Telomere Maintenance Pathways in Lower-Grade Gliomas: Insights from Genetic Subtypes and Telomere Length Dynamics
Abstract
1. Introduction
2. Results
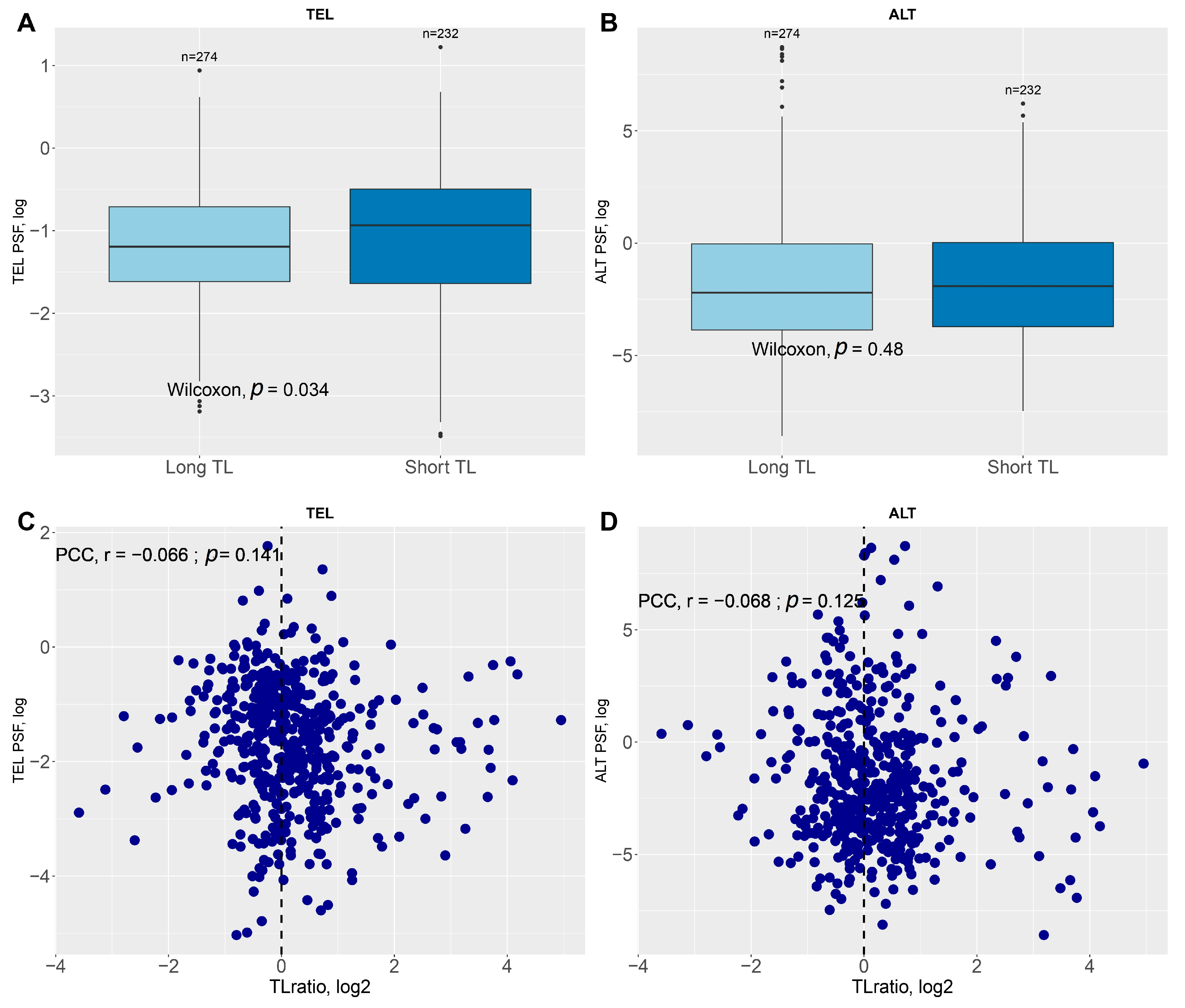
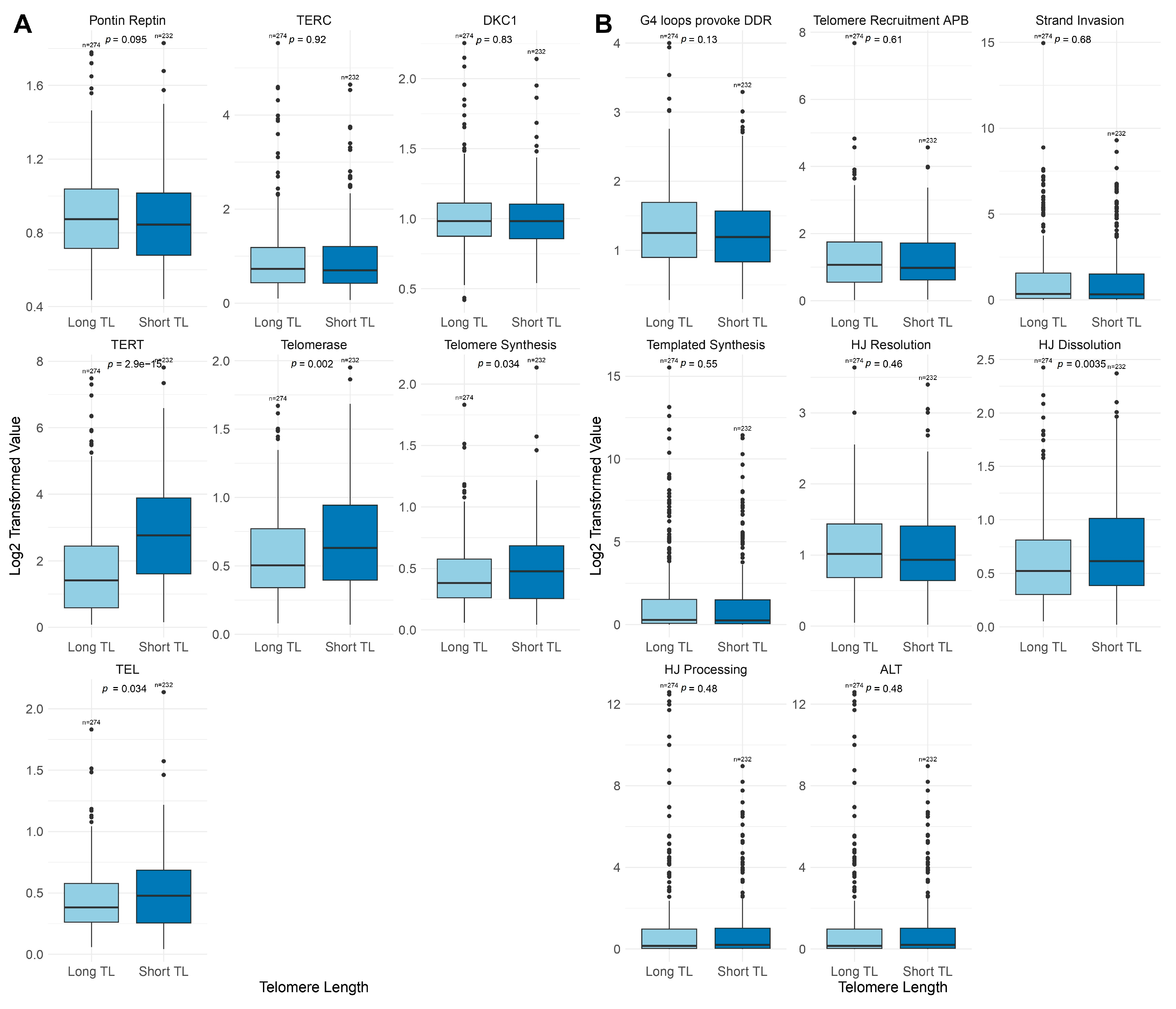
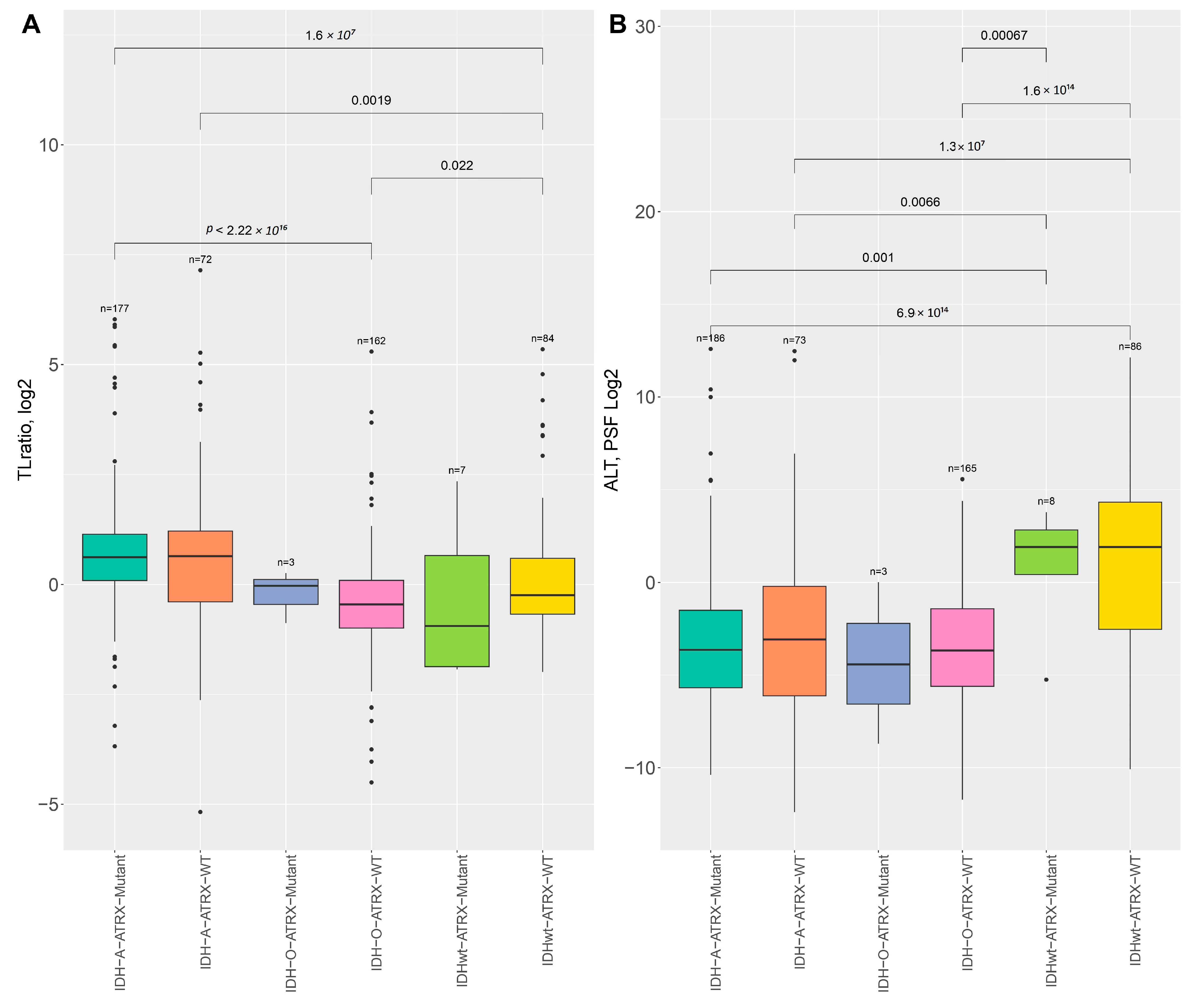
2.1. Association of Telomere Length, TMM, and IDH Mutation in LGG Samples
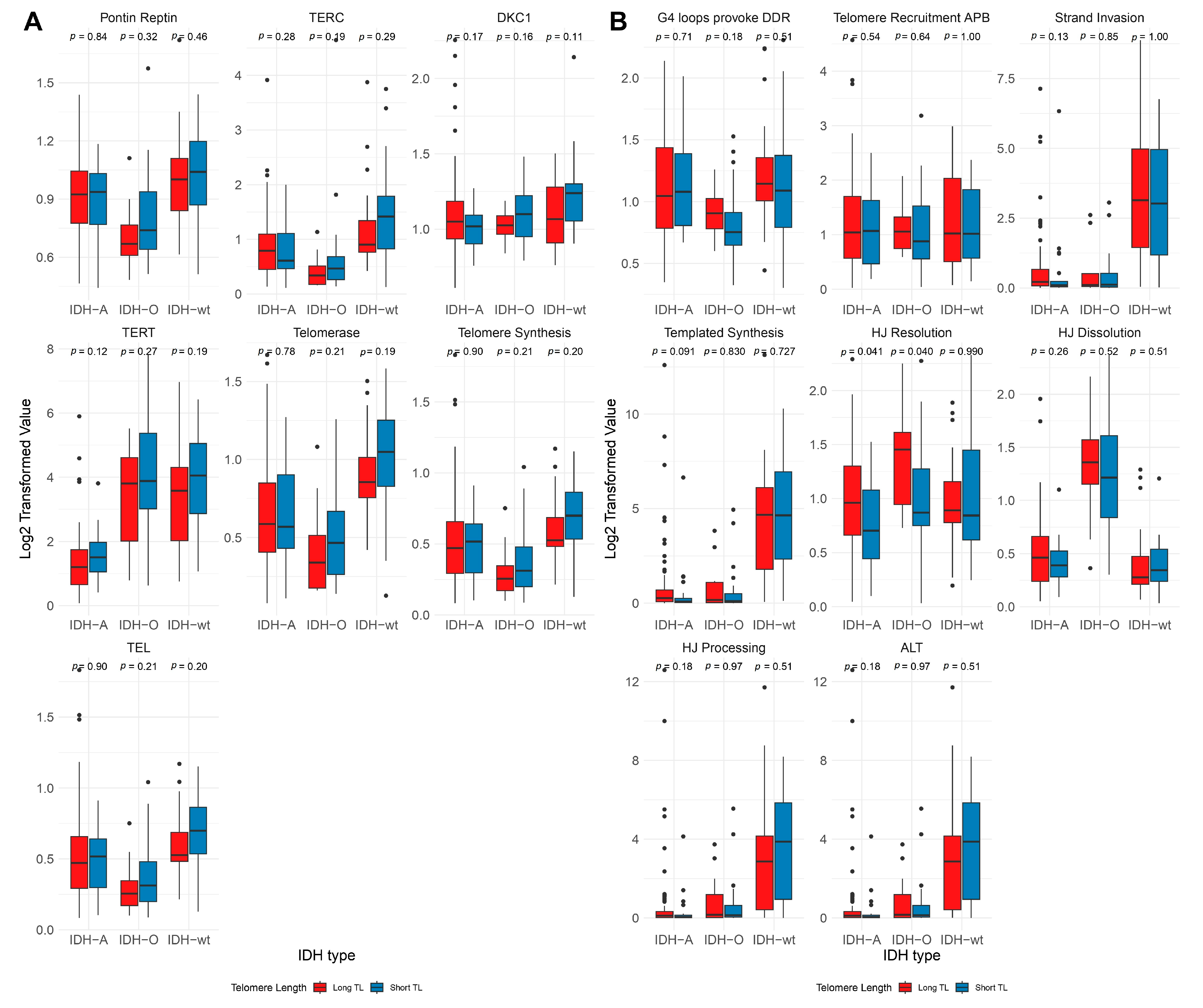
2.2. Telomere Length Variability Across Genetic Subtypes
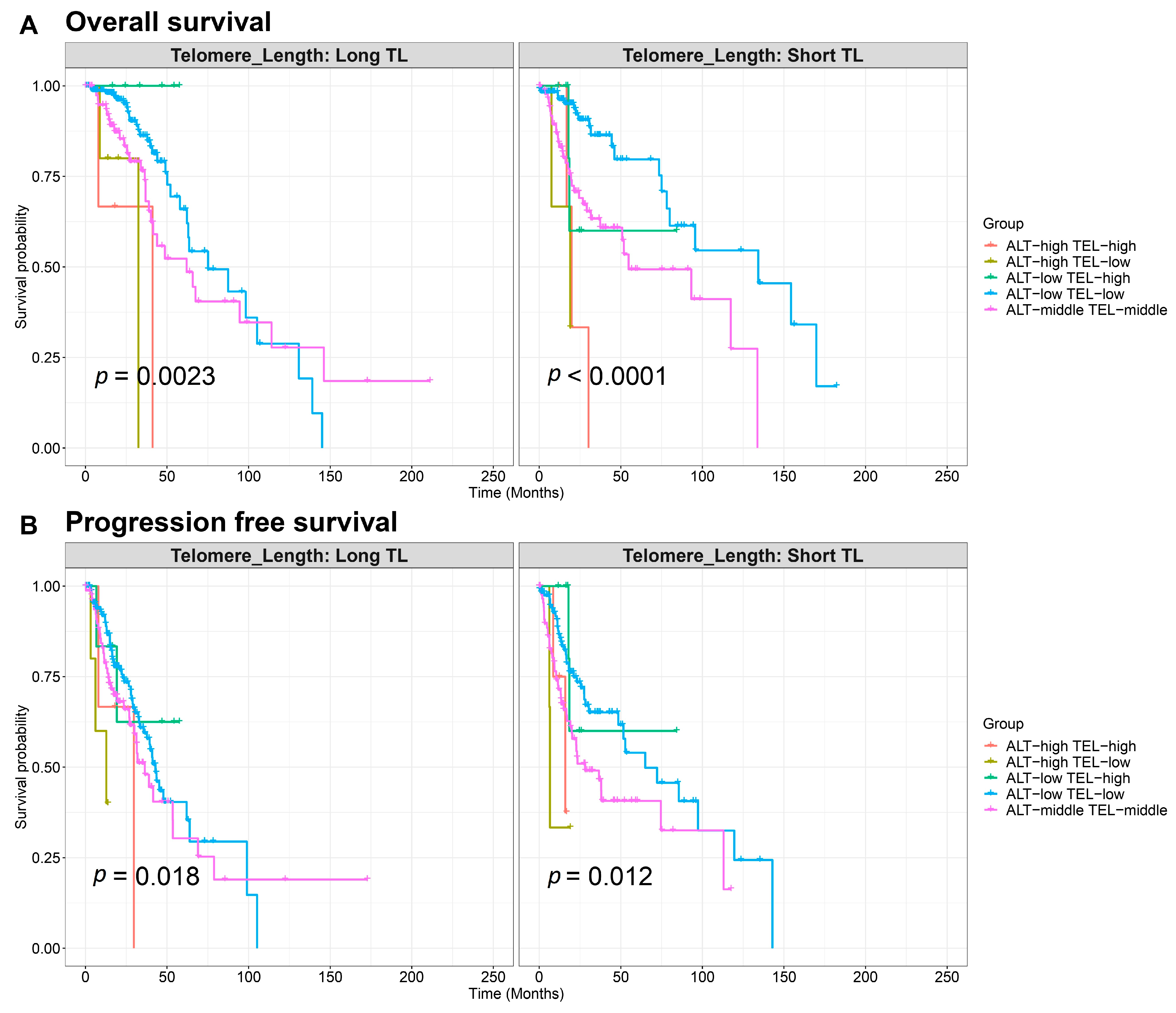
2.3. Survival Analysis Based on Telomere Length and TEL/ALT Pathway Activation
2.4. Validation Through Negative Control Analysis
3. Discussion
3.1. Telomere Length and TMM Pathway Dynamics
3.2. Subtype-Specific Variations in TEL and ALT Pathway Activation
3.3. Telomere Length Variability Across Genetic Subtypes
3.4. Clinical Implications of Telomere Dynamics in LGG
3.5. Translational Relevance of TMM-Targeted Therapies in Gliomas
3.6. Validation and Limitations
4. Materials and Methods
4.1. Data Sources and Preprocessing
4.2. Datasets for Supporting and Validation Analyses
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alternative lengthening of telomeres TMM |
| ATRX | Alpha Thalassemia/Mental Retardation Syndrome X-Linked |
| CGGA | Chinese Glioma Genome Atlas |
| CNS | central nervous system |
| COPD | Chronic Obstructive Pulmonary Disease |
| FC | fold change |
| GBM | glioblastoma |
| GEO | Gene Expression Omnibus |
| HJ | Holiday junction |
| IDH-A | IDH-mutant and 1p/19q-non codeleted astrocytoma |
| IDH-O | IDH-mutant and 1p/19q-codeleted oligodendroglioma |
| IDH-wt | IDH-wildtype glioblastoma |
| LGG | lower-grade gliomas |
| Long TL | long telomere length |
| OS | overall survival |
| PFS | progression-free survival |
| PSF | Pathway Signal Flow |
| Short TL | short telomere length |
| TEL | telomerase-dependent TMM |
| TL | telomere length |
| TMMs | telomere maintenance mechanisms |
| WHO | World Health Organization |
References
- Forst, D.A.; Nahed, B.V.; Loeffler, J.S.; Batchelor, T.T. Low-Grade Gliomas. Oncologist 2014, 19, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System Tumours: A Practical Update on What Neurosurgeons Need to Know—A Minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Zou, L. Alternative Lengthening of Telomeres: From Molecular Mechanisms to Therapeutic Outlooks. Cell Biosci. 2020, 10, 30. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and Telomerase: The Path from Maize, Tetrahymena and Yeast to Human Cancer and Aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- La Torre, D.; Aguennouz, M.; Conti, A.; Giusa, M.; Raffa, G.; Abbritti, R.V.; Germano’, A.; Angileri, F.F. Potential Clinical Role of Telomere Length in Human Glioblastoma. Transl. Med. UniSa 2011, 1, 243–270. [Google Scholar]
- Eustermann, S.; Yang, J.-C.; Law, M.J.; Amos, R.; Chapman, L.M.; Jelinska, C.; Garrick, D.; Clynes, D.; Gibbons, R.J.; Rhodes, D.; et al. Combinatorial Readout of Histone H3 Modifications Specifies Localization of ATRX to Heterochromatin. Nat. Struct. Mol. Biol. 2011, 18, 777–782. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, X.; Ji, T.; Cheng, M.; Wang, R.; Zhang, C.; Liu, M.; Zhang, J.; Zhong, C. The Chromatin Remodeler ATRX: Role and Mechanism in Biology and Cancer. Cancers 2023, 15, 2228. [Google Scholar] [CrossRef]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating Mutations in the TERT Promoter Commonly Occur in Adult Malignant Gliomas and Are Strongly Associated with Total 1p19q Loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sieverling, L.; Hong, C.; Koser, S.D.; Ginsbach, P.; Kleinheinz, K.; Hutter, B.; Braun, D.M.; Cortés-Ciriano, I.; Xi, R.; Kabbe, R.; et al. Genomic Footprints of Activated Telomere Maintenance Mechanisms in Cancer. Nat. Commun. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Hakobyan, M.; Binder, H.; Arakelyan, A. Pan-Cancer Analysis of Telomere Maintenance Mechanisms. J. Biol. Chem. 2024, 300, 107392. [Google Scholar] [CrossRef]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic Analysis of Telomere Length and Somatic Alterations in 31 Cancer Types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Wang, X.-W.; Ciccarino, P.; Rossetto, M.; Boisselier, B.; Marie, Y.; Desestret, V.; Gleize, V.; Mokhtari, K.; Sanson, M.; Labussière, M. IDH Mutations: Genotype-Phenotype Correlation and Prognostic Impact. BioMed Res. Int. 2014, 2014, 540236. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Cheong, J.-H. Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism. Int. J. Mol. Sci. 2021, 22, 11101. [Google Scholar] [CrossRef]
- Livingstone, J.; Shiah, Y.-J.; Yamaguchi, T.N.; Heisler, L.E.; Huang, V.; Lesurf, R.; Gebo, T.; Carlin, B.; Eng, S.; Drysdale, E.; et al. The Telomere Length Landscape of Prostate Cancer. Nat. Commun. 2021, 12, 6893. [Google Scholar] [CrossRef]
- Nersisyan, L.; Hopp, L.; Loeffler-Wirth, H.; Galle, J.; Loeffler, M.; Arakelyan, A.; Binder, H. Telomere Length Maintenance and Its Transcriptional Regulation in Lynch Syndrome and Sporadic Colorectal Carcinoma. Front. Oncol. 2019, 9, 1172. [Google Scholar] [CrossRef]
- Counter, C.M.; Hirte, H.W.; Bacchetti, S.; Harley, C.B. Telomerase Activity in Human Ovarian Carcinoma. Proc. Natl. Acad. Sci. USA 1994, 91, 2900–2904. [Google Scholar] [CrossRef]
- Kammori, M.; Takubo, K.; Nakamura, K.-I.; Furugouri, E.; Endo, H.; Kanauchi, H.; Mimura, Y.; Kaminishi, M. Telomerase Activity and Telomere Length in Benign and Malignant Human Thyroid Tissues. Cancer Lett. 2000, 159, 175–181. [Google Scholar] [CrossRef]
- MacKenzie, D.; Watters, A.K.; To, J.T.; Young, M.W.; Muratori, J.; Wilkoff, M.H.; Abraham, R.G.; Plummer, M.M.; Zhang, D. ALT Positivity in Human Cancers: Prevalence and Clinical Insights. Cancers 2021, 13, 2384. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Nelson, C.B.; Rogers, S.; Cesare, A.J.; Sobinoff, A.P.; Pickett, H.A. Distinct Modes of Telomere Synthesis and Extension Contribute to Alternative Lengthening of Telomeres. iScience 2024, 27, 108655. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Shay, J.W. TERT Promoter Mutations Enhance Telomerase Activation by Long-Range Chromatin Interactions. Cancer Discov. 2016, 6, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Nobusawa, S.; Watanabe, T.; Kleihues, P.; Ohgaki, H. IDH1 Mutations as Molecular Signature and Predictive Factor of Secondary Glioblastomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6002–6007. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH Mutation in Glioma: Molecular Mechanisms and Potential Therapeutic Targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef]
- Mechanisms of Telomere Maintenance and Associated Therapeutic Vulnerabilities in Malignant Gliomas|Neuro-Oncology|Oxford Academic. Available online: https://academic.oup.com/neuro-oncology/article-abstract/26/6/1012/7591242?redirectedFrom=fulltext&login=false (accessed on 20 January 2025).
- Hakobyan, M. Pan-cancer screening for tert and atrx mutationassociated changes in telomere maintenance mechanisms. Ceмнaдцaтaя Гoдичнaя Hayчнaя Koнφepeнция Φизикo-Maтeмaтичecкиe И Ecтecтвeнныe Hayки 2024, 1, 74–79. [Google Scholar]
- de Nonneville, A.; Reddel, R.R. Alternative Lengthening of Telomeres Is Not Synonymous with Mutations in ATRX/DAXX. Nat. Commun. 2021, 12, 1552. [Google Scholar] [CrossRef]
- Feuerbach, L. Formal Reply to “Alternative Lengthening of Telomeres Is Not Synonymous with Mutations in ATRX/DAXX”. Nat. Commun. 2021, 12, 1551. [Google Scholar] [CrossRef]
- Brosnan-Cashman, J.A.; Yuan, M.; Graham, M.K.; Rizzo, A.J.; Myers, K.M.; Davis, C.; Zhang, R.; Esopi, D.M.; Raabe, E.H.; Eberhart, C.G.; et al. ATRX Loss Induces Multiple Hallmarks of the Alternative Lengthening of Telomeres (ALT) Phenotype in Human Glioma Cell Lines in a Cell Line-Specific Manner. PLoS ONE 2018, 13, e0204159. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.L.; Cox, K.E.; Jeitany, M.; Wakimoto, H.; Bryll, A.R.; Ganem, N.J.; Bersani, F.; Pineda, J.R.; Suvà, M.L.; Benes, C.H.; et al. Alternative Lengthening of Telomeres Renders Cancer Cells Hypersensitive to ATR Inhibitors. Science 2015, 347, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Kannan, K.; Inagaki, A.; Silber, J.; Gorovets, D.; Zhang, J.; Kastenhuber, E.R.; Heguy, A.; Petrini, J.H.; Chan, T.A.; Huse, J.T. Whole-Exome Sequencing Identifies ATRX Mutation as a Key Molecular Determinant in Lower-Grade Glioma. Oncotarget 2012, 3, 1194–1203. [Google Scholar] [CrossRef]
- Haase, S.; Garcia-Fabiani, M.B.; Carney, S.; Altshuler, D.; Núñez, F.J.; Méndez, F.M.; Núñez, F.; Lowenstein, P.R.; Castro, M.G. Mutant ATRX: Uncovering a New Therapeutic Target for Glioma. Expert Opin. Ther. Targets 2018, 22, 599–613. [Google Scholar] [CrossRef]
- Willscher, E.; Hopp, L.; Kreuz, M.; Schmidt, M.; Hakobyan, S.; Arakelyan, A.; Hentschel, B.; Jones, D.T.W.; Pfister, S.M.; Loeffler, M.; et al. High-Resolution Cartography of the Transcriptome and Methylome Landscapes of Diffuse Gliomas. Cancers 2021, 13, 3198. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, F.; Liu, L. Alternative Lengthening of Telomeres (ALT) in Tumors and Pluripotent Stem Cells. Genes 2019, 10, 1030. [Google Scholar] [CrossRef]
- Recagni, M.; Bidzinska, J.; Zaffaroni, N.; Folini, M. The Role of Alternative Lengthening of Telomeres Mechanism in Cancer: Translational and Therapeutic Implications. Cancers 2020, 12, 949. [Google Scholar] [CrossRef]
- Kim, S.; Chowdhury, T.; Yu, H.J.; Kahng, J.Y.; Lee, C.E.; Choi, S.A.; Kim, K.-M.; Kang, H.; Lee, J.H.; Lee, S.-T.; et al. The Telomere Maintenance Mechanism Spectrum and Its Dynamics in Gliomas. Genome Med. 2022, 14, 88. [Google Scholar] [CrossRef]
- Lennox, A.L.; Huang, F.; Behrs, M.K.; González-Sales, M.; Bhise, N.; Wan, Y.; Sun, L.; Berry, T.; Feller, F.; Morcos, P.N. Imetelstat, a Novel, First-in-Class Telomerase Inhibitor: Mechanism of Action, Clinical, and Translational Science. Clin. Transl. Sci. 2024, 17, e70076. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, R.; Wang, L.; Yang, Q.; Hu, X.; Fu, Q.; Zhang, X.; Li, W.; Ren, Y. The Emerging Roles of Rad51 in Cancer and Its Potential as a Therapeutic Target. Front. Oncol. 2022, 12, 935593. [Google Scholar] [CrossRef]
- Houillier, C.; Wang, X.; Kaloshi, G.; Mokhtari, K.; Guillevin, R.; Laffaire, J.; Paris, S.; Boisselier, B.; Idbaih, A.; Laigle-Donadey, F.; et al. IDH1 or IDH2 Mutations Predict Longer Survival and Response to Temozolomide in Low-Grade Gliomas. Neurology 2010, 75, 1560–1566. [Google Scholar] [CrossRef]
- Nersisyan, L.; Simonyan, A.; Binder, H.; Arakelyan, A. Telomere Maintenance Pathway Activity Analysis Enables Tissue- and Gene-Level Inferences. Front. Genet. 2021, 12, 662464. [Google Scholar] [CrossRef] [PubMed]
- Nersisyan, L.; Johnson, G.; Riel-Mehan, M.; Pico, A.R.; Arakelyan, A. PSFC: A Pathway Signal Flow Calculator App for Cytoscape. F1000Research 2017, 4, 480. [Google Scholar] [CrossRef] [PubMed]
- Hakobyan, S.; Stepanyan, A.; Nersisyan, L.; Binder, H.; Arakelyan, A. PSF Toolkit: An R Package for Pathway Curation and Topology-Aware Analysis. Front. Genet. 2023, 14, 1264656. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.-N.; Wang, Q.; Li, G.; Zeng, F.; Zhang, Y.; Wu, F.; Chai, R.; Wang, Z.; Zhang, C.; et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Glioma Patients. Genomics Proteomics Bioinformatics 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Li, G.; Chang, X.; Li, S.; Chen, J.; Zhao, Z.; Wang, J.; Jiang, T.; Chai, R. Clinical Management and Survival Outcomes of Patients with Different Molecular Subtypes of Diffuse Gliomas in China (2011–2017): A Multicenter Retrospective Study from CGGA. Cancer Biol. Med. 2022, 19, 1460–1476. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The Sva Package for Removing Batch Effects and Other Unwanted Variation in High-Throughput Experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- RNA-Sequencing across Three Matched Tissues Reveals Shared and Tissue-Specific Gene Expression and Pathway Signatures of COPD|Respiratory Research|Full Text. Available online: https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1032-z#Sec2 (accessed on 20 January 2025).
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Harrell, F.E. Cox Proportional Hazards Regression Model. In Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Harrell, F.E., Ed.; Springer Series in Statistics; Springer: New York, NY, USA, 2001; pp. 465–507. ISBN 978-1-4757-3462-1. [Google Scholar]

| Long TL | Short TL | Long TL Fraction | p (Fisher’s Exact Test) | MTL (Mean +/− SD) | p (t-Test) | |
|---|---|---|---|---|---|---|
| LGG | 274 | 232 | 0.54 | |||
| EXP Group * | ||||||
| IDH-A | 73 | 23 | 0.76 | 6.00 × 10⁻7 | 0.55 ± 1.01 | 0.05683 |
| IDH-O | 12 | 33 | 0.27 | 6.19 × 10⁻7 | −0.41 ± 1.06 | 0.3284 |
| IDH-wt | 22 | 19 | 0.54 | 0.4748 | 0.19 ± 1.05 | 0.7108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakobyan, M.; Binder, H.; Arakelyan, A. Telomere Maintenance Pathways in Lower-Grade Gliomas: Insights from Genetic Subtypes and Telomere Length Dynamics. Int. J. Mol. Sci. 2025, 26, 4175. https://doi.org/10.3390/ijms26094175
Hakobyan M, Binder H, Arakelyan A. Telomere Maintenance Pathways in Lower-Grade Gliomas: Insights from Genetic Subtypes and Telomere Length Dynamics. International Journal of Molecular Sciences. 2025; 26(9):4175. https://doi.org/10.3390/ijms26094175
Chicago/Turabian StyleHakobyan, Meline, Hans Binder, and Arsen Arakelyan. 2025. "Telomere Maintenance Pathways in Lower-Grade Gliomas: Insights from Genetic Subtypes and Telomere Length Dynamics" International Journal of Molecular Sciences 26, no. 9: 4175. https://doi.org/10.3390/ijms26094175
APA StyleHakobyan, M., Binder, H., & Arakelyan, A. (2025). Telomere Maintenance Pathways in Lower-Grade Gliomas: Insights from Genetic Subtypes and Telomere Length Dynamics. International Journal of Molecular Sciences, 26(9), 4175. https://doi.org/10.3390/ijms26094175



_Kim.png)




