Revisiting Secondary Dilative Cardiomyopathy
Abstract
1. Introduction
2. Epidemiology
3. Etiology
4. Secondary Dilated Cardiomyopathy
5. Toxic Cardiomyopathy
6. Chemotherapy-Induced Cardiomyopathy
- Oxidative Stress: Anthracyclines undergo redox cycling, producing reactive oxygen species (ROS) that overwhelm the heart’s antioxidant defenses, leading to lipid peroxidation, mitochondrial damage, and apoptosis of cardiomyocytes [35];
- Topoisomerase IIβ Inhibition: Doxorubicin interferes with topoisomerase IIβ in cardiomyocytes, causing DNA double-strand breaks and triggering cell death pathways [36];
- Mitochondrial Dysfunction: The accumulation of anthracyclines in mitochondria disrupts electron transport chains, leading to energy depletion and further ROS production [37].
- Trastuzumab: A monoclonal antibody targeting HER2 receptors, trastuzumab disrupts cardiomyocyte survival pathways, leading to reversible cardiac dysfunction [39];
- Cyclophosphamide: An alkylating agent that can cause endothelial damage, leading to hemorrhagic myocarditis and subsequent DCM [40];
- 5-Fluorouracil (5-FU): An antimetabolite associated with coronary vasospasm and myocardial ischemia, potentially leading to DCM [41];
- Bevacizumab: An anti-VEGF agent that can induce hypertension and thromboembolic events, contributing to cardiac dysfunction [42].
7. Myocarditis, Inflammatory Cardiomyopathies, and Dilative Cardiomyopathy
8. Peripartum Cardiomyopathy
9. Other Causes of Dilative Cardiomyopathy
9.1. Rheumatic Fever
9.2. Endocrine-Related Dilative Cardiomyopathy
9.3. Sarcoidosis
9.4. Dyssynchronopathy: Left Bundle Branch Block-Induced Cardiomyopathy
9.5. Atrial Fibrillation-Induced Dilated Cardiomyopathy
9.6. Premature Ventricular Complex-Induced Cardiomyopathy
10. Diagnosis
10.1. Anamnesis and Clinical Examination
10.2. Electrocardiogram (ECG)
10.3. Imaging
10.3.1. Echocardiography
10.3.2. Cardiac Magnetic Resonance Imaging (MRI)
10.3.3. Future Directions: Integrating Artificial Intelligence in Imaging
11. Therapy
11.1. Pharmacology
11.2. Surgery
11.3. Emerging Therapies
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brieler, J.; Breeden, M.A.; Tucker, J. Cardiomyopathy: An Overview. Am. Fam. Physician 2017, 96, 640–646. [Google Scholar] [PubMed]
- McNally, E.M.; Mestroni, L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Voinescu, O.R.; Ionac, A.; Sosdean, R.; Ionac, I.; Ana, L.S.; Kundnani, N.R.; Morariu, S.; Puiu, M.; Chirita-Emandi, A. Genotype-Phenotype Insights of Inherited Cardiomyopathies—A Review. Medicina 2024, 60, 543. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Nomura, S. Recent Findings Related to Cardiomyopathy and Genetics. Int. J. Mol. Sci. 2021, 22, 12522. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef]
- Oakley, C. Aetiology, diagnosis, investigation, and management of the cardiomyopathies. BMJ 1997, 315, 1520–1524. [Google Scholar] [CrossRef][Green Version]
- Rakar, S.; Sinagra, G.; Di Lenarda, A.; Poletti, A.; Bussani, R.; Silvestri, F.; Camerini, F.; Heart Muscle Disease Study Group. Epidemiology of dilated cardiomyopathy: A prospective post-mortem study of 5252 necropsies. Eur. Heart J. 1997, 18, 117–123. [Google Scholar] [CrossRef]
- Heymans, S.; Lakdawala, N.K.; Tschöpe, C.; Klingel, K. Dilated cardiomyopathy: Causes, mechanisms, and current and future treatment approaches. Lancet 2023, 402, 998–1011. [Google Scholar] [CrossRef]
- Guha, A.; Caraballo, C.; Jain, P.; Miller, P.E.; Owusu-Guha, J.; Clark, K.A.A.; Velazquez, E.J.; Ahmad, T.; Baldassarre, L.A.; Addison, D.; et al. Outcomes in patients with anthracycline-induced cardiomyopathy undergoing left ventricular assist devices implantation. ESC Heart Fail. 2021, 8, 2866–2875. [Google Scholar] [CrossRef]
- Salzano, A.; Sirico, D.; Arcopinto, M.; Marra, A.M.; Guerra, G.; Rocca, A.; Grieco, A.; Giallauria, F.; Vigorito, C. Terapie antiremodeling: Nuove strategie e prospettive future nell’insufficienza cardiaca cronica post ischemica: Parte II. Anti remodeling therapy: New strategies and future perspective in post-ischemic heart failure. Part II. Monaldi Arch. Chest Dis. 2014, 82, 195–201. [Google Scholar] [PubMed]
- Miranda, J.O.; Costa, L.; Rodrigues, E.; Teles, E.L.; Baptista, M.J.; Areias, J.C. Paediatric dilated cardiomyopathy: Clinical profile and outcome. The experience of a tertiary centre for paediatric cardiology. Cardiol. Young 2015, 25, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Konishi, M.; von Haehling, S. The appropriate dose of angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers in patients with dilated cardiomyopathy. The higher, the better? ESC Heart Fail. 2015, 2, 103–105. [Google Scholar] [CrossRef]
- Azeka, E.; Arshad, A.; Martins, C.; Dominguez, A.C.; Siqueira, A.; Loss, A.S.; Jatene, M.; Miura, N. Case Report: Dilated Cardiomyopathy in a Newborn, a Potential Association With SARS-CoV-2. Front. Pediatr. 2021, 9, 674300. [Google Scholar] [CrossRef]
- Chen, Y.X.; Ding, J.; Zhou, W.E.; Zhang, X.; Sun, X.T.; Wang, X.Y.; Zhang, C.; Li, N.; Shao, G.F.; Hu, S.J.; et al. Identification and Functional Prediction of Long Non-Coding RNAs in Dilated Cardiomyopathy by Bioinformatics Analysis. Front. Genet. 2021, 12, 648111. [Google Scholar] [CrossRef] [PubMed]
- Briceno, N.; Schuster, A.; Lumley, M.; Perera, D. Ischaemic cardiomyopathy: Pathophysiology, assessment and the role of revascularisation. Heart 2016, 102, 397–406. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Heymans, S.R.B. Dilated cardiomyopathy: Second hits knock-down the heart. Eur. Heart J. 2023, 45, 500–501. [Google Scholar] [CrossRef]
- Sivitz, A.; Nagdev, A. Heart failure secondary to dilated cardiomyopathy: A role for emergency physician bedside ultrasonography. Pediatr. Emerg. Care 2012, 28, 163–166. [Google Scholar] [CrossRef]
- Codd, M.B.; Sugrue, D.D.; Gersh, B.J.; Melton, L.J., 3rd. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975-1984. Circulation 1989, 80, 564–572. [Google Scholar] [CrossRef]
- Moges, T.; Shiferaw, Y.; Heye, T. Maternal vitamin D deficiency: A Culprit for Hypocalcaemia Induced Myocardial Failure in a Four-Month Old Infant: A Case Report From Tikur Anbessa Specialized Hospital, Ethiopia. Ethiop. J. Health Sci. 2017, 27, 299–304. [Google Scholar] [CrossRef]
- Daubeney, P.E.; Nugent, A.W.; Chondros, P.; Carlin, J.B.; Colan, S.D.; Cheung, M.; Davis, A.M.; Chow, C.W.; Weintraub, R.G. Clinical features and outcomes of childhood dilated cardiomyopathy: Results from a national population-based study. Circulation 2006, 114, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef] [PubMed]
- Heidendael, J.F.; Den Boer, S.L.; Wildenbeest, J.G.; Dalinghaus, M.; Straver, B.; Pajkrt, D. Intravenous immunoglobulins in children with new onset dilated cardiomyopathy. Cardiol. Young 2018, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Deeg, P.; Liebau, G.; Kochsiek, K. Diagnostic relevance of humoral and cytotoxic immune reactions in primary and secondary dilated cardiomyopathy. Am. J. Cardiol. 1983, 52, 1072–1078. [Google Scholar] [CrossRef]
- Luk, A.; Ahn, E.; Soor, G.S.; Butany, J. Dilated cardiomyopathy: A review. J. Clin. Pathol. 2009, 62, 219–225. [Google Scholar] [CrossRef]
- Rupasinghe, N.; Ranasinghe, P.; Wanninayake, L. Dilated cardiomyopathy due to hypocalcaemia: A case report. J. Med. Case Rep. 2024, 18, 204. [Google Scholar] [CrossRef]
- Khochtali, I.; Hamza, N.; Harzallah, O.; Hamdi, S.; Saad, J.; Golli, M.; Mahjoub, S. Reversible dilated cardiomyopathy caused by hypothyroidism. Int. Arch. Med. 2011, 4, 20. [Google Scholar] [CrossRef]
- Behaghel, A.; Donal, E. Hypocalcaemia-induced transient dilated cardiomyopathy in elderly: A case report. Eur. J. Echocardiogr. 2011, 12, E38. [Google Scholar] [CrossRef][Green Version]
- Munguti, C.M.; Al Rifai, M.; Shaheen, W. A Rare Cause of Cardiomyopathy: A Case of Selenium Deficiency Causing Severe Cardiomyopathy that Improved on Supplementation. Cureus 2017, 9, e1627. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Etiology of alcoholic cardiomyopathy: Mitochondria, oxidative stress and apoptosis. Int. J. Biochem. Cell Biol. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Holmgren, G.; Synnergren, J.; Bogestål, Y.; Améen, C.; Åkesson, K.; Holmgren, S.; Lindahl, A.; Sartipy, P. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology 2015, 328, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Hantson, P. Mechanisms of toxic cardiomyopathy. Clin. Toxicol. 2019, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, S.; Sharma, S. Hair Spray Induced Cardiomyopathy. Eur. J. Case Rep. Intern. Med. 2022, 9, 003313. [Google Scholar] [CrossRef]
- Di Lisi, D.; Manno, G.; Madaudo, C.; Filorizzo, C.; Intravaia, R.C.M.; Galassi, A.R.; Incorvaia, L.; Russo, A.; Novo, G. Chemotherapy-related cardiac dysfunction: The usefulness of myocardial work indices. Int. J. Cardiovasc. Imaging 2023, 39, 1845–1853. [Google Scholar] [CrossRef]
- Shakir, D.K.; Rasul, K.I. Chemotherapy induced cardiomyopathy: Pathogenesis, monitoring and management. J. Clin. Med. Res. 2009, 1, 8–12. [Google Scholar] [CrossRef]
- Cui, N.; Wu, F.; Lu, W.J.; Bai, R.; Ke, B.; Liu, T.; Li, L.; Lan, F.; Cui, M. Doxorubicin-induced cardiotoxicity is maturation dependent due to the shift from topoisomerase IIα to IIβ in human stem cell derived cardiomyocytes. J. Cell. Mol. Med. 2019, 23, 4627–4639. [Google Scholar] [CrossRef]
- Ramaccini, D.; Montoya-Uribe, V.; Aan, F.J.; Modesti, L.; Potes, Y.; Wieckowski, M.R.; Krga, I.; Glibetić, M.; Pinton, P.; Giorgi, C.; et al. Mitochondrial Function and Dysfunction in Dilated Cardiomyopathy. Front. Cell Dev. Biol. 2020, 8, 624216. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Ghosh, R.K.; Wongsaengsak, S.; Bandyopadhyay, D.; Ghosh, G.C.; Aronow, W.S.; Fonarow, G.C.; Lenihan, D.J.; Bhatt, D.L. Cardiovascular Toxicities of Immune Checkpoint Inhibitors. JACC 2019, 74, 1714–1727. [Google Scholar] [CrossRef]
- Karmakar, S.; Dixit, R.; Nath, A.; Kumar, S.; Karmakar, S. Dilated cardiomyopathy following trastuzumab chemotherapy. Indian J. Pharmacol. 2012, 44, 131–133. [Google Scholar] [CrossRef]
- Dhesi, S.; Chu, M.P.; Blevins, G.; Paterson, I.; Larratt, L.; Oudit, G.Y.; Kim, D.H. Cyclophosphamide-Induced Cardiomyopathy: A Case Report, Review, and Recommendations for Management. J. Investig. Med. High Impact Case Rep. 2013, 1, 2324709613480346. [Google Scholar] [CrossRef]
- Yuan, C.; Parekh, H.; Allegra, C.; George, T.J.; Starr, J.S. 5-FU induced cardiotoxicity: Case series and review of the literature. Cardio-Oncology 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jain, A. A Case of Bevacizumab-Induced Dilated Cardiomyopathy in High-Grade Endometrial Cancer. Heart Lung Circ. 2018, 27, S69. [Google Scholar] [CrossRef]
- Danti, M.; Sbarbati, S.; Alsadi, N.; Di Filippo, A.; Gangitano, G.; Giglio, L.; Salvini, V.; Amoruso, M.; Camastra, G.S.; Ansalone, G.; et al. Cardiac magnetic resonance imaging: Diagnostic value and utility in the follow-up of patients with acute myocarditis mimicking myocardial infarction. Radiol. Med. 2009, 114, 229–238. [Google Scholar] [CrossRef]
- Baumgratz, J.F.; Vila, J.H.; Silva, J.P.; Fonseca, L.D.; Rodrigues, E.A.; Knobel, E. Cardiogenic shock due to cytomegalovirus myocarditis: Successful clinical treatment. Rev. Bras. Cir. Cardiovasc. 2010, 25, 149–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vinci, A.; Caforio, A.L. A case of peripartum heart failure. Clin. Manag. Issues 2008, 2, 7–15. [Google Scholar] [CrossRef][Green Version]
- Lauriero, F.; Vita, C.V.; Perazzolo, A.; Sanseverino, G.; Moliterno, E.; Rovere, G.; Marano, R.; Natale, L. Acute Myocarditis and Inflammatory Cardiomyopathies: Insights From Cardiac Magnetic Resonance Findings. Echocardiography 2025, 42, e70099. [Google Scholar] [CrossRef]
- Trachtenberg, B.H.; Hare, J.M. Inflammatory Cardiomyopathic Syndromes. Circ. Res. 2017, 121, 803–818. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C. Immunosuppressive therapy in myocarditis. Circ. J. 2015, 79, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Bozkurt, B.; Cooper, L.T.; Aggarwal, N.R.; Basso, C.; Bhave, N.M.; Caforio, A.L.P.; Ferreira, V.M.; Heidecker, B.; Kontorovich, A.R.; et al. 2024 ACC Expert Consensus Decision Pathway on Strategies and Criteria for the Diagnosis and Management of Myocarditis. JACC 2025, 85, 391–431. [Google Scholar] [CrossRef]
- Sliwa, K.; Petrie, M.C.; Hilfiker-Kleiner, D.; Mebazaa, A.; Jackson, A.; Johnson, M.R.; van der Meer, P.; Mbakwem, A.; Bauersachs, J. Long-term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: Practical guidance paper from the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 951–962. [Google Scholar] [CrossRef]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Baruteau, A.E.; Donal, E.; Daubert, J.C. Letter by Baruteau et al regarding article, ‘Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy’. Circulation 2011, 123, e8–e9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elkayam, U. Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis, and management. J. Am. Coll. Cardiol. 2011, 58, 659–670. [Google Scholar] [CrossRef]
- Kažukauskienė, I.; Baltrūnienė, V.; Jakubauskas, A.; Žurauskas, E.; Maneikienė, V.V.; Daunoravičius, D.; Čelutkienė, J.; Ručinskas, K.; Grabauskienė, V. Prevalence and prognostic relevance of myocardial inflammation and cardiotropic viruses in non-ischemic dilated cardiomyopathy. Cardiol. J. 2022, 29, 441–453. [Google Scholar] [CrossRef]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Petramala, L.; Concistrè, A.; Olmati, F.; Saracino, V.; Chimenti, C.; Frustaci, A.; Russo, M.A.; Letizia, C. Cardiomyopathies and Adrenal Diseases. Int. J. Mol. Sci. 2020, 21, 5047. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, S.; Boro, H.; Farooqui, F.A.; Alam, S. Endocrine causes of heart failure: A clinical primer for cardiologists. Indian Heart J. 2021, 73, 14–21. [Google Scholar] [CrossRef]
- Lisco, G.; Giagulli, V.A.; Iovino, M.; Zupo, R.; Guastamacchia, E.; De Pergola, G.; Iacoviello, M.; Triggiani, V. Endocrine system dysfunction and chronic heart failure: A clinical perspective. Endocrine 2022, 75, 360–376. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, N.; Hua, W.; Cheng, S.; Niu, H.; Chen, X.; Gu, M.; Cai, C.; Liu, X.; Huang, H.; et al. Nomogram predicting death and heart transplantation before appropriate ICD shock in dilated cardiomyopathy. ESC Heart Fail. 2022, 9, 1269–1278. [Google Scholar] [CrossRef]
- Kian, W.; Zemel, M.; Kestenbaum, E.H.; Alguayn, W.; Shvarts, B.; Sharb, A.A.; Yakobson, A. Cardiomyopathy etiologies, symptoms and management. In Cardiomyopathy-Disease of the Heart Muscle; IntechOpen: London, UK, 2021. [Google Scholar]
- Lau, E.W.; Bonnemeier, H.; Baldauf, B. Left bundle branch block-Innocent bystander, silent menace, or both. Heart Rhythm. 2024. [Google Scholar] [CrossRef]
- Ponnusamy, S.S.; Vijayaraman, P.; Ellenbogen, K.A. Left Bundle Branch Block-associated Cardiomyopathy: A New Approach. Arrhythm Electrophysiol. Rev. 2024, 13, e15. [Google Scholar] [CrossRef]
- Gatti, P.; Lind, S.; Kristjánsdóttir, I.; Azari, A.; Savarese, G.; Anselmino, M.; Linde, C.; Gadler, F. Prognosis of CRT-treated and CRT-untreated unselected population with LBBB in Stockholm County. EP Eur. 2023, 25, euad192. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, M.; Zhang, M.; Liu, D.; Li, Z.; Du, J.; Hou, Y. Treatment of atrial fibrillation: A comprehensive review and practice guide. Cardiovasc. J. Afr. 2020, 31, 153–158. [Google Scholar] [CrossRef]
- Zafeiropoulos, S.; Doundoulakis, I.; Bekiaridou, A.; Farmakis, I.T.; Papadopoulos, G.E.; Coleman, K.M.; Giannakoulas, G.; Zanos, S.; Tsiachris, D.; Duru, F.; et al. Rhythm vs Rate Control Strategy for Atrial Fibrillation: A Meta-Analysis of Randomized Controlled Trials. JACC Clin. Electrophysiol. 2024, 10 Pt 1, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Panizo, J.G.; Barra, S.; Mellor, G.; Heck, P.; Agarwal, S. Premature Ventricular Complex-induced Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2018, 7, 128–134. [Google Scholar] [CrossRef]
- Latchamsetty, R.; Bogun, F. Premature Ventricular Complex–Induced Cardiomyopathy. JACC Clin. Electrophysiol. 2019, 5, 537–550. [Google Scholar] [CrossRef]
- Marcus, G.M. Evaluation and Management of Premature Ventricular Complexes. Circulation 2020, 141, 1404–1418. [Google Scholar] [CrossRef]
- Mazzaccara, C.; Limongelli, G.; Petretta, M.; Vastarella, R.; Pacileo, G.; Bonaduce, D.; Salvatore, F.; Frisso, G. A common polymorphism in the SCN5A gene is associated with dilated cardiomyopathy. J. Cardiovasc. Med. 2018, 19, 344–350. [Google Scholar] [CrossRef]
- Rastogi, P.; Dua, A.; Attri, S.; Sharma, H. Hypothyroidism-induced reversible dilated cardiomyopathy. J. Postgrad. Med. 2018, 64, 177–179. [Google Scholar] [CrossRef]
- Seidel, F.; Holtgrewe, M.; Al-Wakeel-Marquard, N.; Opgen-Rhein, B.; Dartsch, J.; Herbst, C.; Beule, D.; Pickardt, T.; Klingel, K.; Messroghli, D.; et al. Pathogenic Variants Associated With Dilated Cardiomyopathy Predict Outcome in Pediatric Myocarditis. Circ. Genom. Precis. Med. 2021, 14, e003250. [Google Scholar] [CrossRef] [PubMed]
- Kalekar, T.; Gupta, A.; Kumar, M. 3-Tesla cardiac magnetic resonance imaging in primary dilated cardiomyopathy. Afr. J. Thorac. Crit. Care Med. 2024, 30, e844. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Delgado, V.; Bucciarelli-Ducci, C.; Galli, E.; Haugaa, K.H.; Charron, P.; Voigt, J.-U.; Cardim, N.; Masci, P.G.; Galderisi, M.; et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: An expert consensus document from the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 1075–1093. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Todiere, G.; Barison, A.; Fabiani, I.; Panichella, G.; Genovesi, D.; Bonino, L.; Clemente, A.; Cademartiri, F.; et al. Role of Imaging in Cardiomyopathies. Card. Fail. Rev. 2023, 9, e08. [Google Scholar] [CrossRef]
- Casas, G.; Rodríguez-Palomares, J.F. Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis. J. Clin. Med. 2022, 11, 578. [Google Scholar] [CrossRef]
- Iovănescu, M.L.; Florescu, D.R.; Marcu, A.S.; Donoiu, I.; Militaru, S.; Florescu, C.; Istrătoaie, O.; Militaru, C. The Dysfunctional Right Ventricle in Dilated Cardiomyopathies: Looking from the Right Point of View. J. Cardiovasc. Dev. Dis. 2022, 9, 359. [Google Scholar] [CrossRef]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Alpendurada, F.; Guha, K.; Ismail, N.A.; Raza, S.; Khwaja, J.; Brown, T.D.; Morarji, K.; et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013, 128, 1623–1633. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, H.; Wang, Y.; Yu, Y.; Chen, R. A pilot study of S100A4, S100A8/A9, and S100A12 in dilated cardiomyopathy: Novel biomarkers for diagnosis or prognosis? ESC Heart Fail. 2024, 11, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, S.; Jing, R.; Jin, H.; Hu, Y.; Wang, J.; Gu, M.; Niu, H.; Zhang, S.; Chen, L.; et al. Plasma Metabolomic Profiles Differentiate Patients With Dilated Cardiomyopathy and Ischemic Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 597546. [Google Scholar] [CrossRef]
- Klüser, L.; Maier, E.T.; Wess, G. Evaluation of a high-sensitivity cardiac troponin I assay compared to a first-generation cardiac troponin I assay in Doberman Pinschers with and without dilated cardiomyopathy. J. Vet. Intern. Med. 2019, 33, 54–63. [Google Scholar] [CrossRef]
- Shao, X.N.; Sun, Y.J.; Xiao, K.T.; Zhang, Y.; Zhang, W.B.; Kou, Z.F.; Cheng, J.L. Texture analysis of magnetic resonance T1 mapping with dilated cardiomyopathy: A machine learning approach. Medicine 2018, 97, e12246. [Google Scholar] [CrossRef]
- Sun, M.; Kilaru, V.; Majeed, H.; Patel, S.; Mihilli, A.; Acosta, G. Heart in Disguise: Unmasking a Novel Gene Deletion in Dilated Cardiomyopathy. Cureus 2024, 16, e55170. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Wu, C.; Wang, F. Multimodal MRI Analysis of Cervical Cancer on the Basis of Artificial Intelligence Algorithm. Contrast Media Mol. Imaging 2021, 2021, 1673490. [Google Scholar] [CrossRef] [PubMed]
- Wolterink, J.M.; Leiner, T.; Viergever, M.A.; Isgum, I. Automatic Segmentation and Disease Classification Using Cardiac Cine MR Images. arXiv 2017, arXiv:1708.01141. [Google Scholar] [CrossRef]
- Duffy, G.; Cheng, P.P.; Yuan, N.; He, B.; Kwan, A.C.; Shun-Shin, M.J.; Alexander, K.M.; Ebinger, J.; Lungren, M.P.; Rader, F.; et al. High-Throughput Precision Phenotyping of Left Ventricular Hypertrophy with Cardiovascular Deep Learning. arXiv 2021, arXiv:2106.12511. [Google Scholar] [CrossRef] [PubMed]
- Madaudo, C.; Parlati, A.L.M.; Di Lisi, D.; Carluccio, R.; Sucato, V.; Vadalà, G.; Nardi, E.; Macaione, F.; Cannata, A.; Manzullo, N.; et al. Artificial intelligence in cardiology: A peek at the future and the role of ChatGPT in cardiology practice. J. Cardiovasc. Med. 2024, 25, 766–771. [Google Scholar] [CrossRef]
- Willenheimer, R. The current role of beta-blockers in chronic heart failure: With special emphasis on the CIBIS III trial. Eur. Heart J. Suppl. 2009, 11 (Suppl. A), A15–A20. [Google Scholar] [CrossRef]
- Wikstrand, J. MERIT-HF-description of the trial. Basic Res. Cardiol. 2000, 95 (Suppl. S1), I90–I97. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Liebau, G. Therapy of dilated cardiomyopathy with digitalis, diuretics and vasodilators. Herz 1985, 10, 138–142. [Google Scholar]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.A.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004, 350, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Pereira, N.L.; Shah, D.K.; Boilson, B.; Schirger, J.A.; Kushwaha, S.S.; Joyce, L.D.; Park, S.J. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ. Heart Fail. 2011, 4, 266–275. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Gallina, S.; Di Mauro, M.; Gaeta, F.; Iacò, A.L.; D’Alessandro, S.; Mazzei, V.; Di Giammarco, G. Mitral valve procedure in dilated cardiomyopathy: Repair or replacement? Ann. Thorac. Surg. 2001, 71, 1146–1152. [Google Scholar] [CrossRef]
- Jiao, R.; Liu, Y.; Yang, W.J.; Zhu, X.Y.; Li, J.; Tang, Q.Z. Effects of stem cell therapy on dilated cardiomyopathy. Saudi Med. J. 2014, 35, 1463–1468. [Google Scholar] [PubMed]
- Matsuura, N.; Saitou, K.; Hidaka, H. Generalized pitting edema in a patient with dilated cardiomyopathy secondary to hypothyroidism. CMAJ 2023, 195, E10–E13. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Dziedzic, R.; Surdacki, A.; Chyrchel, B. Thyroid Hormones-An Underestimated Player in Dilated Cardiomyopathy? J. Clin. Med. 2021, 10, 3618. [Google Scholar] [CrossRef]
- Jeon, G.J.; Song, B.G.; Park, Y.H.; Kang, G.H.; Chun, W.J.; Oh, J.H. Acute Stroke and Limb Ischemia Secondary to Catastrophic Massive Intracardiac Thrombus in a 40-Year-Old Patient With Dilated Cardiomyopathy. Cardiol. Res. 2012, 3, 37–40. [Google Scholar] [CrossRef]
- Barbiero, G.; Cognolato, D.; Casarin, A.; Guarise, A. Intra-arterial thrombolysis of acute hand ischaemia with or without microcatheter: Preliminary experience and comparison with the literature. Radiol. Med. 2011, 116, 919–931. [Google Scholar] [CrossRef]
- Crosetti, E.; Caracciolo, A.; Molteni, G.; Sprio, A.E.; Berta, G.N.; Presutti, L.; Succo, G. Unravelling the risk factors that underlie laryngeal surgery in elderly. Acta Otorhinolaryngol. Ital. 2016, 36, 185–193. [Google Scholar] [CrossRef]
- Jakulla, R.S.; Gunta, S.P.; Huded, C.P. Heart Failure after Aortic Valve Replacement: Incidence, Risk Factors, and Implications. J. Clin. Med. 2023, 12, 6048. [Google Scholar] [CrossRef]
- Bientinesi, R.; Recupero, S.M.; Palermo, G.; D’Agostino, D.; Bassi, P.F.; Sacco, E. Chirurgia dell’incontinenza urinaria maschile: A che punto siamo? Surgery for male urinary incontinence: Where are we now and what is in the pipeline? Urologia 2015, 82, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, G.; Chen, Y.; Liu, S.; Chen, F.; Xie, L.; Wang, W.; Zhang, Y.; Wang, T.; Lai, X.; et al. Human umbilical cord mesenchymal stem cells alleviate interstitial fibrosis and cardiac dysfunction in a dilated cardiomyopathy rat model by inhibiting TNF α and TGF β1/ERK1/2 signaling pathways. Mol. Med. Rep. 2018, 17, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, Y.; Li, X.; Gong, J.; Shen, L.; Zhang, R. A new discovered gene mutation in a child with dilated cardiomyopathy. Cardiol. Young 2021, 31, 1530–1531. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Wang, Z.; Wang, X.; Yang, Z.; Bao, L. Efficacy and safety of stem cell therapy in patients with dilated cardiomyopathy: A systematic appraisal and meta-analysis. J. Transl. Med. 2019, 17, 221. [Google Scholar] [CrossRef]
- Levett, J.J.; Georgiopoulos, M.; Martel, S.; Mugheiry, W.A.; Stavropoulos, N.A.; Vega-Arroyo, M.; Demetriades, A.K. Pharmacological treatment of degenerative cervical myelopathy: A critical review of current evidence. Neurospine 2024, 21, 375–400. [Google Scholar] [CrossRef]
- Conti, F.; Sili, A.; Vellone, E.; Alvaro, R. Le motivazioni al posizionamento di un accesso vascolare centrale ad inserzione periferica: l’esperienza di un picc team. Scenar. Il Nurs. Nella Sopravvivenza 2018, 30, 4–10. [Google Scholar] [CrossRef]
- Harding, D.; Chong, M.H.A.; Lahoti, N.; Bigogno, C.M.; Prema, R.; Mohiddin, S.A.; Marelli-Berg, F. Dilated cardiomyopathy and chronic cardiac inflammation: Pathogenesis, diagnosis and therapy. J. Intern. Med. 2023, 293, 23–47. [Google Scholar] [CrossRef]
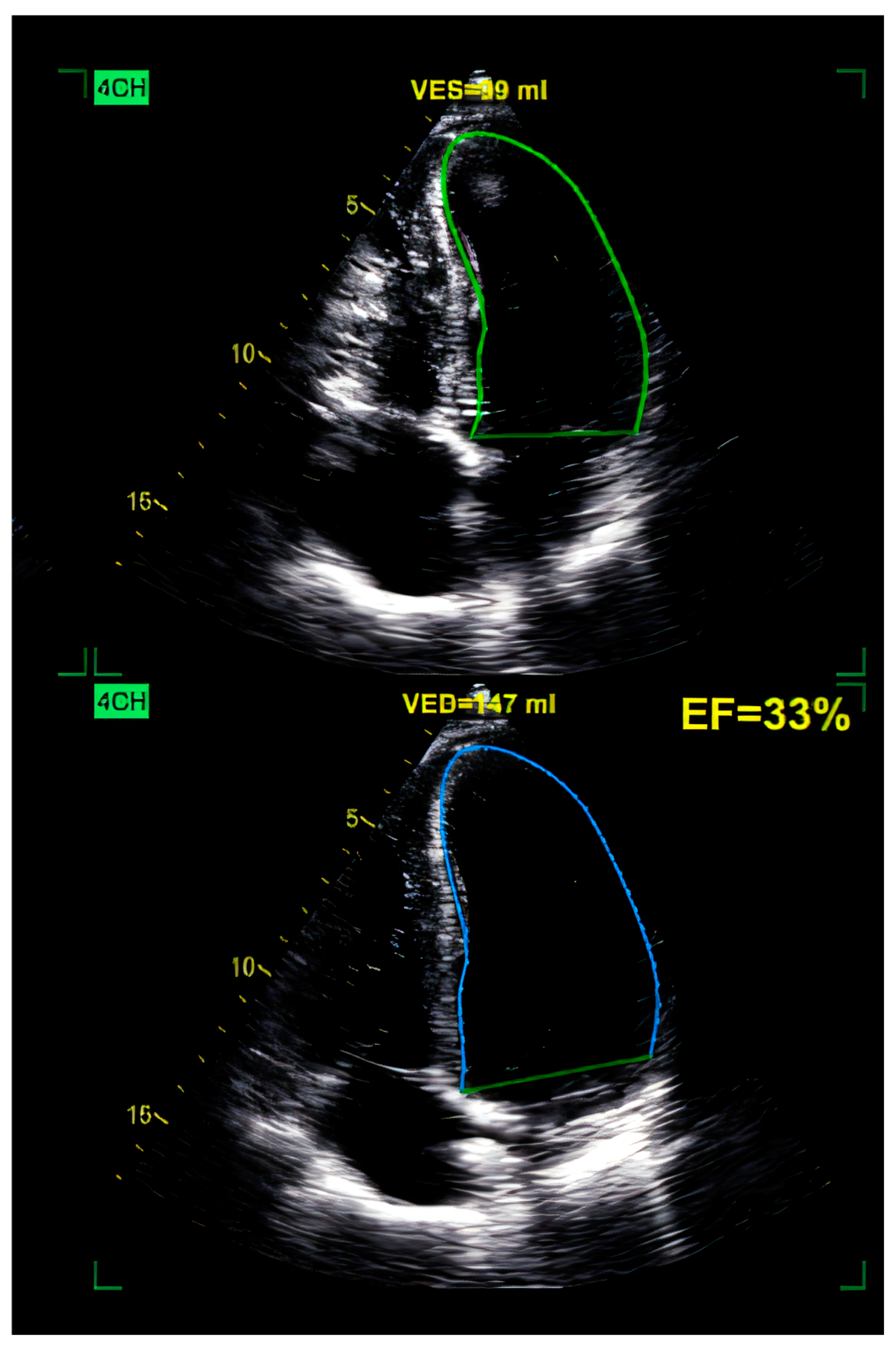
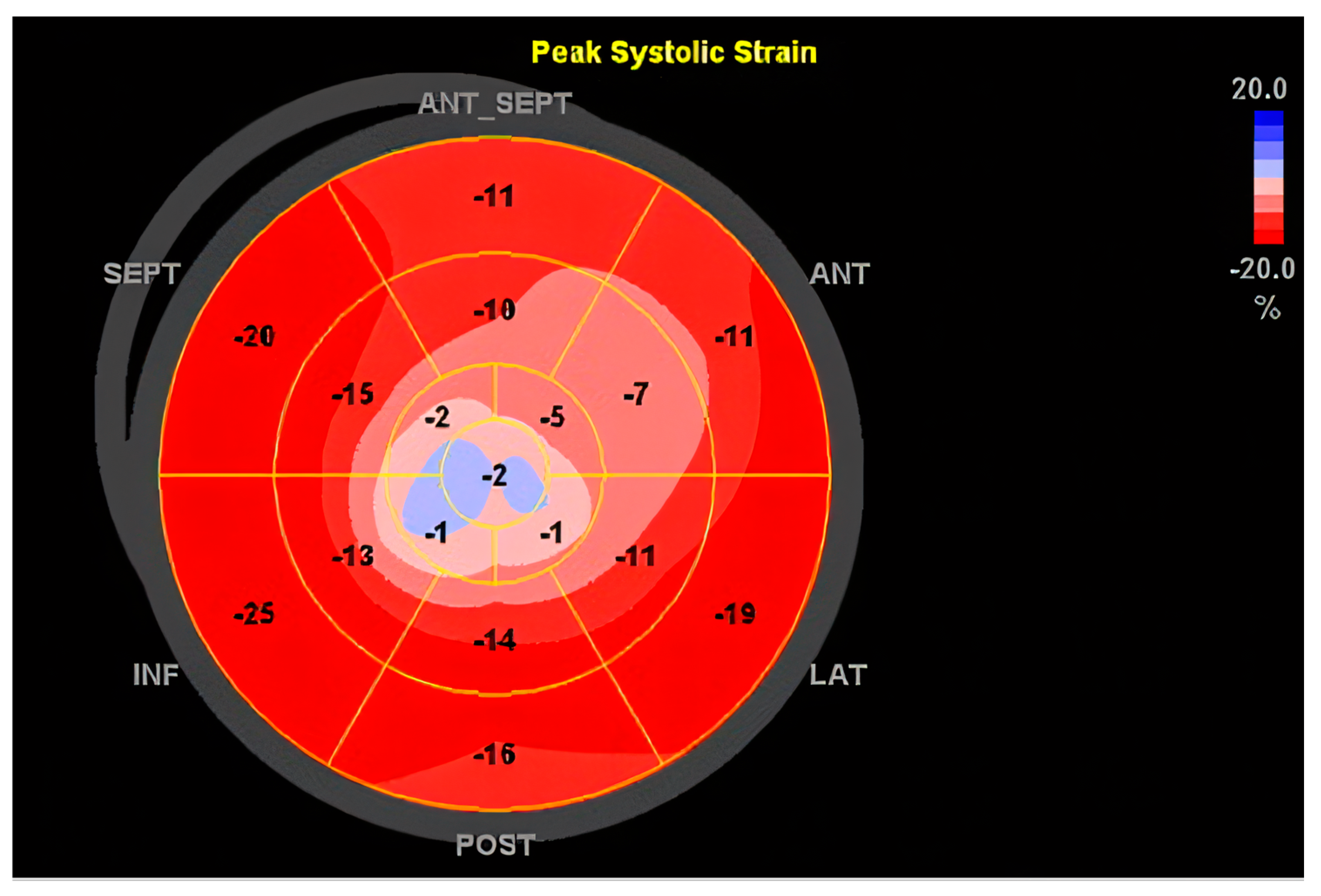
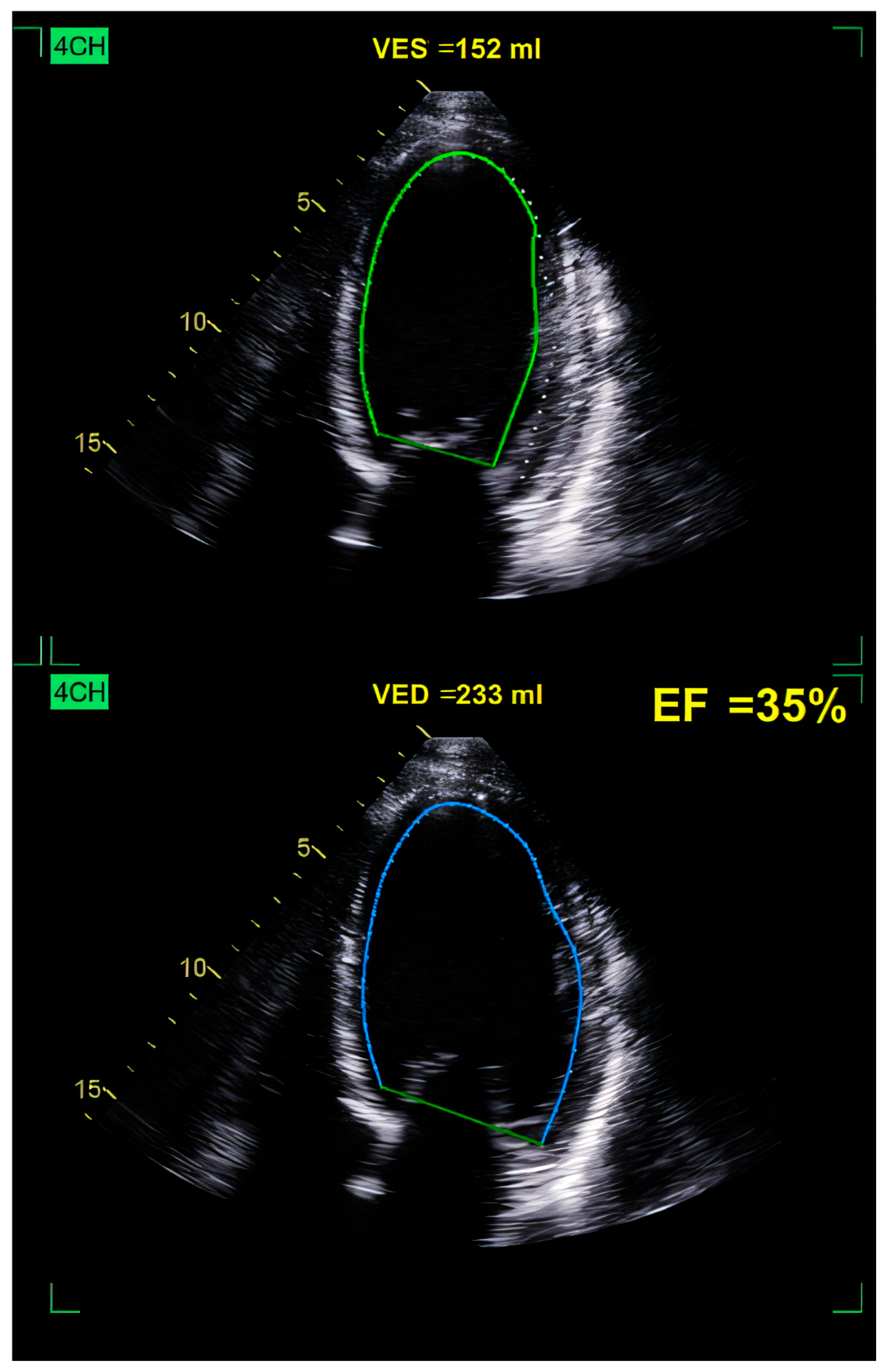

| Category | Details |
|---|---|
| Regional Prevalence | Higher in tropical/less developed regions (Briceno, Schuster et al., 2016 [16]) |
| Contribution to Heart Failure | Represents 30–50% of DCM cases (Heymans, Lakdawala et al., 2023 [9]) |
| Developed Countries—Common Causes | Linked to hypertension, coronary artery disease (Sivitz and Nagdev, 2012 [18]) |
| Developing Countries—Common Causes | Infectious causes like Chagas disease prevalent in Latin America |
| Hospitalized Patients | Accounts for 10–20% among hospitalized HF patients (Verdonschot and Heymans, 2023 [17]) |
| Specific Risk Factors | Chemotherapy (e.g., anthracyclines), alcohol abuse, systemic inflammatory diseases, older age, male sex |
| Nutritional Deficiencies | Vitamin D deficiency; example from Ethiopia (Moges, Shiferaw et al., 2017 [20]) |
| Socioeconomic Disparities | Limited access to nutrition and healthcare in low-income communities (Moges, Shiferaw et al., 2017 [20]) |
| Etiological Class | Definition | Common Causes | Diagnostic Tools | Estimated Incidence |
|---|---|---|---|---|
| Toxic | Cardiomyopathy resulting from exposure to harmful substances, including drugs, alcohol, and chemicals. | Anthracyclines (e.g., doxorubicin), alcohol, heavy metals, illicit drugs | History of toxin exposure, ECG, echocardiography, cardiac MRI | Common in cancer patients (up to 9% for anthracyclines); varies by exposure type |
| Infectious | Myocardial damage due to viral, bacterial, or parasitic infections. | Coxsackievirus, Cytomegalovirus, Trypanosoma cruzi (Chagas disease) | Serology, cardiac MRI, endomyocardial biopsy | Significant in endemic areas (e.g., Chagas: 30% of infected individuals) |
| Autoimmune | Cardiac inflammation and dysfunction due to immune system attacking the myocardium. | Systemic lupus erythematosus, sarcoidosis, post-viral immune activation | Autoantibodies, inflammatory markers, CMR, biopsy | Low to moderate; varies with underlying autoimmune disease prevalence |
| Endocrine | Cardiomyopathy secondary to hormonal imbalances or deficiencies. | Hypothyroidism, hyperthyroidism, adrenal insufficiency | Hormonal panels (TSH, cortisol), echocardiography | Variable; hypothyroidism and adrenal insufficiency-induced DCM are rare but reversible |
| Peripartum | DCM occurring during the last trimester of pregnancy or postpartum period. | Pregnancy-associated hormonal changes and oxidative stress | Echocardiography, BNP levels, clinical correlation with pregnancy | Incidence ranges from 1 in 1000 to 1 in 3000 live births globally |
| Nutritional | Cardiomyopathy due to deficiency of key nutrients essential for cardiac function. | Vitamin D, selenium, calcium deficiencies; post-bariatric surgery malnutrition | Blood nutrient levels, echocardiography, clinical history | Rare; more frequent in low-resource settings and post-bariatric surgery patient |
| Chemotherapy-Induced | Cardiac dysfunction secondary to antineoplastic treatments leading to systolic impairment and ventricular dilation. | Anthracyclines, trastuzumab, cyclophosphamide, ICIs, 5-FU, bevacizumab | Cardiac MRI, biomarkers (troponin, BNP), echocardiography (strain imaging), clinical monitoring | Up to 9% in patients receiving anthracyclines; varies by agent and patient risk factors |
| Dyssynchronopathy (LBBB-Induced DCM) | Cardiomyopathy due to electromechanical dyssynchrony from left bundle branch block (LBBB), reversible with resynchronization. | Isolated LBBB, post-surgical conduction block | ECG, echocardiography with dyssynchrony analysis, cardiac MRI | Subset of heart failure with reduced EF; improves with CRT in selected patients |
| AF-Induced DCM | Tachycardia-induced cardiomyopathy due to persistent atrial fibrillation. | Chronic uncontrolled AF | ECG, Holter monitoring, echocardiography | Up to 25% of patients with AF-related heart failure show LV recovery with rhythm control |
| VES-Induced DCM | Cardiomyopathy due to high burden of ventricular ectopic beats; reversible after arrhythmia control. | Frequent monomorphic PVCs (>10,000/day), idiopathic or structural | ECG, Holter, echocardiography, electrophysiological study | ~5% of patients with high PVC burden may develop reversible DCM |
| Imaging Technique | Strengths | Limitations |
|---|---|---|
| Transthoracic Echocardiography (TTE) | Widely available, non-invasive, real-time assessment of cardiac function and chamber size | Operator-dependent, limited by poor acoustic windows, limited tissue characterization |
| Cardiac Magnetic Resonance (CMR) | High-resolution imaging, tissue characterization (e.g., fibrosis), accurate volume, and EF measurements | Costly, limited availability, contraindications in patients with metal implants or severe renal dysfunction |
| Cardiac CT | Excellent for coronary artery visualization and calcium scoring; quick scan times | Radiation exposure, less informative for functional assessment, limited soft tissue detail |
| Nuclear Imaging | Assesses perfusion, metabolism, and receptor expression; useful for specific causes like sarcoidosis | Radiation exposure, lower spatial resolution, limited availability, expensive |
| Therapy | Indications | Benefits | Limitations |
|---|---|---|---|
| Thyroid Hormone Replacement (Levothyroxine) | DCM due to hypothyroidism | Reduces LV size, improves ejection fraction, and may reverse DCM | Requires careful hormone monitoring and dose adjustment |
| Glucocorticoid Therapy (Corticosteroids) | DCM due to adrenal insufficiency | Can result in full cardiac function recovery | Diagnosis of adrenal insufficiency may be delayed or missed |
| Anticoagulation Therapy | Presence of ventricular thrombi or high thromboembolic risk | Reduces thromboembolic complications, improves prognosis | Risk of bleeding; requires monitoring of coagulation status |
| ACE Inhibitors and Beta-Blockers | Heart failure symptoms and blood pressure control in DCM | Improves symptoms, reduces mortality in heart failure | Does not address the underlying cause of secondary DCM directly |
| Surgical Procedure | Indications | Strengths | Limitations |
|---|---|---|---|
| Mitral valve repair | Mitral insufficiency due to DCM | Improves ejection fraction, limits heart failure symptoms | Surgical risks are not suitable for all patients |
| Aortic valve replacement | Aortic stenosis leading to DCM | Restores cardiac function, lowers all-cause mortality | Invasive, requires thorough assessment for suitability |
| Heart transplant | End-stage heart failure unresponsive to other treatments | Improves survival and quality of life in end-stage DCM | Limited donor availability, immune suppression required |
| Catheter ablation | Atrial fibrillation/frequent PVCs | May restore sinus rhythm and reduce long-term chances of ischemic events | Surgical risks are not suitable for all patients |
| Cardiac resynchronization therapy (CRT) | Dyssynchronopathy due to LBBB | Restores normal electrical conduction, improves EF | Surgical risks, not suitable for all patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundnani, N.R.; Di Luca, F.; Meche, V.; Sharma, A.; Popa, M.-D.; Nicula-Neagu, M.; Voinescu, O.R.; Iacob, M.; Duda-Seiman, D.-M.; Dragan, S.R. Revisiting Secondary Dilative Cardiomyopathy. Int. J. Mol. Sci. 2025, 26, 4181. https://doi.org/10.3390/ijms26094181
Kundnani NR, Di Luca F, Meche V, Sharma A, Popa M-D, Nicula-Neagu M, Voinescu OR, Iacob M, Duda-Seiman D-M, Dragan SR. Revisiting Secondary Dilative Cardiomyopathy. International Journal of Molecular Sciences. 2025; 26(9):4181. https://doi.org/10.3390/ijms26094181
Chicago/Turabian StyleKundnani, Nilima Rajpal, Federico Di Luca, Vlad Meche, Abhinav Sharma, Mihaela-Diana Popa, Marioara Nicula-Neagu, Oana Raluca Voinescu, Mihai Iacob, Daniel-Marius Duda-Seiman, and Simona Ruxanda Dragan. 2025. "Revisiting Secondary Dilative Cardiomyopathy" International Journal of Molecular Sciences 26, no. 9: 4181. https://doi.org/10.3390/ijms26094181
APA StyleKundnani, N. R., Di Luca, F., Meche, V., Sharma, A., Popa, M.-D., Nicula-Neagu, M., Voinescu, O. R., Iacob, M., Duda-Seiman, D.-M., & Dragan, S. R. (2025). Revisiting Secondary Dilative Cardiomyopathy. International Journal of Molecular Sciences, 26(9), 4181. https://doi.org/10.3390/ijms26094181








