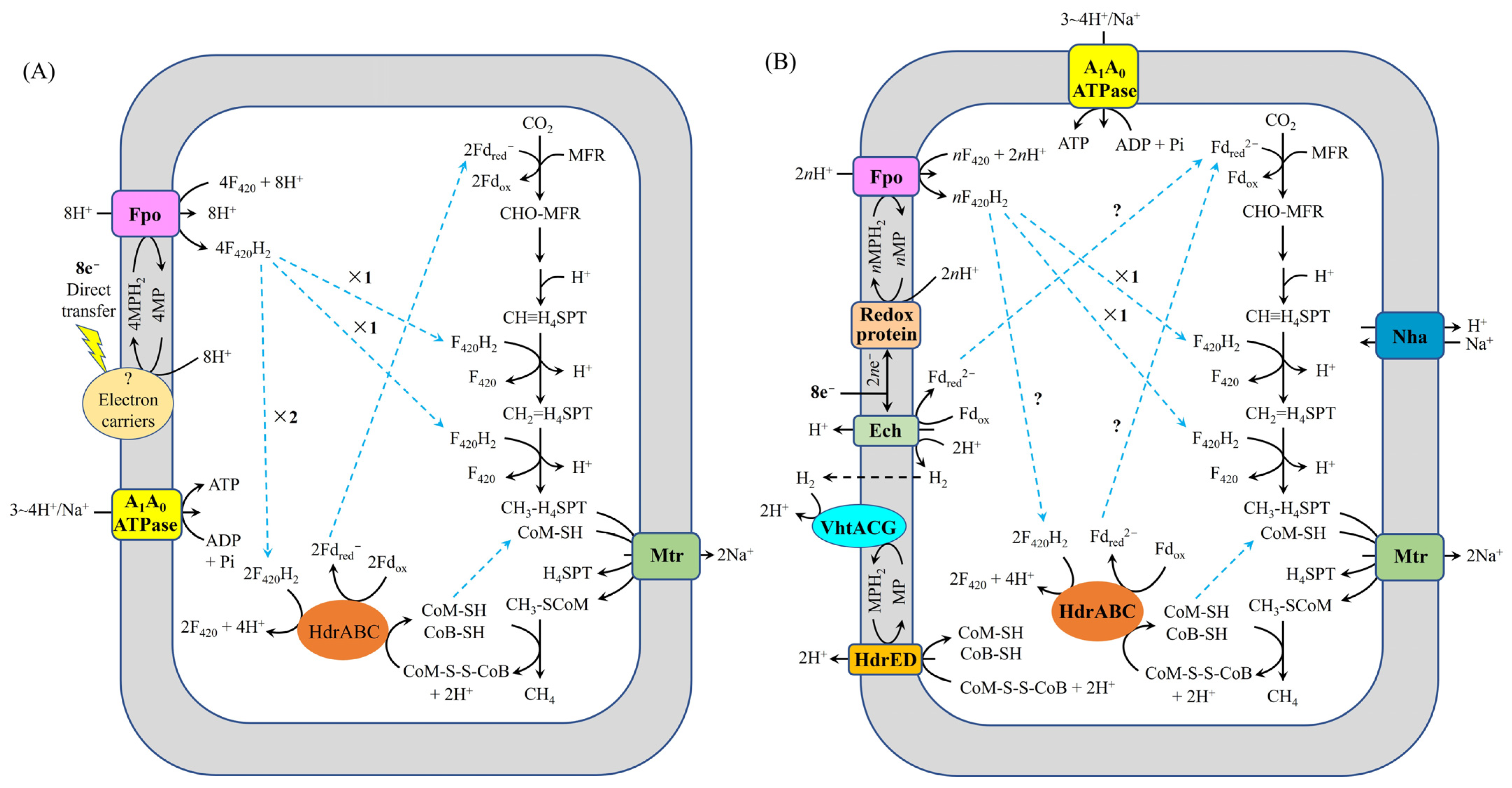
- Methanogens capable of conserving energy via Fpo, such as type I Methanosarcina spp., including M. bakeri 800, may utilize the mechanism for carbon dioxide reduction to methane illustrated in Figure 2. During DIET, Fpo, HdrED, FrhABG, HdrABC, VhtACG, and Ech in these methanogens are likely to contribute to extracellular electron uptake.
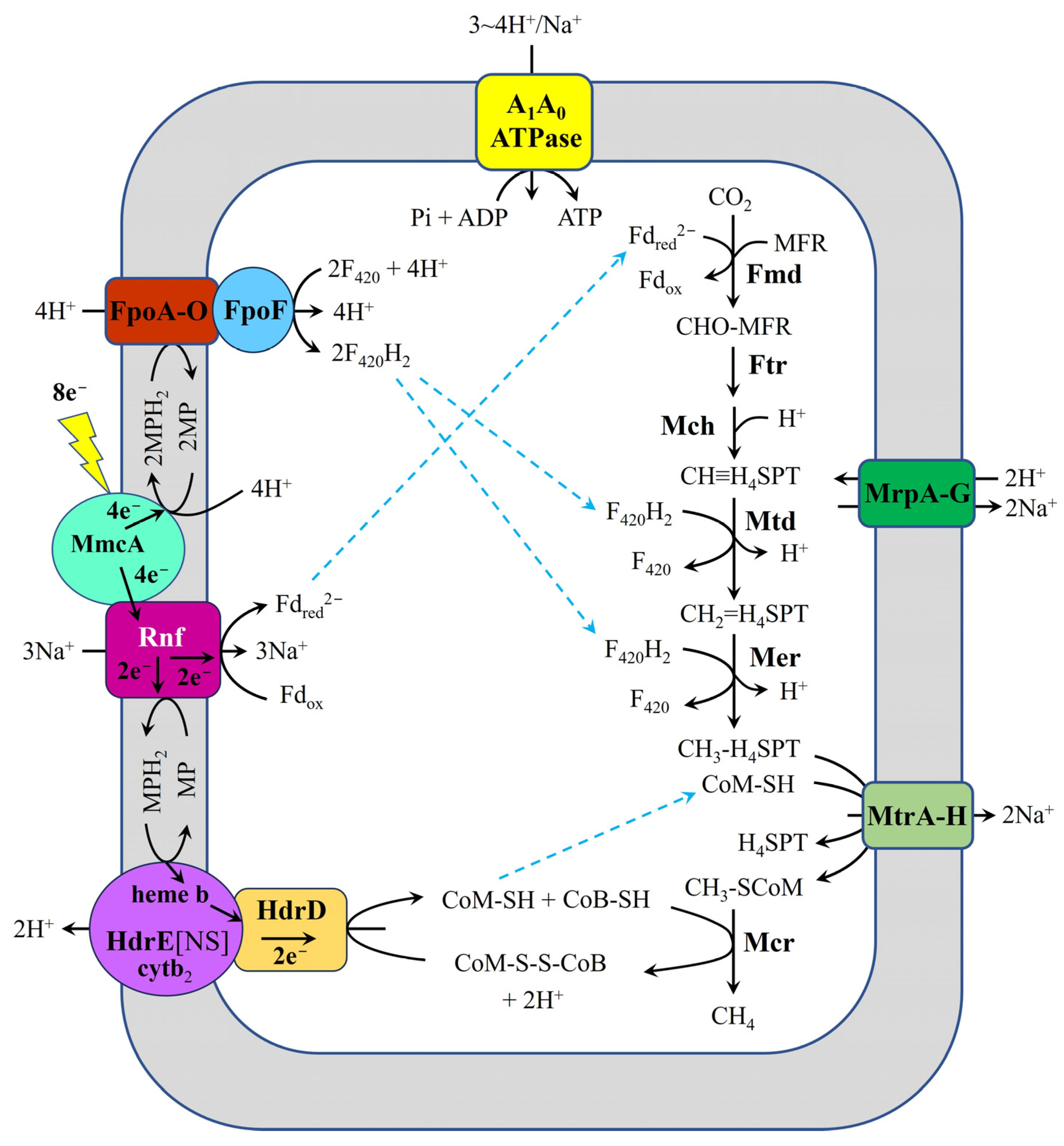
- For methanogens that conserve energy through Rnf, like type II Methanosarcina spp., such as M. acetivorans WWM1(Δhpt) and Methanosarcina horonobensis HB-1, the mechanism for carbon dioxide reduction to methane is depicted in Figure 3. In these organisms, Fpo, Rnf, and HdrED are likely involved in extracellular electron uptake during DIET. Additionally, HdrABC may also facilitate the acquisition of extracellular electrons.
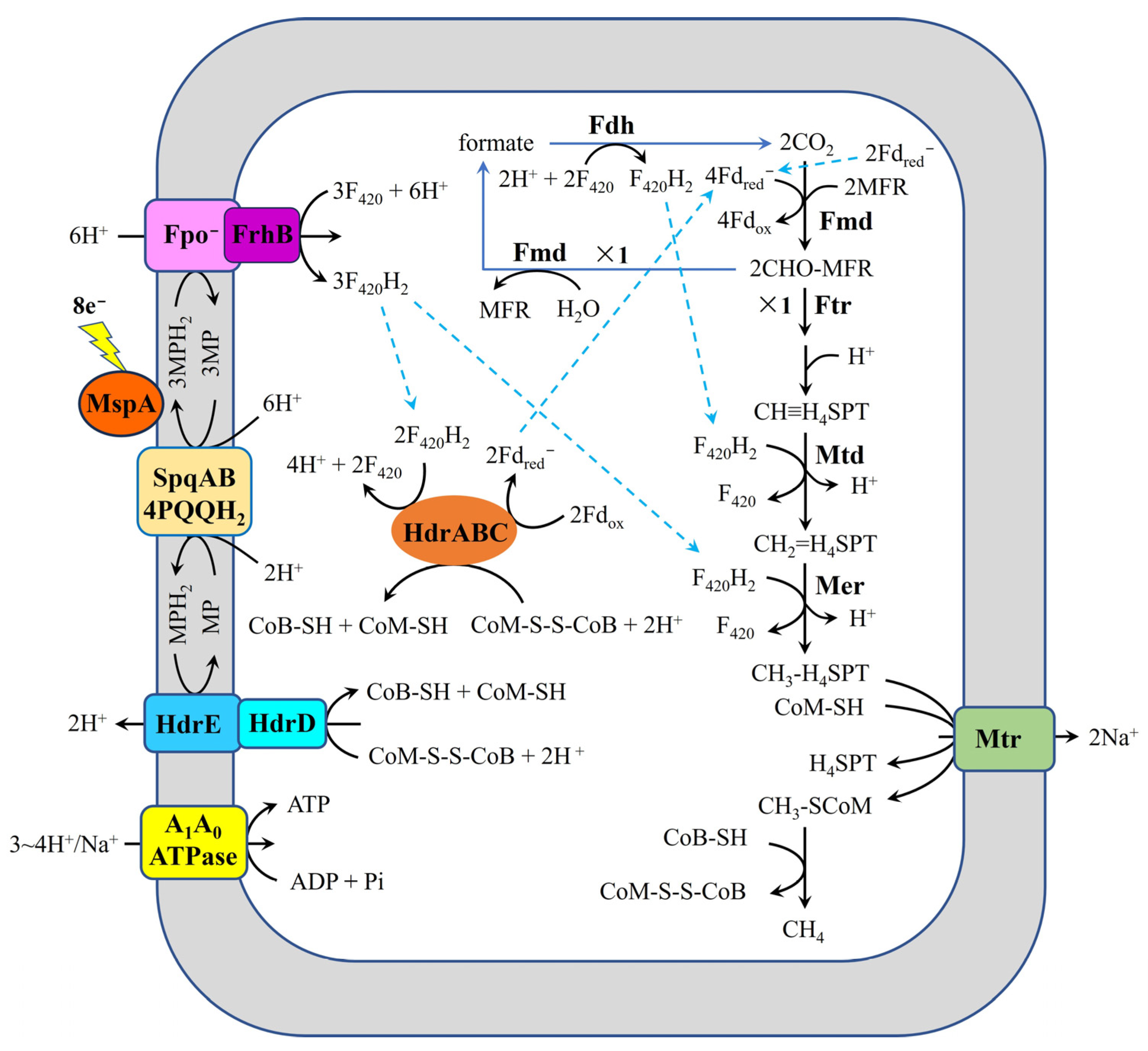
- Methanogens that conserve energy through Fpo−, like Methanothrix spp. (formerly named Methanosaeta spp.), including M. harundinacea 6Ac and M. thermoacetophila, may follow the mechanism for carbon dioxide reduction to methane that is shown in Figure 7. During DIET, the Fpo−, HdrED, and HdrABC-FrhB complexes are potentially responsible for extracellular electron uptake in these methanogens.
7. Conclusions
Funding
Conflicts of Interest
References
- Bryant, M.P.; Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Mikrobiol. 1967, 59, 20–31. [Google Scholar] [CrossRef] [PubMed]
- de-Bok, F.A.; Luijten, M.L.; Stams, A.J. Biochemical evidence for formate transfer in syntrophic propionate-oxidizing cocultures of Syntrophobacter fumaroxidans and Methanospirillum hungatei. Appl. Environ. Microbiol. 2002, 68, 4247–4252. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef]
- Schink, B.; Stams, A.J.M. Syntrophism among prokaryotes. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 309–335. [Google Scholar]
- Mcinerney, M.J.; Struchtemeyer, C.G.; Sieber, J.; Mouttaki, H.; Stams, A.J.; Schink, B.; Rohlin, L.; Gunsalus, R.P. Physiology, ecology, phylogeny, and genomics of microorganisms capable of syntrophic metabolism. Ann. N. Y. Acad. Sci. 2008, 1125, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Stams, A.J. The ecology and biotechnology of sulphate reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef]
- Smith, R.L.; Ceazan, M.L.; Brooks, M.H. Autotrophic, hydrogen-oxidizing, denitrifying bacteria in groundwater, potential agents for bioremediation of nitrate contamination. Appl. Environ. Microbiol. 1998, 60, 1949–1955. [Google Scholar] [CrossRef]
- Strohm, T.O.; Griffin, B.; Zumft, W.G.; Schink, B. Growth yields in bacterial denitrification and nitrate ammonification. Appl. Environ. Microbiol. 2007, 73, 1420–1424. [Google Scholar] [CrossRef]
- Smith, R.L.; Miller, D.N.; Brooks, M.H.; Widdowson, M.A.; Killingstad, M.W. In situ stimulation of groundwater denitrification with formate to remediate nitrate contamination. Environ. Sci. Technol. 2001, 35, 196–203. [Google Scholar] [CrossRef]
- Ginige, M.P.; Keller, J.; Blackall, L.L. Investigation of an acetate-fed denitrifying microbial community by stable isotope probing, full-cycle rRNA analysis, and fluorescent in situ hybridization-microautoradiography. Appl. Environ. Microbiol. 2005, 71, 8683–8691. [Google Scholar] [CrossRef]
- McInerney, M.; Bryant, M. Anaerobic degradation of lactate by syntrophic associations of Methanosarcina barkeri and Desulfovibrio species and effect of H2 on acetate degradation. Appl. Environ. Microbiol. 1981, 41, 346–354. [Google Scholar] [CrossRef]
- Doloman, A.; Boeren, S.; Miller, C.D.; Sousa, D.Z. Stimulating effect of trichococcus flocculiformis on a coculture of Syntrophomonas wolfei and Methanospirillum hungatei. Appl. Environ. Microbiol. 2022, 88, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Awata, T.; Zhang, D.; Katayama, A. Denitrification by Pseudomonas stutzeri coupled with CO2 reduction by Sporomusa ovata with hydrogen as an electron donor assisted by solid-phase humin. J. Biosci. Bioeng. 2016, 122, 307–313. [Google Scholar] [CrossRef]
- Ozuolmez, D.; Na, H.; Lever, M.A.; Kjeldsen, K.U.; Jørgensen, B.B.; Plugge, C.M. Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: Teamwork or coexistence? Front. Microbiol. 2015, 6, 492. [Google Scholar] [CrossRef]
- Heijthuijsen, J.H.F.G.; Hansen, T.A. Interspecies hydrogen transfer in co-cultures of methanol-utilizing acidogens and sulfate-reducing or methanogenic bacteria. FEMS Microbiol. Lett. 1986, 38, 57–64. [Google Scholar] [CrossRef][Green Version]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef]
- Ha, P.T.; Lindemann, S.R.; Shi, L.; Dohnalkova, A.C.; Fredrickson, J.K.; Madigan, M.T.; Beyenal, H. Syntrophic anaerobic photosynthesis via direct interspecies electron transfer. Nat. Commun. 2017, 8, 13924. [Google Scholar] [CrossRef]
- Yee, M.O.; Rotaru, A.E. Extracellular electron uptake in Methanosarcinales is independent of multiheme c-type cytochromes. Sci. Rep. 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Smith, J.A.; Li, M.; Holmes, D.E. Methane production by Methanothrix thermoacetophila via direct interspecies electron transfer with Geobacter metallireducens. mBio 2023, 14, e0036023. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Wang, L.; Li, X.; Zhang, J.; Jiang, H. Geobacter grbiciae—A new electron donor in the formation of co-cultures via direct interspecies electron transfer. Microbiol. Res. 2023, 14, 1774–1787. [Google Scholar] [CrossRef]
- Kalathil, S.; Rahaman, M.; Lam, E.; Augustin, T.L.; Greer, H.F.; Reisner, E. Solar-Driven Methanogenesis through Microbial Ecosystem Engineering on Carbon Nitride. Angew. Chem. Int. Ed. 2024, 63, e202409192. [Google Scholar] [CrossRef]
- Lovley, D.R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011, 4, 4896–4906. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Link between capacity for current production and syntrophic growth in Geobacter species. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef]
- Huang, L.Y.; Liu, X.; Zhang, Z.S.; Ye, J.; Rensing, C.; Zhou, S.G.; Nealson, K.H. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri in an electric syntrophic coculture. ISME J. 2022, 16, 370–377. [Google Scholar] [CrossRef]
- Walker, D.J.F.; Nevin, K.P.; Holmes, D.E.; Rotaru, A.E.; Ward, J.E.; Woodard, T.L.; Zhu, J.X.; Ueki, T.; Nonnenmann, S.S.; McInerney, M.J.; et al. Syntrophus conductive pili demonstrate that common hydrogen-donating syntrophs can have a direct electron transfer option. ISME J. 2020, 14, 837–846. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Sha, C.; Zhao, X.M.; Du, J.X.; Zou, L.; Yong, Y.C. Unnatural Direct Interspecies Electron Transfer Enabled by Living Cell-Cell Click Chemistry. Angew. Chem. Int. Ed. 2024, 63, e202402318. [Google Scholar] [CrossRef]
- Holmes, D.E.; Zhou, J.J.; Ueki, T.; Woodard, T.; Lovley, D.R. Mechanisms for Electron Uptake by Methanosarcina acetivorans during Direct Interspecies Electron Transfer. mBio 2021, 12, e0234421. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.Y.; Rensing, C.; Ye, J.; Nealson, K.H.; Zhou, S.G. Syntrophic interspecies electron transfer drives carbon fixation and growth by Rhodopseudomonas palustris under dark, anoxic conditions. Sci. Adv. 2021, 7, eabh1852. [Google Scholar] [CrossRef]
- Yee, M.O.; Snoeyenbos-West, O.L.; Thamdrup, B.; Ottosen, L.D.M.; Rotaru, A.E. Extracellular Electron Uptake by Two Methanosarcina Species. Front. Microbiol. 2019, 7, 29. [Google Scholar] [CrossRef]
- McGlynn, S.E.; Chadwick, G.L.; Kempes, C.P.; Orphan, V.J. Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 2015, 526, 531–535. [Google Scholar] [CrossRef]
- Holmes, D.E.; Rotaru, A.E.; Ueki, T.; Shrestha, P.M.; Ferry, J.G.; Lovley, D.R. Electron and Proton Flux for Carbon Dioxide Reduction in Methanosarcina barkeri During Direct Interspecies Electron Transfer. Front. Microbiol. 2018, 9, 3109. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Research on Stoichiometrical Production of Methane by Microbes via Direct Interspecies Electron Transfer. Master’s Thesis, Inner Mongolia University of Science and Technology, Baotou, China, 2020. [Google Scholar]
- Wang, L.; Nevin, K.P.; Woodard, T.L.; Mu, B.Z.; Lovley, D.R. Expanding the Diet for DIET: Electron Donors Supporting Direct Interspecies Electron Transfer (DIET) in Defined Co-Cultures. Front. Microbiol. 2016, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Welte, C.; Deppenmeier, U. Re-evaluation of the function of the F420 dehydrogenase in electron transport of Methanosarcina mazei. FEBS J. 2011, 278, 1277–1287. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Arnulf, K.; Thomas, H.; Jennifer, F.; Laura, V.N.; Reinhard, R. Cytochromes c in Archaea-distribution, maturation, cell architecture, and the special case of Ignicoccus hospitalis. Front. Microbiol. 2015, 6, 439. [Google Scholar]
- Kamlage, B.; Blaut, M. Characterization of Cytochromes from Methanosarcina Strain Göl and Their Involvement in Electron Transport during Growth on Methanol. J. Bacteriol. 1992, 174, 3921–3927. [Google Scholar] [CrossRef][Green Version]
- Tietze, M.; Beuchle, A.; Lamla, I.; Orth, N.; Dehler, M.; Greiner, G.; Beifuss, U. Redox Potentials of Methanophenazine and CoB-S-S-CoM, Factors Involved in Electron Transport in Methanogenic Archaea. Chembiochem 2003, 4, 333–335. [Google Scholar] [CrossRef]
- Zhu, J.X.; Zheng, H.J.; Ai, G.M.; Zhang, G.S.; Liu, D.; Liu, X.L.; Dong, X.Z. The Genome Characteristics and Predicted Function of Methyl-Group Oxidation Pathway in the Obligate Aceticlastic Methanogens, Methanosaeta spp. PLoS ONE 2012, 7, e36756. [Google Scholar] [CrossRef] [PubMed]
- Welte, C.; Deppenmeier, U. Membrane-bound electron transport in Methanosaeta thermophila. J. Bacteriol. 2011, 193, 2868–2870. [Google Scholar] [CrossRef]
- Welte, C.; Deppenmeier, U. Bioenergetics and anaerobic respiratory chains of aceticlastic methanogens. BBA 2014, 1837, 1130–1147. [Google Scholar] [CrossRef]
- Welte, C.; Kallnik, V.; Grapp, M.; Bender, G.; Ragsdale, S.; Deppenmeier, U. Function of Ech Hydrogenase in Ferredoxin-Dependent, Membrane-Bound Electron Transport in Methanosarcina mazei. J. Bacteriol. 2010, 192, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, M.; Ferry, J.G. A Ferredoxin- and F420H2-dependent, electron-bifurcating, heterodisulfide reductase with homologs in the domains Bacteria and Archaea. mBio 2017, 8, e0228516. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ferry, J.G. Electron Bifurcation and Confurcation in Methanogenesis and Reverse Methanogenesis. Front. Microbiol. 2018, 9, 1322. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.K.; Goenrich, M.; Schick, M.; Hiromoto, T.; Shima, S. Hydrogenases from Methanogenic Archaea, Nickel, a Novel Cofactor, and H2 Storage. Annu. Rev. Biochem. 2010, 79, 507–536. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Liu, Y. Harnessing Carbonaceous materials’ multifaceted roles for enhanced anaerobic digestion performance. Chem. Eng. J. 2023, 477, 146931. [Google Scholar] [CrossRef]
- Deppenmeier, U. Redox-driven proton translocation in methanogenic Archaea. Cell. Mol. Life Sci. 2002, 59, 1513–1533. [Google Scholar] [CrossRef]
- Buan, N.R.; Metcalf, W.W. Methanogenesis by Methanosarcina acetivorans involves two structurally and functionally distinct classes of heterodisulfide reductase. Mol. Microbiol. 2010, 75, 843–853. [Google Scholar] [CrossRef]
- Ma, K.; Liu, X.; Dong, X. Methanosaeta harundinacea sp. nov., a novel acetate-scavenging methanogen isolated from a UASB reactor. Int. J. Syst. Evol. Microbiol. 2006, 56, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Lie, T.J.; Costa, K.C.; Lupa, B.; Korpole, S.; Whitman, W.B.; Leigh, J.A. Essential anaplerotic role for the energy-converting hydrogenase Eha in hydrogenotrophic methanogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 15473–15478. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Speth, D.R.; Graaf RMd Camp, H.J.O.; Jetten, M.S.M.; Welte, C.U. A metagenomics-based metabolic model of nitrate-dependent anaerobic oxidation of methane by Methanoperedens-like archaea. Front. Microbiol. 2015, 6, 1423. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Leu, A.O.; Xie, G.J.; Guo, J.H.; Feng, Y.X.; Zhao, J.X.; Tyson, G.W.; Yuan, Z.G.; Hu, S.H. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J. 2018, 12, 1929–1939. [Google Scholar] [CrossRef]
- Leu, A.O.; Cai, C.; McIlroy, S.J.; Southam, G.; Orphan, V.J.; Yuan, Z.; Hu, S.; Tyson, G.W. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae. ISME J. 2020, 14, 1030–1041. [Google Scholar] [CrossRef]

| Microbes | Extracellular Electron Uptake | MP Reduction | F420 Reduction | CoM-S-S-CoB Reduction | Fdox Reduction | Reference |
|---|---|---|---|---|---|---|
| M. barkeri 800 | Unknow | Unknow | Fpo with MPH2 | HdrABC with F420H2, HdrED with MPH2, interaction of VhtACG and HdrED with H2 | HdrABC with F420H2, Ech direct with extracellular electron, Ech with H2 from oxidation of F420H2 by FrhABG | [28,35,36] |
| M. acetivorans | MmcA | MmcA, Rnf with electrons from MmcA | Fpo with MPH2 | HdrED with MPH2 | Rnf with electrons from MmcA | [31] |
| M. thermoacetophila | MspA | SpqAB with PQQH2 | Fpo−-FrhB complex with MPH2, interaction of Fmd and Fdh with Fdred2− | HdrABC with F420H2 and HdrED with MPH2 | HdrABC with F420H2 and oxidation of acetate | [23] |
| M. harundinacea 6Ac | Unknow | Cyt c? | HdrABC-FrhB complex with Fdred2−, CoM-SH and CoB-SH | HdrED with MPH2 | Fpo− with MPH2 | This article |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Shan, X.; Xu, Y.; Xi, Q.; Jiang, H.; Li, X. Analysis of Mechanisms for Electron Uptake by Methanothrix harundinacea 6Ac During Direct Interspecies Electron Transfer. Int. J. Mol. Sci. 2025, 26, 4195. https://doi.org/10.3390/ijms26094195
Wang L, Shan X, Xu Y, Xi Q, Jiang H, Li X. Analysis of Mechanisms for Electron Uptake by Methanothrix harundinacea 6Ac During Direct Interspecies Electron Transfer. International Journal of Molecular Sciences. 2025; 26(9):4195. https://doi.org/10.3390/ijms26094195
Chicago/Turabian StyleWang, Lei, Xiaoman Shan, Yanhui Xu, Quan Xi, Haiming Jiang, and Xia Li. 2025. "Analysis of Mechanisms for Electron Uptake by Methanothrix harundinacea 6Ac During Direct Interspecies Electron Transfer" International Journal of Molecular Sciences 26, no. 9: 4195. https://doi.org/10.3390/ijms26094195
APA StyleWang, L., Shan, X., Xu, Y., Xi, Q., Jiang, H., & Li, X. (2025). Analysis of Mechanisms for Electron Uptake by Methanothrix harundinacea 6Ac During Direct Interspecies Electron Transfer. International Journal of Molecular Sciences, 26(9), 4195. https://doi.org/10.3390/ijms26094195





