(Pro)renin Receptor Blockade Prevents Increases in Systolic Blood Pressure, Sodium Retention, and αENaC Protein Expression in the Kidney of 2K1C Goldblatt Mice
Abstract
1. Introduction
2. Results
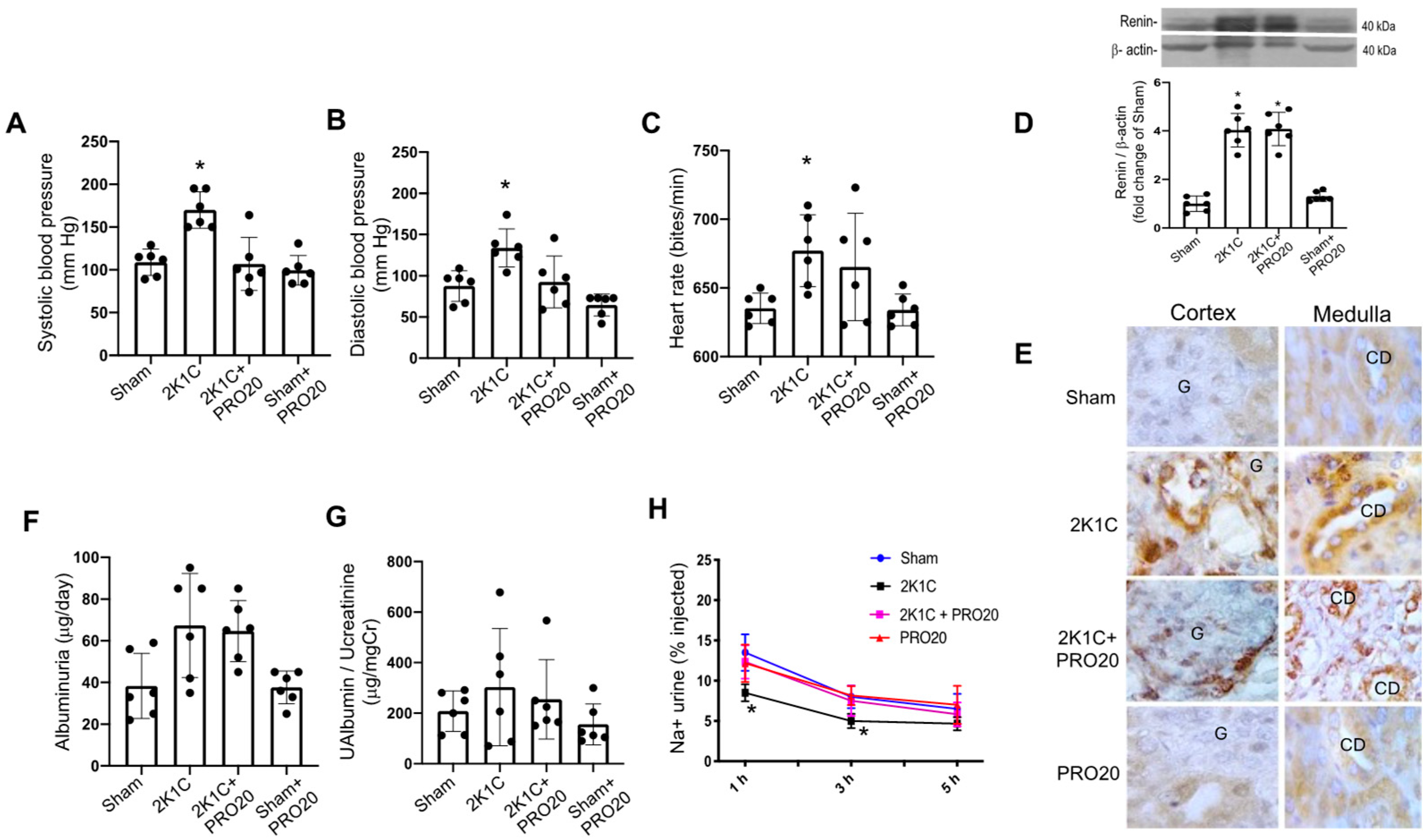
2.1. K1C Goldblatt Model Showed Increased Blood Pressure and Reduced Natriuretic Ability, However, PRO20 Prevented This Effect
2.2. Kidney Histological Characteristics
2.3. Intrarenal ACE Activity and Ang II Levels
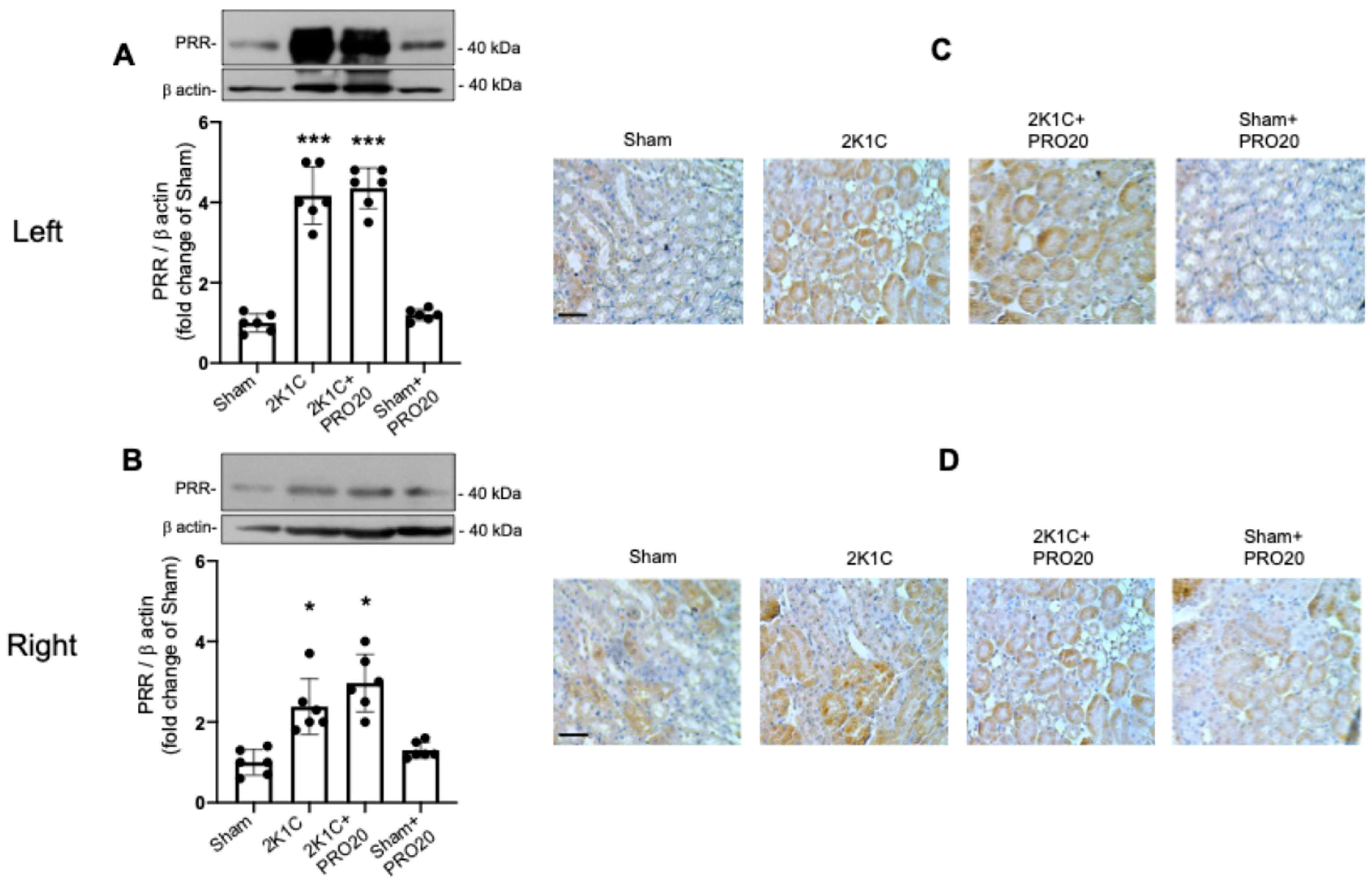
2.4. Upregulation of the PRR in 2K1C and 2K1C + PRO20 Mice
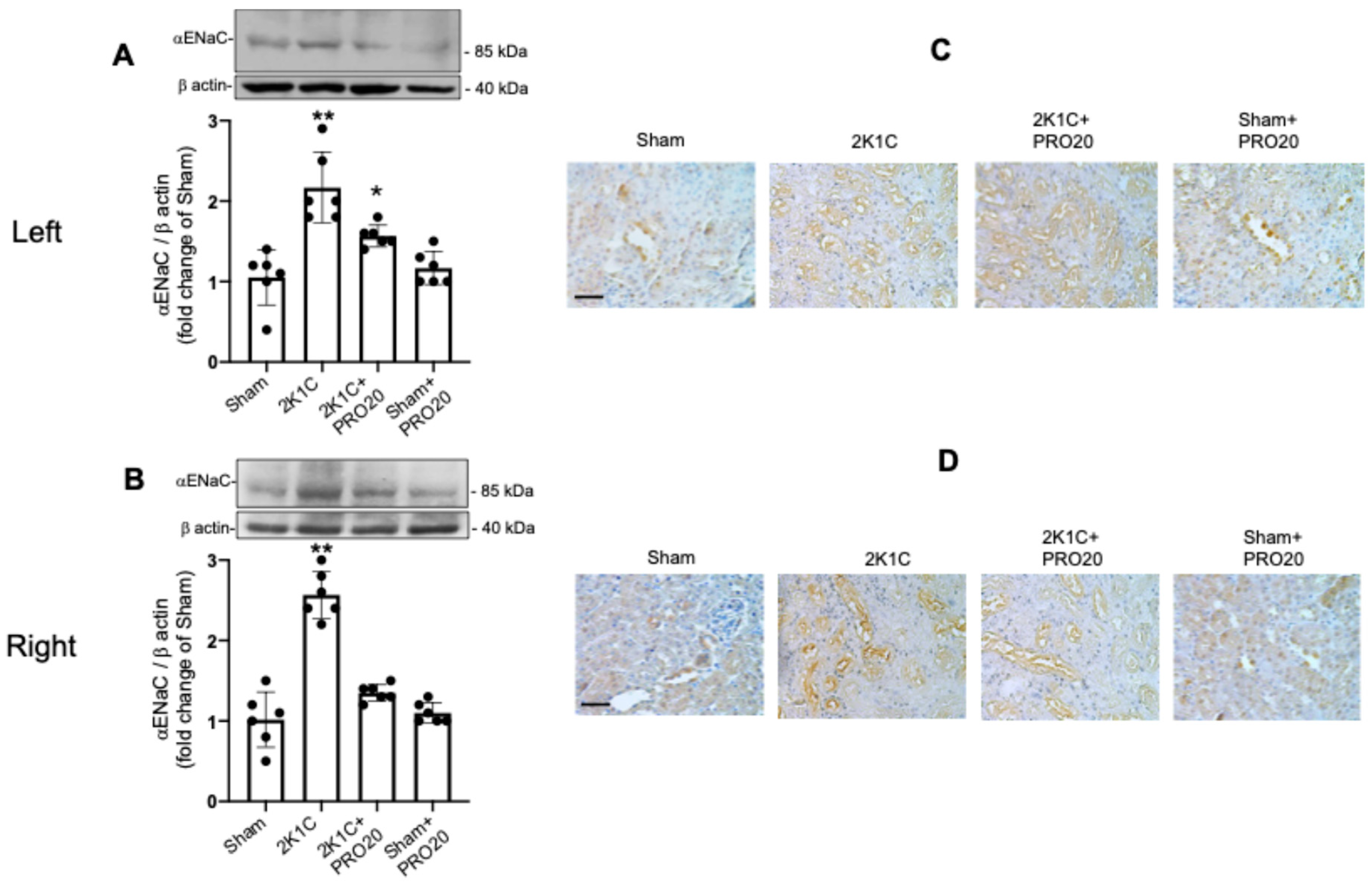
2.5. Upregulation of αENaC in the Non-Clipped Kidneys of 2K1C Mice Is Prevented by PRO20
3. Discussion
4. Materials and Methods
4.1. Animals
K1C Goldblatt Surgery
4.2. PRO20 Peptide Administration
4.3. Blood Pressure Measurements
4.4. Urine Flow, Water Intake, Food Intake, and 24-Hour Sodium Excretion
4.5. Saline Challenge Test
4.6. Immunoblotting Analyses
4.7. Histology
4.8. Immunostaining of Renin, PRR and αENaC
4.9. Angiotensin II Kidney Content
4.10. ACE Enzymatic Activity
4.11. Urinary Creatinine
4.12. Urinary Albumin
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fountain, J.H.; Lappin, S.L. Physiology, Renin Angiotensin System; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Ferrario, C.M. Importance of the renin-angiotensin-aldosterone system (RAS) in the physiology and pathology of hypertension. An overview. Drugs 1990, 39 (Suppl. 2), 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Whelton, P.K. Elevated systolic blood pressure and risk of cardiovascular and renal disease: Overview of evidence from observational epidemiologic studies and randomized controlled trials. Am. Heart J. 1999, 138, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Erdine, S.; Aran, S.N. Current status of hypertension control around the world. Clin. Exp. Hypertens. 2004, 26, 731–738. [Google Scholar] [CrossRef]
- Gonzalez-Villalobos, R.A.; Satou, R.; Semprun-Prieto, L.C.; Katsurada, A.; Seth, D.M.; Kobori, H.; Navar, L.G. Effects of ACE inhibition on intrarenal angiotensin II content and blood pressure during angiotensin II-induced hypertension. Hypertension 2008, 52, E125. [Google Scholar]
- Mamenko, M.; Zaika, O.; Pochynyuk, O. Direct regulation of ENaC by bradykinin in the distal nephron. Implications for renal sodium handling. Curr. Opin. Nephrol. Hypertens. 2014, 23, 122–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, Y.Y.; Kobori, H.; Nakano, D.; Hitomi, H.; Mori, H.; Masaki, T.; Sun, Y.X.; Zhi, N.; Zhang, L.; Huang, W.; et al. Aberrant activation of the intrarenal renin-angiotensin system in the developing kidneys of type 2 diabetic rats. Horm. Metab. Res. 2013, 45, 338–343. [Google Scholar] [CrossRef]
- Giani, J.F.; Shah, K.H.; Khan, Z.; Bernstein, E.A.; Shen, X.Z.; McDonough, A.A.; Gonzalez-Villalobos, R.A.; Bernstein, K.E. The intrarenal generation of angiotensin II is required for experimental hypertension. Curr. Opin. Pharmacol. 2015, 21, 73–81. [Google Scholar] [CrossRef]
- Zhuang, Z.; Bai, Q.; Liang, Y.; Zheng, D.; Wang, Y. Increased urinary angiotensinogen precedes the onset of albuminuria in normotensive type 2 diabetic patients. Int. J. Clin. Exp. Pathol. 2015, 8, 11464–11469. [Google Scholar]
- Nishiyama, A.; Konishi, Y.; Ohashi, N.; Morikawa, T.; Urushihara, M.; Maeda, I.; Hamada, M.; Kishida, M.; Hitomi, H.; Shirahashi, N.; et al. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2011, 26, 170–177. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Liu, L.; Lara, L.S.; Seth, D.M.; Navar, L.G.; Prieto, M.C. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 2011, 57, 594–599. [Google Scholar] [CrossRef]
- van den Heuvel, M.; Batenburg, W.W.; Jainandunsing, S.; Garrelds, I.M.; van Gool, J.M.G.; Feelders, R.A.; van den Meiracker, A.H.; Danser, A.H.J. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. J. Hypertens. 2011, 29, 2147–2155. [Google Scholar] [CrossRef]
- Redublo Quinto, B.M.; Camargo de Andrade, M.C.; Ronchi, F.A.; Santos, E.L.; Alves Correa, S.A.; Shimuta, S.I.; Pesquero, J.B.; Mortara, R.A.; Casarini, D.E. Expression of angiotensin I-converting enzymes and bradykinin B-2 receptors in mouse inner medullary-collecting duct cells. Int. Immunopharmacol. 2008, 8, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Navar, L.G.; Harrison-Bernard, L.M. Intrarenal angiotensin II augmentation in angiotensin II dependent hypertension. Hypertens. Res. 2000, 23, 291–301. [Google Scholar] [CrossRef]
- Gonzalez-Villalobos, R.A.; Satou, R.; Ohashi, N.; Semprun-Prieto, L.C.; Katsurada, A.; Kim, C.; Upchurch, G.M.; Prieto, M.C.; Kobori, H.; Navar, L.G. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am. J. Physiol. Ren. 2010, 298, F150–F157. [Google Scholar] [CrossRef]
- Gonzalez-Villalobos, R.A.; Satou, R.; Seth, D.M.; Semprun-Prieto, L.C.; Katsurada, A.; Kobori, H.; Navar, L.G. Angiotensin-Converting Enzyme-Derived Angiotensin II Formation During Angiotensin II-Induced Hypertension. Hypertension 2009, 53, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Urushihara, M.; Xu, J.H.; Berenson, G.S.; Navar, L.G. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J. Hypertens. 2010, 28, 1422–1428. [Google Scholar] [CrossRef]
- Navar, L.G.; Lewis, L.; Hymel, A.; Braam, B.; Mitchell, K.D. Tubular Fluid Concentrations and Kidney Contents of Angiotensin-I and Angiotensin-Ii in Anesthetized Rats. J. Am. Soc. Nephrol. 1994, 5, 1153–1158. [Google Scholar] [CrossRef]
- Shao, W.; Seth, D.M.; Navar, L.G. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am. J. physiology. Ren. Physiol. 2009, 296, F1067–F1071. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Bernard, L.M.; Zhuo, J.L.; Kobori, H.; Ohishi, M.; Navar, L.G. Intrarenal AT(1) receptor and ACE binding in ANG II-induced hypertensive rats. Am. J. Physiol. Ren. 2002, 282, F19–F25. [Google Scholar] [CrossRef]
- Zaika, O.; Mamenko, M.; Staruschenko, A.; Pochynyuk, O. Direct Activation of ENaC by Angiotensin II: Recent Advances and New Insights. Curr. Hypertens. Rep. 2013, 15, 17–24. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Liu, L.; Lara, L.S.; Bourgeois, C.R.; Ibaceta-Gonzalez, C.; Salinas-Parra, N.; Gogulamudi, V.R.; Seth, D.M.; Prieto, M.C. PKC-alpha-dependent augmentation of cAMP and CREB phosphorylation mediates the angiotensin II stimulation of renin in the collecting duct. Am. J. Physiol. Ren. Physiol. 2015, 309, F880–F888. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Carrasquero, M.C.; Kobori, H.; Ozawa, Y.; Guttierrez, A.; Seth, D.; Navar, L.G. AT(1) receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am. J. Physiol. Ren. 2005, 289, F632–F637. [Google Scholar] [CrossRef]
- Ichihara, A.; Kaneshiro, Y.; Suzuki, F. Prorenin receptor blockers: Effects on cardiovascular complications of diabetes and hypertension. Expert Opin. Investig. Drugs 2006, 15, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, N.; Stuart, D.; Calquin, M.; Quadri, S.; Wang, S.; Van Hoek, A.N.; Siragy, H.M.; Ichihara, A.; Kohan, D.E. Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am. J. Physiol. Ren. Physiol. 2015, 309, F48–F56. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.C.; Reverte, V.; Mamenko, M.; Kuczeriszka, M.; Veiras, L.C.; Rosales, C.B.; McLellan, M.; Gentile, O.; Jensen, V.B.; Ichihara, A.; et al. Collecting duct prorenin receptor knockout reduces renal function, increases sodium excretion, and mitigates renal responses in ANG II-induced hypertensive mice. Am. J. Physiol. Ren. Physiol. 2017, 313, F1243–F1253. [Google Scholar] [CrossRef]
- Nguyen, G.; Contrepas, A. Physiology and pharmacology of the (pro)renin receptor. Curr. Opin. Pharmacol. 2008, 8, 127–132. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Zamora, L.; Reyes-Martinez, C.; Salinas-Parra, N.; Roldan, N.; Cuevas, C.A.; Figueroa, S.; Gonzalez-Vergara, A.; Prieto, M.C. (Pro)renin receptor activation increases profibrotic markers and fibroblast-like phenotype through MAPK-dependent ROS formation in mouse renal collecting duct cells. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1134–1144. [Google Scholar] [CrossRef]
- Hennrikus, M.; Gonzalez, A.A.; Prieto, M.C. The prorenin receptor in the cardiovascular system and beyond. Am. J. Physiol. Heart C 2018, 314, H139–H145. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Lara, L.S.; Luffman, C.; Seth, D.M.; Prieto, M.C. Soluble Form of the (Pro) Renin Receptor Is Augmented in the Collecting Duct and Urine of Chronic Angiotensin II-Dependent Hypertensive Rats. Hypertension 2011, 57, 859–864. [Google Scholar] [CrossRef]
- Guan, S.; Fox, J.; Mitchell, K.D.; Navar, L.G. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension 1992, 20, 763–767. [Google Scholar] [CrossRef]
- Wiesel, P.; Mazzolai, L.; Nussberger, J.; Pedrazzini, T. Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension 1997, 29, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, H.S.; Morrill, A.L.; Ploth, D.W. Pressure dependence of exaggerated natriuresis in two-kidney, one clip Goldblatt hypertensive rats. Kidney Int. 1985, 27, 731–738. [Google Scholar] [CrossRef]
- Kopkan, L.; Huskova, Z.; Sporkova, A.; Varcabova, S.; Honetschlagerova, Z.; Hwang, S.H.; Tsai, H.J.; Hammock, B.D.; Imig, J.D.; Kramer, H.J.; et al. Soluble epoxide hydrolase inhibition exhibits antihypertensive actions independently of nitric oxide in mice with renovascular hypertension. Kidney Blood Press. Res. 2012, 35, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, P.; Nunez-Allimant, C.; Silva, K.; Cid-Salinas, C.; Leon, A.C.; Vallotton, Z.; Lorca, R.A.; Oliveira, L.C.G.; Casarini, D.E.; Cespedes, C.; et al. OXGR1-Dependent (Pro)Renin Receptor Upregulation in Collecting Ducts of the Clipped Kidney Contributes to Na+ Balance in Goldblatt Hypertensive Mice. Int. J. Mol. Sci. 2024, 25, 10045. [Google Scholar] [CrossRef]
- Prieto, M.C.; Botros, F.T.; Kavanagh, K.; Navar, L.G. Prorenin receptor in distal nephron segments of 2-kidney, 1-clip goldblatt hypertensive rats. Ochsner J. 2013, 13, 26–32. [Google Scholar]
- Wang, F.; Lu, X.; Peng, K.; Fang, H.; Zhou, L.; Su, J.; Nau, A.; Yang, K.T.; Ichihara, A.; Lu, A.; et al. Antidiuretic Action of Collecting Duct (Pro)Renin Receptor Downstream of Vasopressin and PGE2 Receptor EP4. J. Am. Soc. Nephrol. 2016, 27, 3022–3034. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lu, X.H.; Liu, M.; Feng, Y.M.; Zhou, S.F.; Yang, T.X. Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med. 2015, 13, 278. [Google Scholar] [CrossRef]
- Peng, K.; Lu, X.; Wang, F.; Nau, A.; Chen, R.; Zhou, S.F.; Yang, T. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. Am. J. Physiol. Ren. Physiol. 2017, 312, F245–F253. [Google Scholar] [CrossRef]
- Fang, H.; Xu, C.M.; Lu, A.H.; Zou, C.J.; Xie, S.Y.; Chen, Y.T.; Zhou, L.; Liu, M.; Wang, L.; Wang, W.D.; et al. (Pro)renin receptor mediates albumin-induced cellular responses: Role of site-1 protease-derived soluble (pro)renin receptor in renal epithelial cells. Am. J. Physiol. Cell Physiol. 2017, 313, C632–C643. [Google Scholar] [CrossRef]
- Danser, A.H.J. The Role of the (Pro)renin Receptor in Hypertensive Disease. Am. J. Hypertens. 2015, 28, 1187–1196. [Google Scholar] [CrossRef]
- Ramkumar, N.; Stuart, D.; Mironova, E.; Abraham, N.; Gao, Y.; Wang, S.; Lakshmipathi, J.; Stockand, J.D.; Kohan, D.E. Collecting duct principal, but not intercalated, cell prorenin receptor regulates renal sodium and water excretion. Am. J. Physiol. Ren. Physiol. 2018, 315, F607–F617. [Google Scholar] [CrossRef]
- Ramkumar, N.; Stuart, D.; Mironova, E.; Bugay, V.; Wang, S.P.; Abraham, N.; Ichihara, A.; Stockand, J.D.; Kohan, D.E. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am. J. Physiol. Ren. 2016, 311, F186–F194. [Google Scholar] [CrossRef]
- Alawi, L.F.; Dhakal, S.; Emberesh, S.E.; Sawant, H.; Hosawi, A.; Thanekar, U.; Grobe, N.; Elased, K.M. Effects of Angiotensin II Type 1A Receptor on ACE2, Neprilysin and KIM-1 in Two Kidney One Clip (2K1C) Model of Renovascular Hypertension. Front. Pharmacol. 2020, 11, 602985. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Carrasquero, M.C.; Botros, F.T.; Pagan, J.; Kobori, H.; Seth, D.M.; Casarini, D.E.; Navar, L.G. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension 2008, 51, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, F.; Liu, M.; Yang, K.T.; Nau, A.; Kohan, D.E.; Reese, V.; Richardson, R.S.; Yang, T. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: Involvement of Nox4-derived hydrogen peroxide. Am. J. Physiol. Ren. Physiol. 2016, 310, F1243–F1250. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lu, A.; Lu, X.; Zhang, L.; Fang, H.; Zhou, L.; Yang, T. Activation of Renal (Pro)Renin Receptor Contributes to High Fructose-Induced Salt Sensitivity. Hypertension 2017, 69, 339–348. [Google Scholar] [CrossRef]
- Xu, Q.B.; Jensen, D.D.; Peng, H.; Feng, Y.M. The critical role of the central nervous system (pro)renin receptor in regulating systemic blood pressure. Pharmacol. Ther. 2016, 164, 126–134. [Google Scholar] [CrossRef]
- Rosivall, L.; Rinder, D.F.; Champion, J.; Khosla, M.C.; Navar, L.G.; Oparil, S. Intrarenal Angiotensin-I Conversion at Normal and Reduced Renal Blood-Flow in the Dog. Am. J. Physiol. 1983, 245, F408–F415. [Google Scholar] [CrossRef]
- Shao, W.; Miyata, K.; Katsurada, A.; Satou, R.; Seth, D.M.; Rosales, C.B.; Prieto, M.C.; Mitchell, K.D.; Navar, L.G. Increased angiotensinogen expression, urinary angiotensinogen excretion, and tissue injury in nonclipped kidneys of two-kidney, one-clip hypertensive rats. Am. J. Physiol. Ren. Physiol. 2016, 311, F278–F290. [Google Scholar] [CrossRef]
- Prieto, M.C.; Gonzalez, A.A.; Visniauskas, B.; Navar, L.G. The evolving complexity of the collecting duct renin-angiotensin system in hypertension. Nat. Rev. Nephrol. 2021, 17, 481–492. [Google Scholar] [CrossRef]
- Ramkumar, N.; Stuart, D.; Rees, S.; Van Hoek, A.; Sigmund, C.D.; Kohan, D.E. Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am. J. Physiol. Ren. 2014, 307, F931–F938. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.; Hamming, I.; Sadaghiani, S.; Steinmetz, O.M.; Meyer-Schwesinger, C.; Fehr, S.; Stahl, R.A.K.; Garrelds, I.M.; Danser, A.H.J.; van Goor, H.; et al. Antihypertensive therapy upregulates renin and (pro) renin receptor in the clipped kidney of Goldblatt hypertensive rats. Kidney Int. 2007, 72, 725–730. [Google Scholar] [CrossRef][Green Version]
- Cousin, C.; Bracquart, D.; Contrepas, A.; Corvol, P.; Muller, L.; Nguyen, G. Soluble Form of the (Pro) Renin Receptor Generated by Intracellular Cleavage by Furin Is Secreted in Plasma. Hypertension 2009, 53, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Luo, R.; Peng, K.; Liu, X.; Xu, C.; Lu, X.; Soodvilai, S.; Yang, T. Soluble (pro)renin receptor regulation of ENaC involved in aldosterone signaling in cultured collecting duct cells. Am. J. Physiol. Ren. Physiol. 2020, 318, F817–F825. [Google Scholar] [CrossRef]
- Ryuzaki, M.; Ichihara, A.; Ohshima, Y.; Sakoda, M.; Kurauchi-Mito, A.; Narita, T.; Kinouchi, K.; Murohashi-Bokuda, K.; Nishiyama, A.; Itoh, H. Involvement of activated prorenin in the pathogenesis of slowly progressive nephropathy in the non-clipped kidney of two kidney, one-clip hypertension. Hypertens Res. 2011, 34, 301–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muller, D.N.; Klanke, B.; Feldt, S.; Cordasic, N.; Hartner, A.; Schmieder, R.E.; Luft, F.C.; Hilgers, K.F. (Pro) renin receptor peptide inhibitor "handle-region" peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension 2008, 51, 676–681. [Google Scholar] [CrossRef]
- Correa, J.W.N.; Boaro, K.R.; Sene, L.B.; Polidoro, J.Z.; Salles, T.A.; Martins, F.L.; Bendhack, L.M.; Girardi, A.C.C. Antiproteinuric and Hyperkalemic Mechanisms Activated by Dual Versus Single Blockade of the RAS in Renovascular Hypertensive Rats. Front. Physiol. 2021, 12, 656460. [Google Scholar] [CrossRef]
- Ichihara, A.; Kaneshiro, Y.; Takemitsu, T.; Sakoda, M.; Suzuki, F.; Nakagawa, T.; Nishiyama, A.; Inagami, T.; Hayashi, M. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension 2006, 47, 894–900. [Google Scholar] [CrossRef]
- Mahmud, H.; Candido, W.M.; van Genne, L.; Vreeswijk-Baudoin, I.; Yu, H.J.; van de Sluis, B.; van Deursen, J.; van Gilst, W.H.; Sillje, H.H.W.; de Boer, R.A. Cardiac Function and Architecture Are Maintained in a Model of Cardiorestricted Overexpression of the Prorenin-Renin Receptor. PLoS ONE 2014, 9, e89929. [Google Scholar] [CrossRef]
- Mahmud, H.; Sillje, H.H.W.; Cannon, M.V.; van Gilst, W.H.; de Boer, R.A. Regulation of the (pro)renin-renin receptor in cardiac remodelling. J. Cell. Mol. Med. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Cassis, L.A.; Police, S.B.; Yiannikouris, F.; Thatcher, S.E. Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 2008, 10, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Achard, V.; Boullu-Ciocca, S.; Desbriere, R.; Nguyen, G.; Grino, M. Renin receptor expression in human adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R274–R282. [Google Scholar] [CrossRef] [PubMed]
- Satou, R.; Penrose, H.; Navar, L.G. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr. Hypertens. Rep. 2018, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Araos, P.; Prado, C.; Lozano, M.; Figueroa, S.; Espinoza, A.; Berger, T.; Mak, T.W.; Jaisser, F.; Pacheco, R.; Michea, L.; et al. Dendritic cells are crucial for cardiovascular remodeling and modulate neutrophil gelatinase-associated lipocalin expression upon mineralocorticoid receptor activation. J. Hypertens. 2019, 37, 1482–1492. [Google Scholar] [CrossRef]
- Narumi, K.; Hirose, T.; Sato, E.; Mori, T.; Kisu, K.; Ishikawa, M.; Totsune, K.; Ishii, T.; Ichihara, A.; Nguyen, G.; et al. A functional (pro)renin receptor is expressed in human lymphocytes and monocytes. Am. J. Physiol. Ren. 2015, 308, F487–F499. [Google Scholar] [CrossRef]
- Friedland, J.; Silverstein, E. A sensitive fluorimetric assay for serum angiotensin-converting enzyme. Am. J. Clin. Pathol. 1976, 66, 416–424. [Google Scholar] [CrossRef]
- Ronchi, F.A.; Irigoyen, M.C.; Casarini, D.E. Association of somatic and N-domain angiotensin-converting enzymes from Wistar rat tissue with renal dysfunction in diabetes mellitus. J. Renin Angiotensin Aldosterone Syst. JRAAS 2007, 8, 34–41. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, P.; Cid-Salinas, C.; León, A.C.; Castillo-Geraldo, J.; de Oliveira, L.C.G.; Yokota, R.; Vallotton, Z.; Casarini, D.E.; Prieto, M.C.; Lorca, R.A.; et al. (Pro)renin Receptor Blockade Prevents Increases in Systolic Blood Pressure, Sodium Retention, and αENaC Protein Expression in the Kidney of 2K1C Goldblatt Mice. Int. J. Mol. Sci. 2025, 26, 4177. https://doi.org/10.3390/ijms26094177
Cárdenas P, Cid-Salinas C, León AC, Castillo-Geraldo J, de Oliveira LCG, Yokota R, Vallotton Z, Casarini DE, Prieto MC, Lorca RA, et al. (Pro)renin Receptor Blockade Prevents Increases in Systolic Blood Pressure, Sodium Retention, and αENaC Protein Expression in the Kidney of 2K1C Goldblatt Mice. International Journal of Molecular Sciences. 2025; 26(9):4177. https://doi.org/10.3390/ijms26094177
Chicago/Turabian StyleCárdenas, Pilar, Catalina Cid-Salinas, Allison C. León, Juan Castillo-Geraldo, Lilian Caroline Gonçalves de Oliveira, Rodrigo Yokota, Zoe Vallotton, Dulce Elena Casarini, Minolfa C. Prieto, Ramón A. Lorca, and et al. 2025. "(Pro)renin Receptor Blockade Prevents Increases in Systolic Blood Pressure, Sodium Retention, and αENaC Protein Expression in the Kidney of 2K1C Goldblatt Mice" International Journal of Molecular Sciences 26, no. 9: 4177. https://doi.org/10.3390/ijms26094177
APA StyleCárdenas, P., Cid-Salinas, C., León, A. C., Castillo-Geraldo, J., de Oliveira, L. C. G., Yokota, R., Vallotton, Z., Casarini, D. E., Prieto, M. C., Lorca, R. A., & Gonzalez, A. A. (2025). (Pro)renin Receptor Blockade Prevents Increases in Systolic Blood Pressure, Sodium Retention, and αENaC Protein Expression in the Kidney of 2K1C Goldblatt Mice. International Journal of Molecular Sciences, 26(9), 4177. https://doi.org/10.3390/ijms26094177





