Biochemical and Ultrasonographic Parameters Predicting Long-Term Hypothyroidism After Subacute Thyroiditis
Abstract
1. Introduction
2. Results
2.1. Univariate Analysis of Variables at Disease Presentation
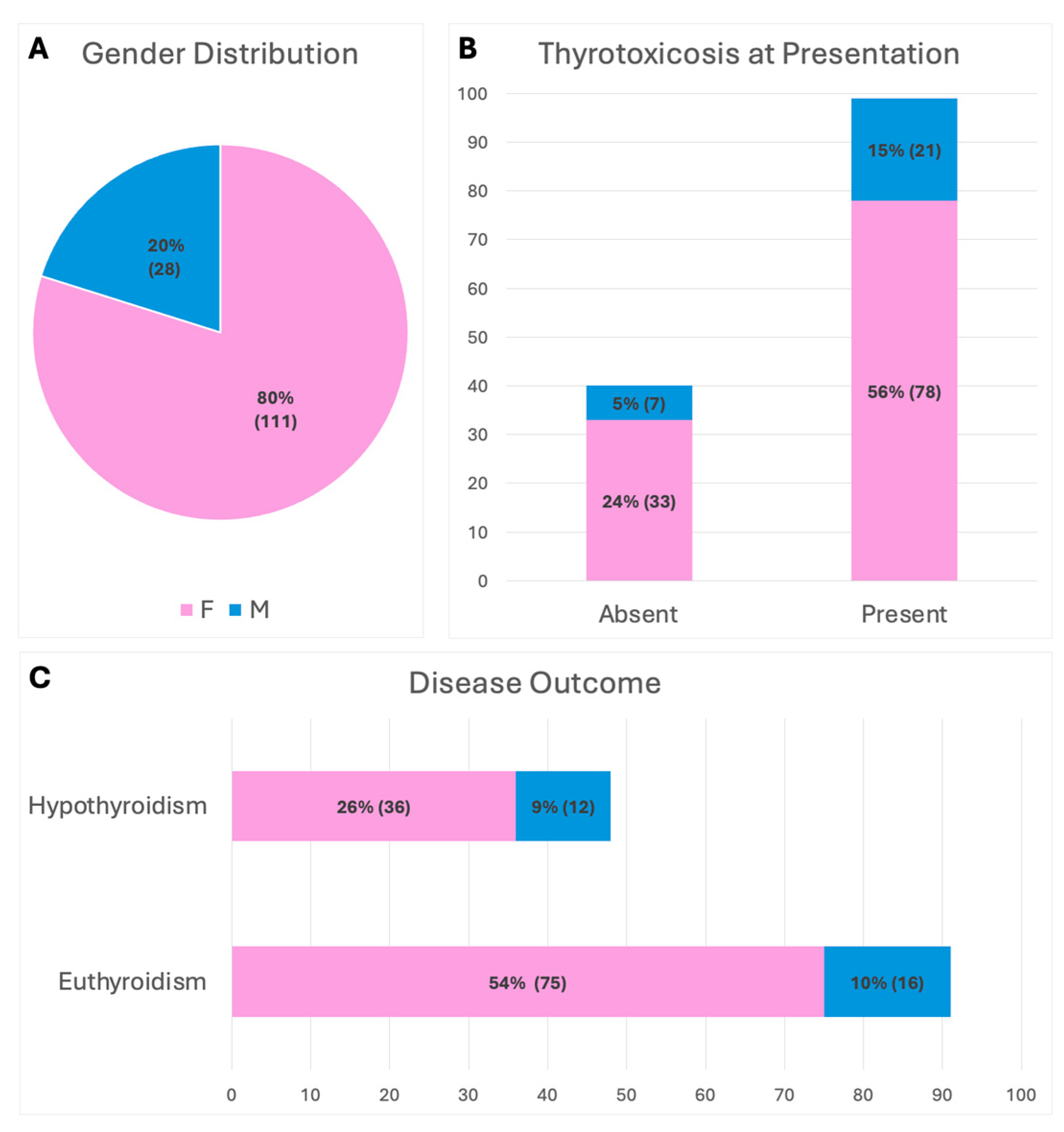
2.1.1. Clinical Variables and Inflammatory Index
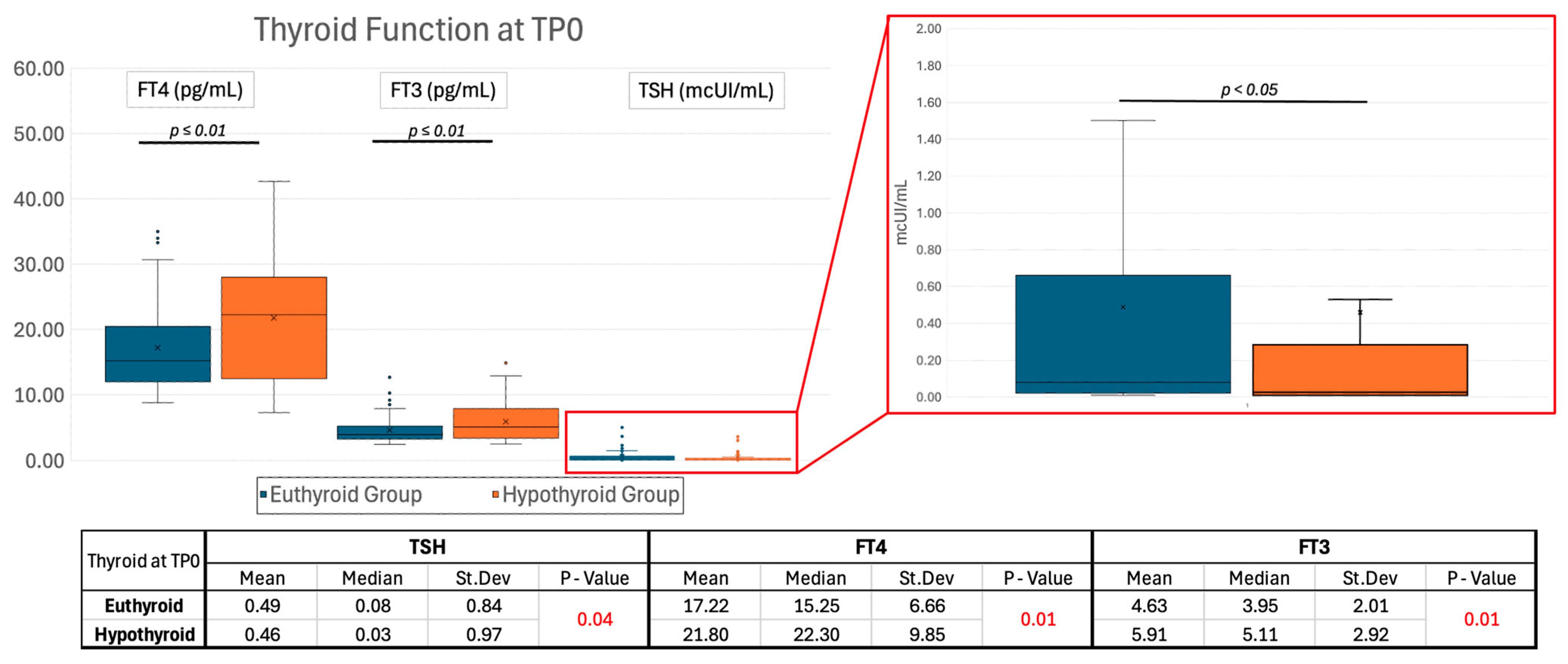
2.1.2. Thyroid Function
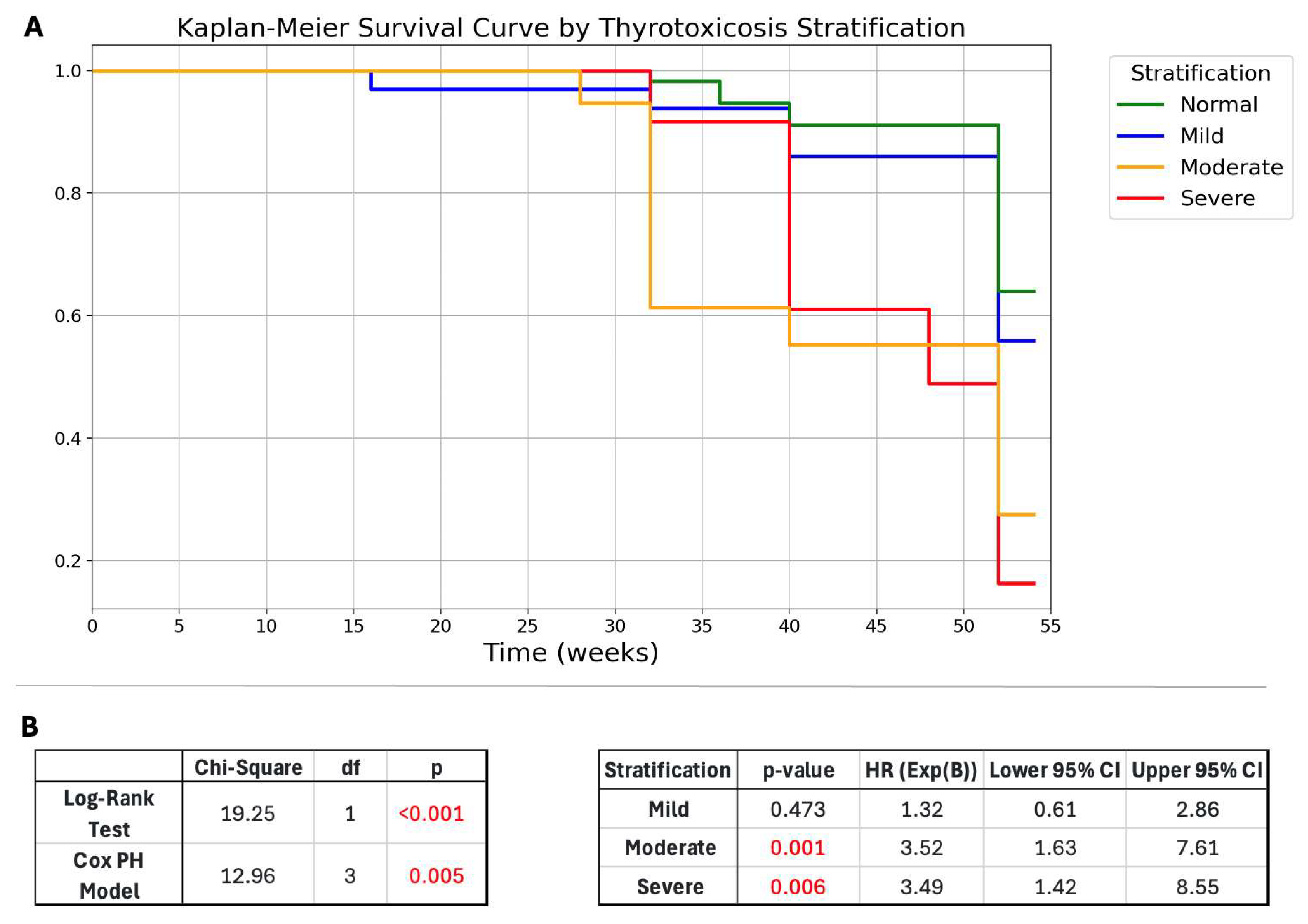
2.1.3. Disease Severity Stratification
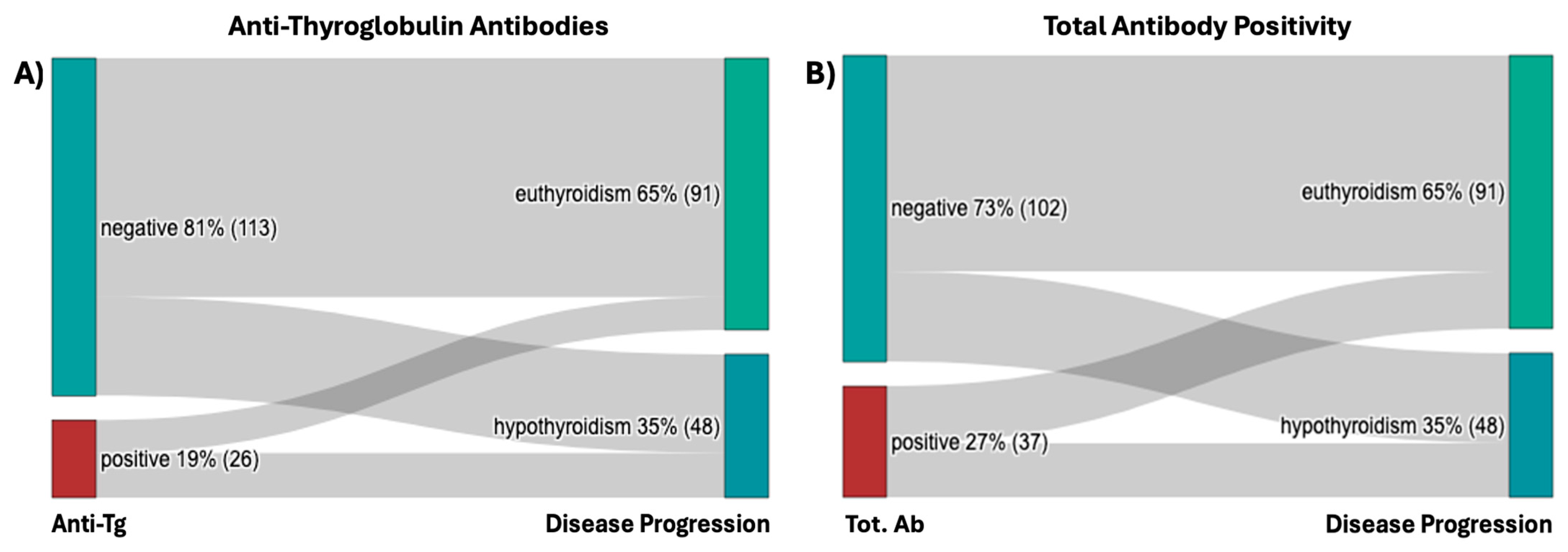
2.1.4. Autoantibodies
2.1.5. Ultrasound (US)
2.1.6. Therapy
2.2. Univariate Analysis of Variables During Disease Follow-Up
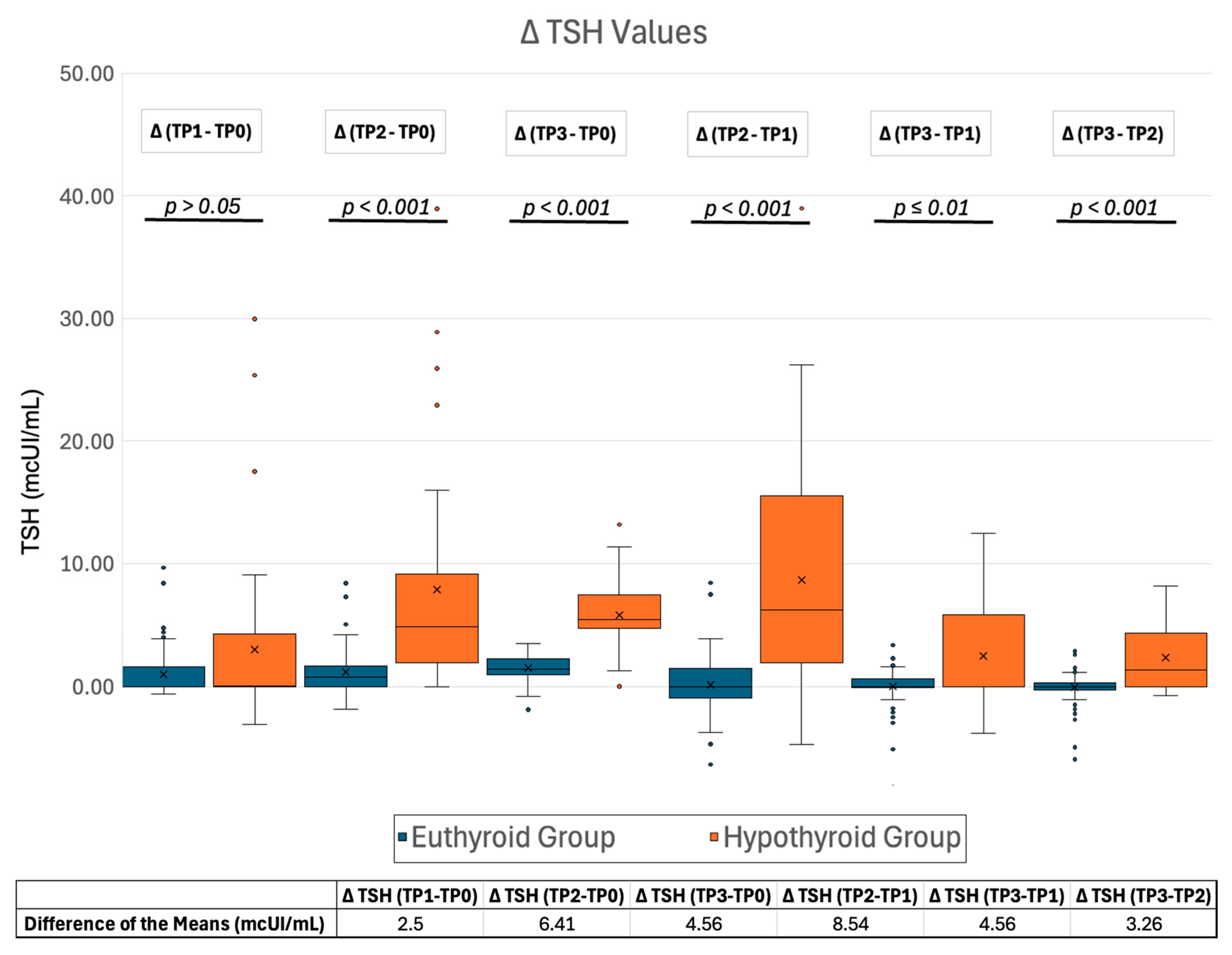
2.2.1. TSH Changes During Follow-Up
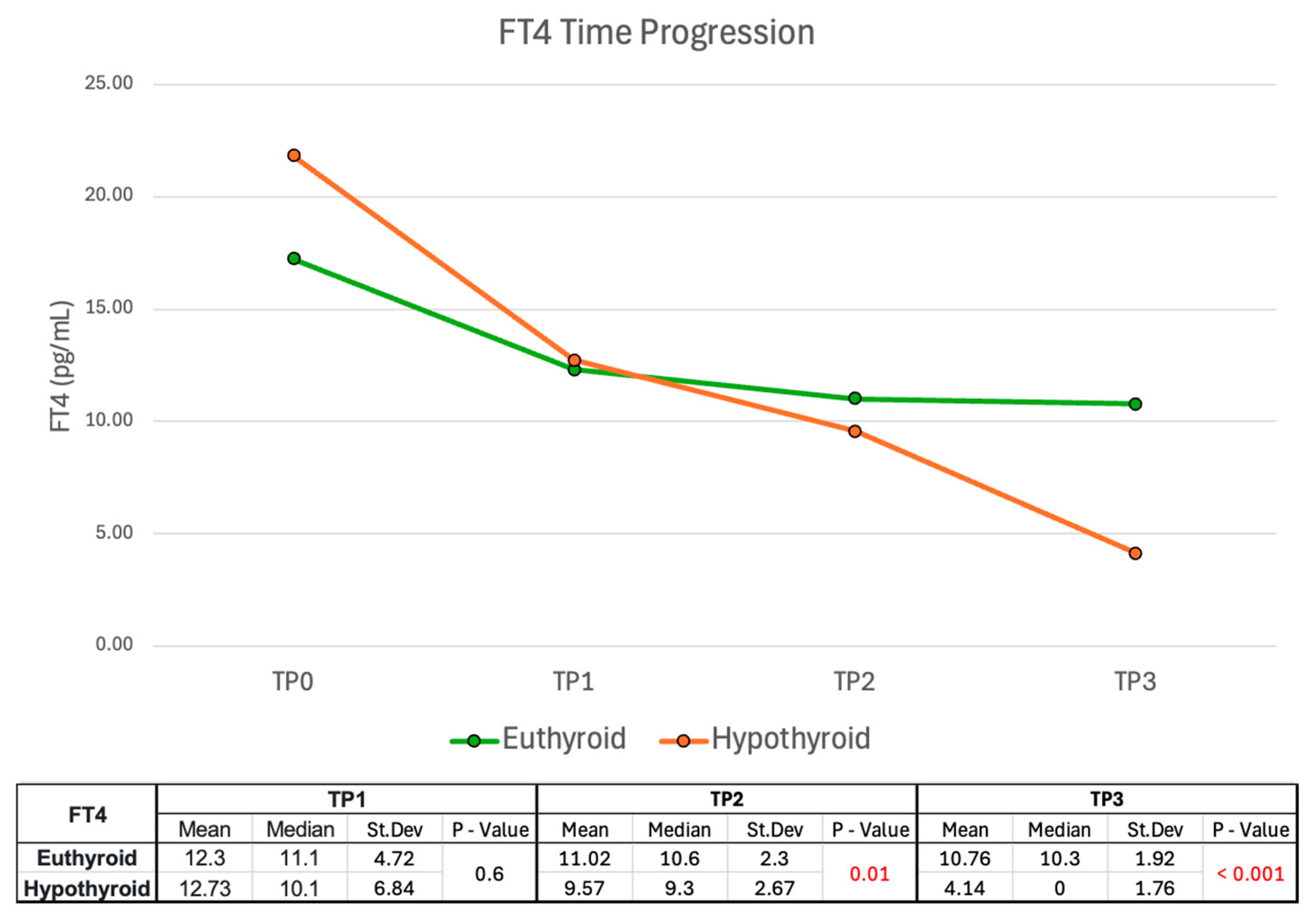
2.2.2. FT4 Time Progression
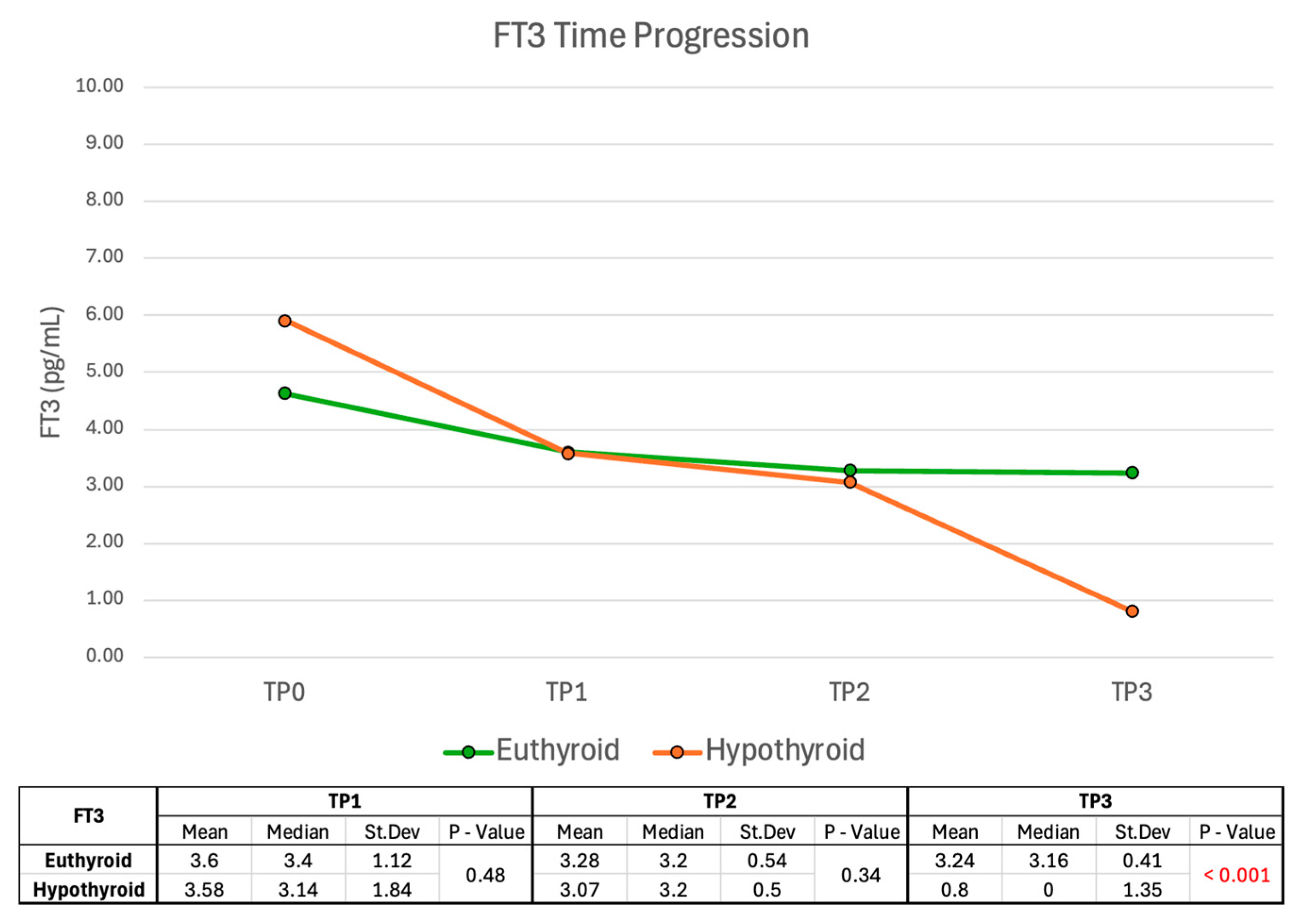
2.2.3. FT3 Time Progression
2.2.4. LT4 Supplementation at TP3
2.2.5. Ultrasound (US)
2.3. Multivariable Analysis
3. Discussion
4. Materials and Methods
4.1. Study Cohort and Evaluation Criteria
- Clinical parameters: age at diagnosis, Body Mass Index (BMI), smoking history, family history of thyroid disorders, maximum body temperature (afebrile if <37 °C, low-grade fever if between 37 °C and 38 °C, fever if >38 °C), pain in the thyroid lodge (0 if absent, 1 if mild, 2 if moderate and 3 if severe), month of onset and mean heart rate (HR).
- Biochemical parameters: TSH, FT4, FT3, thyroglobulin, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), white blood cell count (WBC), anti-thyroglobulin (anti-Tg) autoantibodies (Abs), anti-thyroperoxidase (Anti-TPO) Abs.
- Instrumental parameters: decreased, increased, or normal tracer uptake at thyroid scintigraphy (if available), US parameters (thyroid size, presence of hypoechogenic areas, vascularization).
- Mild thyrotoxicosis: FT4 1–1.5 times the upper limit of normal.
- Moderate thyrotoxicosis: FT4 1.5–2 times the upper limit of normal.
- Severe thyrotoxicosis: FT4 2–3 times the upper limit of normal.
- -
- they were included in the “hypothyroid group” if they experienced a new rise in TSH levels (>4 μUI/mL) following LT4 reduction or withdrawal at the last follow-up;
- -
- they were included in the “euthyroid group” if TSH levels following LT4 reduction or withdrawal were ≤4 μUI/mL at the last follow-up.
4.2. Treatment Protocols
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stasiak, M.; Lewinski, A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev. Endocr. Metab. Disord. 2021, 22, 1027–1039. [Google Scholar] [CrossRef]
- Dimos, G.; Pappas, G.; Akritidis, N. Subacute thyroiditis in the course of novel H1N1 influenza infection. Endocrine 2010, 37, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Desailloud, R.; Hober, D. Viruses and thyroiditis: An update. Virol. J. 2009, 6, 5. [Google Scholar] [CrossRef]
- Martínez-Artola, Y.; Poncino, D.; García, M.L.; Munné, M.S.; González, J.; García, D.S. Acute hepatitis e virus infection and association with a subacute thyroiditis. Ann. Hepatol. 2015, 14, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Brancatella, A.; Ricci, D.; Cappellani, D.; Viola, N.; Sgro, D.; Santini, F.; Latrofa, F. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J. Clin. Endocrinol. Metab. 2020, 105, e3742–e3746. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Pitoia, F.; Esposito, K.; Piccardo, A.; Trimboli, P. Impact of COVID-19 on the thyroid gland: An update. Rev. Endocr. Metab. Disord. 2020, 22, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Oyibo, S.O. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19). Cureus 2021, 13, e16045. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Woo, Y.C.; Hung, I.F.N.; Lam, K.S.L. Thyroid dysfunction in COVID-19. Nat. Rev. Endocrinol. 2024, 20, 336–348. [Google Scholar] [CrossRef]
- Nyulassy, S.; Hnilica, P.; Buc, M.; Guman, M.; Hirschova, V.; Stefanovic, J. Subacute (de quervain’s) thyroiditis: Association with Hla-Bw35 antigen and abnormalities of the complement system, immunoglobulins and other serum proteins. J. Clin. Endocrinol. Metab. 1977, 45, 270–274. [Google Scholar] [CrossRef]
- Stasiak, M.; Tymoniuk, B.; Stasiak, B.; Lewinski, A. The risk of recurrence of subacute thyroiditis is HLA-dependent. Int. J. Mol. Sci. 2019, 20, 1089. [Google Scholar] [CrossRef]
- Lanzo, N.; Patera, B.; Fazzino, G.; Gallo, D.; Lai, A.; Piantanida, E.; Ippolito, S.; Tanda, M. The old and the new in subacute thyroiditis: An integrative review. Endocrines 2022, 3, 391–410. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, S.; Cui, X.J.; Huang, M.S.; Wang, S.W.; Li, Y.G.; Zhao, L.; Wan, W.N.; Li, Y.S.; Shan, Z.Y.; et al. Two-years prospective follow-up study of subacute thyroiditis. Front. Endocrinol. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Weetman, A. Autoimmune thyroid disease. Endocrine 2020, 68, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H. Multivariable analysis: A primer for readers of medical research. Ann. Intern. Med. 2003, 138, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Reboldi, G.; Angeli, F.; Verdecchia, P. Multivariable analysis in cerebrovascular research: Practical notes for the clinician. Cerebrovasc. Dis. 2013, 35, 187–193. [Google Scholar] [CrossRef]
- Fatourechi, V.; Aniszewski, J.P.; Fatourechi, G.Z.; Atkinson, E.J.; Jacobsen, S.J. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted county, minnesota, study. J. Clin. Endocrinol. Metab. 2003, 88, 2100–2105. [Google Scholar] [CrossRef]
- Erdem, N.; Erdogan, M.; Ozbek, M.; Karadeniz, M.; Cetinkalp, S.; Ozgen, A.G.; Saygili, F.; Yilmaz, C.; Tuzun, M.; Kabalak, T. Demographic and clinical features of patients with subacute thyroiditis: Results of 169 patients from a single university center in turkey. J. Endocrinol. Investig. 2007, 30, 546–550. [Google Scholar] [CrossRef]
- Nishihara, E.; Amino, N.; Ohye, H.; Ota, H.; Ito, M.; Kubota, S.; Fukata, S.; Miyauchi, A. Extent of hypoechogenic area in the thyroid is related with thyroid dysfunction after subacute thyroiditis. J. Endocrinol. Investig. 2009, 32, 33–36. [Google Scholar] [CrossRef]
- Benbassat, C.A.; Olchovsky, D.; Tsvetov, G.; Shimon, I. Subacute thyroiditis: Clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J. Endocrinol. Investig. 2007, 30, 631–635. [Google Scholar] [CrossRef]
- Harding, A.T.; Heaton, N.S. The impact of estrogens and their receptors on immunity and inflammation during infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef]
- Gorges, J.; Ulrich, J.; Keck, C.; Muller-Wieland, D.; Diederich, S.; Janssen, O.E. Long-term outcome of subacute thyroiditis. Exp. Clin. Endocrinol. Diabetes 2019, 128, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 american thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Di Donna, V.; Locantore, P.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Factors predicting time to TSH normalization and persistence of TSH suppression after total thyroidectomy for graves’ disease. Front. Endocrinol. 2019, 10, 95. [Google Scholar] [CrossRef]
- Ciftel, S.; Tuzun, Z. Could the systemic immune inflammation index predict diagnosis, recovery time, hypothyroidism, and recurrence rates in subacute thyroiditis? Int. J. Gen. Med. 2023, 16, 1375–1382. [Google Scholar] [CrossRef]
- Nishihara, E.; Amino, N.; Kudo, T.; Kohsaka, K.; Ito, M.; Fukata, S.; Nishikawa, M.; Nakamura, H.; Miyauchi, A. Moderate frequency of anti-thyroglobulin antibodies in the early phase of subacute thyroiditis. Eur. Thyroid. J. 2019, 8, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Latrofa, F.; Ricci, D.; Montanelli, L.; Altea, M.A.; Pucci, A.; Pinchera, A.; Vitti, P. Thyroglobulin autoantibodies of patients with subacute thyroiditis are restricted to a major B cell epitope. J. Endocrinol. Investig. 2012, 35, 712–714. [Google Scholar] [CrossRef]
- Brancatella, A.; Viola, N.; Rutigliano, G.; Sgro, D.; Santini, F.; Latrofa, F. Subacute thyroiditis during the SARS-CoV-2 pandemic. J. Endocr. Soc. 2021, 5, bvab130. [Google Scholar] [CrossRef]
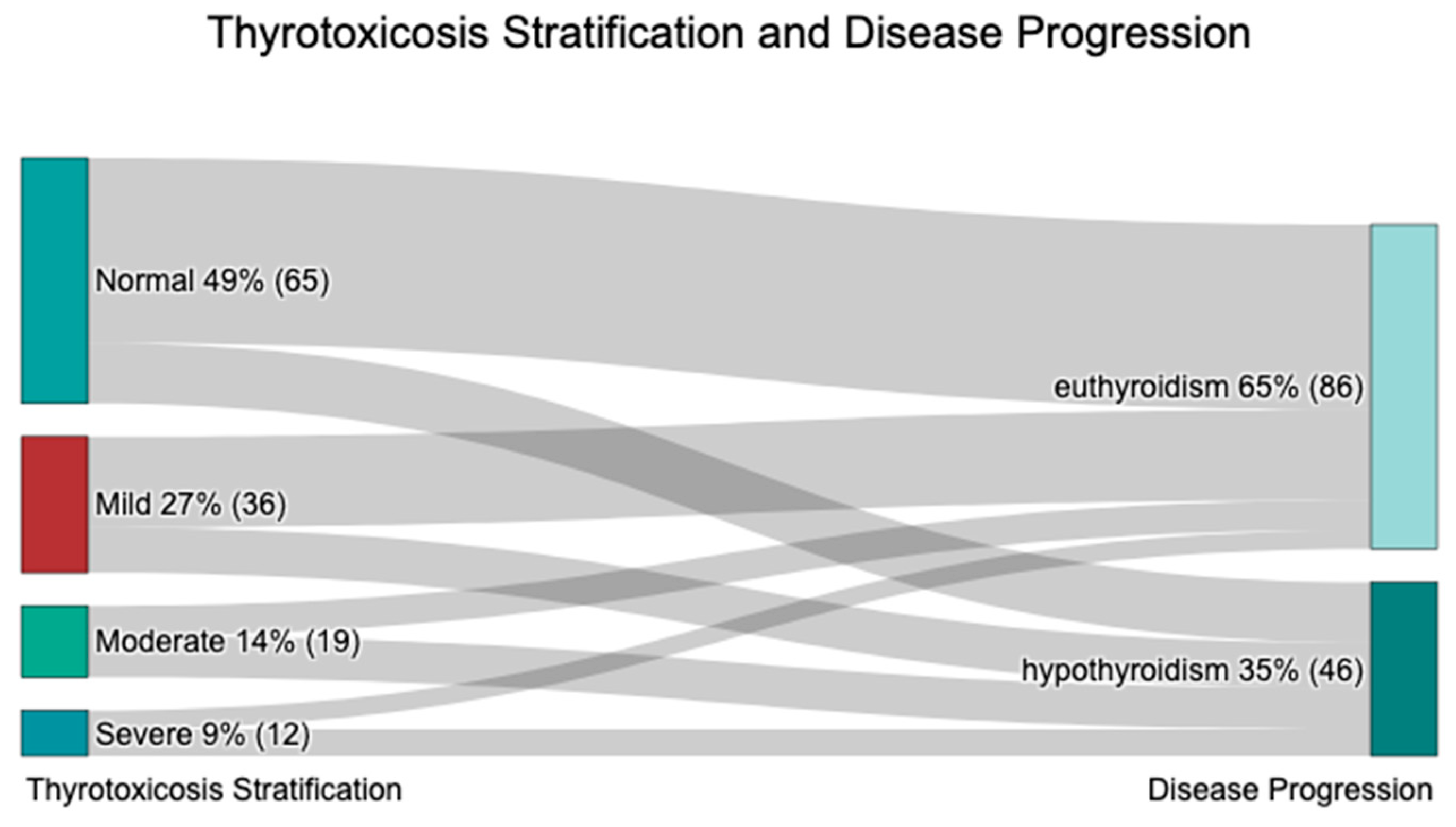

| Thyrotoxicosis Severity | Euthyroid | Hypothyroid | Grand Total | |
|---|---|---|---|---|
| Normal FT4 | 75.38% (49) | 24.62% (16) | 100% (65) | |
| Mild | 66.67% (24) | 33.33% (12) | 100% (36) | |
| Moderate | 42.11% (8) | 57.89% (11) | 100% (19) | |
| Severe | 41.67% (5) | 58.33% (7) | 100% (12) | |
| Grand Total | 65.15% (86) | 34.85% (46) | 100% (132) | |
| p-value | 0.003 |
| Abs at TP0 | ||||
|---|---|---|---|---|
| Anti-Tg | Euthyroid | Hypothyroid | Grand Total | |
| Negative | 70.80% (80) | 29.20% (33) | 100% (113) | |
| Positive | 42.31% (11) | 57.69% (15) | 100% (26) | |
| Grand Total | 65.47% (91) | 34.53% (48) | 100% (139) | |
| p-value | 0.0059 | |||
| Anti-TPO | Euthyroid | Hypothyroid | Grand Total | |
| Negative | 66.13% (82) | 33.87% (42) | 100% (124) | |
| Positive | 60.00% (9) | 40.00% (6) | 100% (15) | |
| Grand Total | 65.47% (91) | 34.53% (48) | 100% (139) | |
| p-value | 0.64 | |||
| Combined | Euthyroid | Hypothyroid | Grand Total | |
| Negative | 70.59% (72) | 29.41% (30) | 100% (102) | |
| Positive | 51.35% (19) | 51.35% (18) | 100% (37) | |
| Grand Total | 65.47% (91) | 34.53% (48) | 100% (139) | |
| p-value | 0.035 |
| Anti-Tg | Diminished | Increased | Normal | Grand Total | |
| Negative | 0.91% (1) | 54.55% (60) | 44.55% (49) | 100% (110) | |
| Positive | 0.00% (0) | 61.54% (16) | 38.46% (10) | 100% (26) | |
| Grand Total | 0.74% (1) | 55.88% (76) | 43.38% (59) | 100% (136) | |
| p-value | 0.55 | ||||
| Anti-TPO | Diminished | Increased | Normal | Grand Total | |
| Negative | 0.00% (0) | 54.10% (66) | 45.90% (56) | 100% (122) | |
| Positive | 7.14% (1) | 71.43% (10) | 21.43% (3) | 100% (14) | |
| Grand Total | 0.74% (1) | 55.88% (76) | 43.38% (59) | 100% (136) | |
| p-value | 0.12 | ||||
| Combined | Diminished | Increased | Normal | Grand Total | |
| Negative | 0.00% (0) | 54.00% (54) | 46.00% (46) | 100% (100) | |
| Positive | 2.78% (1) | 61.11% (22) | 36.11% (13) | 100% (36) | |
| Grand Total | 0.74% (1) | 55.88% (76) | 43.38% (59) | 100% (136) | |
| p-value | 0.63 |
| US findings at TP0 | ||||
|---|---|---|---|---|
| Dimensions | Euthyroid | Hypothyroid | Grand Total | |
| Diminished | 100% (1) | 0% (0) | 100% (1) | |
| Increased | 69.74% (53) | 30.26% (23) | 100% (76) | |
| Normal | 59.32% (35) | 40.68% (24) | 100% (59) | |
| Grand Total | 65.44% (89) | 34.56% (47) | 100% (136) | |
| p-value | 0.21 | |||
| Hypoechoic areas | Euthyroid | Hypothyroid | Grand Total | |
| Absent | 71.43% (5) | 28.57% (2) | 100% (7) | |
| Single | 75.00% (3) | 25.00% (1) | 100% (4) | |
| Multiple, monolateral | 66.66% (10) | 33.33% (5) | 100% (15) | |
| Multiple, bilateral | 65.18% (73) | 34.82% (39) | 100% (112) | |
| Grand Total | 65.94% (91) | 34.06% (47) | 100% (138) | |
| p-value | 0.98 | |||
| Nodules | Euthyroid | Hypothyroid | Grand Total | |
| Absent | 69.49% (41) | 30.51% (18) | 100% (59) | |
| Present | 66.66% (34) | 33.33% (17) | 100% (51) | |
| Pseudo-nodular | 57.14% (16) | 48.86% (12) | 100% (28) | |
| Grand Total | 65.94% (91) | 34.06% (47) | 100% (138) | |
| p-value | 0.52 | |||
| Vascularization | Euthyroid | Hypothyroid | Grand Total | |
| Increased | 59.26% (16) | 40.74% (11) | 100% (27) | |
| Normal | 73.03% (65) | 26.97% (24) | 100% (89) | |
| Reduced | 45.45% (10) | 54.55% (12) | 100% (22) | |
| Grand Total | 65.94% (91) | 34.06% (47) | 100% (138) | |
| p-value | 0.036 |
| US findings at TP3 | ||||
|---|---|---|---|---|
| Dimensions | Euthyroid | Hypothyroid | Grand Total | |
| Diminished | 33.33% (6) | 66.66% (12) | 100% (18) | |
| Increased | 100% (2) | 0.00% (0) | 100% (2) | |
| Normal | 69.83% (81) | 30.17% (35) | 100% (116) | |
| Grand Total | 65.44% (89) | 34.56% (47) | 100% (136) | |
| p-value | 0.003 | |||
| Hypoechoic areas | Euthyroid | Hypothyroid | Grand Total | |
| Absent | 79.49% (31) | 20.51% (8) | 100% (39) | |
| Present | 59.60% (59) | 40.40% (40) | 100% (99) | |
| Grand Total | 65.22% (90) | 34.78% (48) | 100% (138) | |
| p-value | 0.027 | |||
| Nodules | Euthyroid | Hypothyroid | Grand Total | |
| Absent | 62.90% (39) | 37.10% (23) | 100% (62) | |
| Present | 65.45% (36) | 34.55% (19) | 100% (55) | |
| Pseudo-nodular | 71.43% (15) | 28.57% (6) | 100% (21) | |
| Grand Total | 65.22% (90) | 34.78% (48) | 100% (138) | |
| p-value | 0.297 | |||
| Vascularization | Euthyroid | Hypothyroid | Grand Total | |
| Increased | 80.00% (8) | 20.00% (2) | 100% (10) | |
| Normal | 63.49% (80) | 36.51% (46) | 100% (126) | |
| Reduced | 100% (2) | 0.00% (0) | 100% (2) | |
| Grand Total | 65.22% (90) | 34.78% (48) | 100% (138) | |
| p-value | 0.334 | |||
| Resolution | Euthyroid | Hypothyroid | Grand Total | |
| Not achieved | 59.60% (59) | 40.40% (40) | 100% (99) | |
| Achieved | 84.38% (27) | 15.63% (5) | 100% (32) | |
| Grand Total | 65.65% (86) | 34.35% (45) | 100% (131) | |
| p-value | 0.01 |
| Coefficient B | Standard Error | z | p | Odds Ratio | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| Constant | −1.9 | 0.59 | 3.23 | 0.001 | 0.15 | 0.05–0.47 |
| Anti-Tg Abs positivity | 2.23 | 1.14 | 1.95 | 0.051 | 9.33 | 0.99–87.95 |
| US—reduced vascularization | 1.08 | 0.96 | 1.12 | 0.262 | 2.93 | 0.45–19.22 |
| US—increased vascularization | −0.08 | 0.89 | 0.08 | 0.932 | 0.93 | 0.16–5.35 |
| Δ TSH (TP2-TP1) | 0.26 | 0.1 | 2.71 | 0.007 | 1.3 | 1.07–1.57 |
| US—reduced dimensions | 1.83 | 1.12 | 1.64 | 0.102 | 6.21 | 0.7–55.43 |
| US—increased dimensions | −19.02 | 7380.73 | 0 | 0.998 | 0 | 0–infinity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsello, A.; Cacciamani Fanelli, P.M.; Lener, L.; Cera, G.; Locantore, P.; Pontecorvi, A.; Corsello, S.M.; Paragliola, R.M. Biochemical and Ultrasonographic Parameters Predicting Long-Term Hypothyroidism After Subacute Thyroiditis. Int. J. Mol. Sci. 2025, 26, 4176. https://doi.org/10.3390/ijms26094176
Corsello A, Cacciamani Fanelli PM, Lener L, Cera G, Locantore P, Pontecorvi A, Corsello SM, Paragliola RM. Biochemical and Ultrasonographic Parameters Predicting Long-Term Hypothyroidism After Subacute Thyroiditis. International Journal of Molecular Sciences. 2025; 26(9):4176. https://doi.org/10.3390/ijms26094176
Chicago/Turabian StyleCorsello, Andrea, Piergiacomo Maria Cacciamani Fanelli, Luisa Lener, Gianluca Cera, Pietro Locantore, Alfredo Pontecorvi, Salvatore Maria Corsello, and Rosa Maria Paragliola. 2025. "Biochemical and Ultrasonographic Parameters Predicting Long-Term Hypothyroidism After Subacute Thyroiditis" International Journal of Molecular Sciences 26, no. 9: 4176. https://doi.org/10.3390/ijms26094176
APA StyleCorsello, A., Cacciamani Fanelli, P. M., Lener, L., Cera, G., Locantore, P., Pontecorvi, A., Corsello, S. M., & Paragliola, R. M. (2025). Biochemical and Ultrasonographic Parameters Predicting Long-Term Hypothyroidism After Subacute Thyroiditis. International Journal of Molecular Sciences, 26(9), 4176. https://doi.org/10.3390/ijms26094176








