Buxus natalensis (Oliv.) Hutch (Buxaceae) Exhibits Its Anticancer Potential by Stimulating ROS Production and Caspase-p53-BCL-2-Dependent Apoptosis in Hepatocellular Carcinoma and Prostate Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
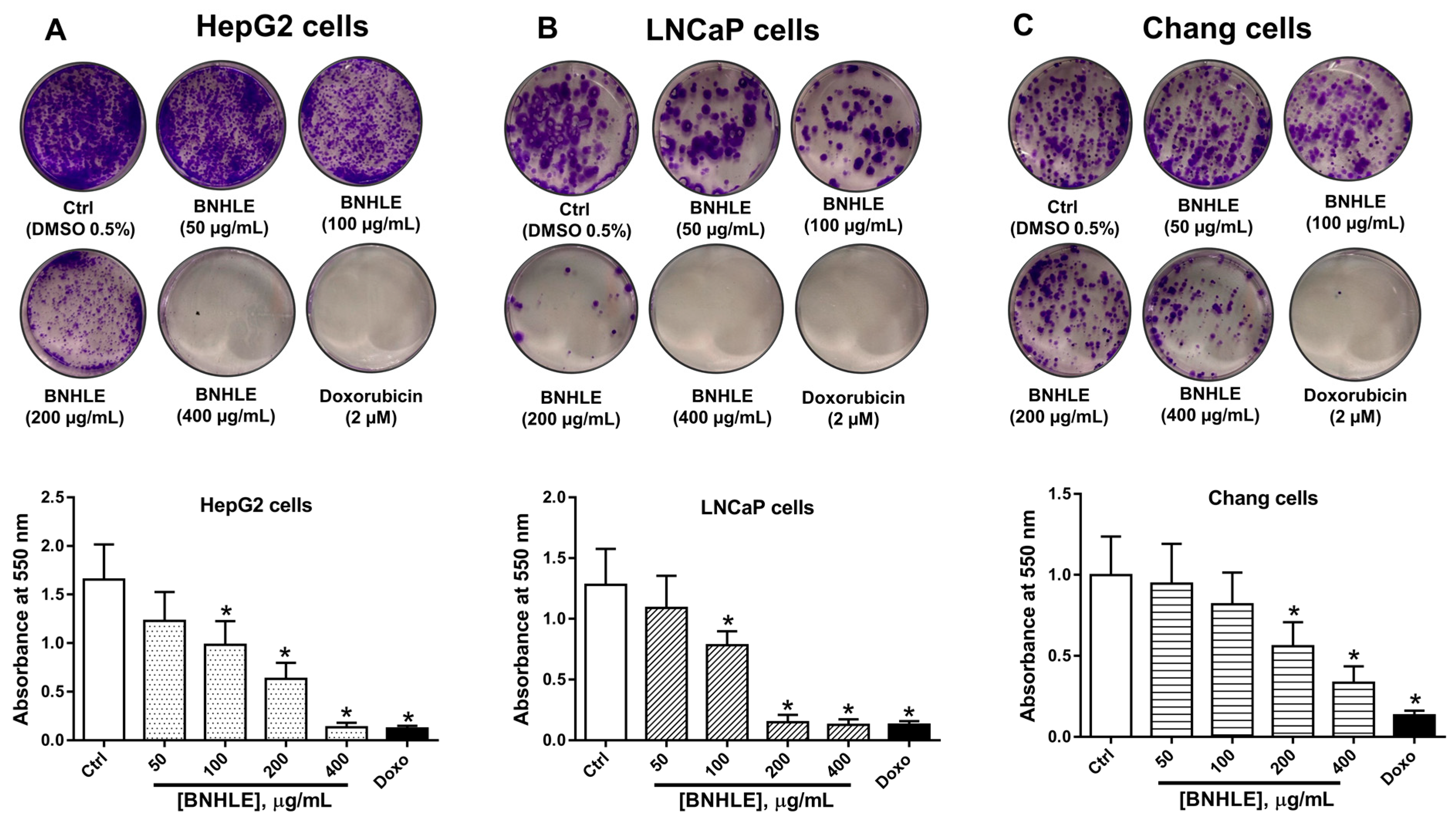
2.1. Buxus natalensis Leaf Extracts Trigger Differential Cytotoxic Effect Between Cancer Cell Lines and Normal Cell Line
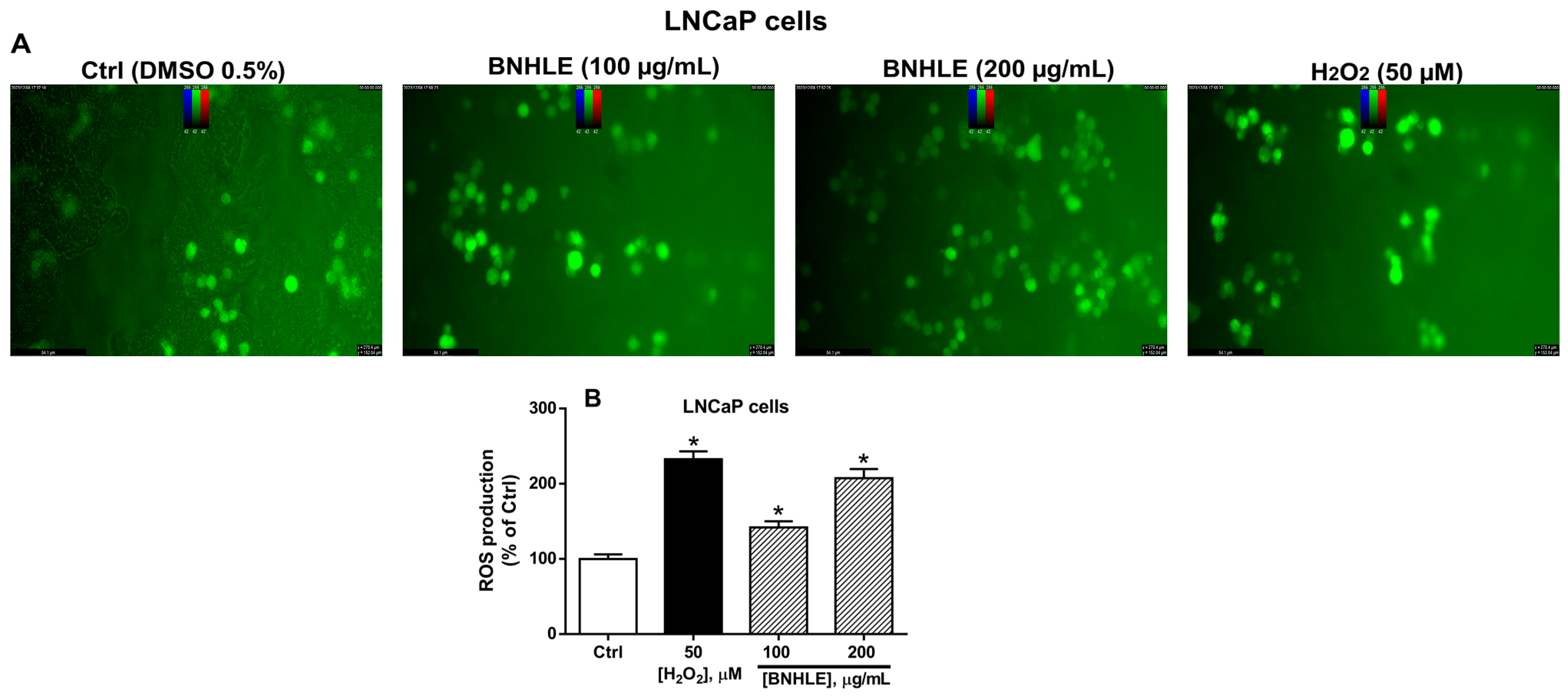
2.2. Buxus natalensis Hydroethanolic Leaf Extract Induces the Intracellular Production of Reactive Oxygen Species in Cancer Cells
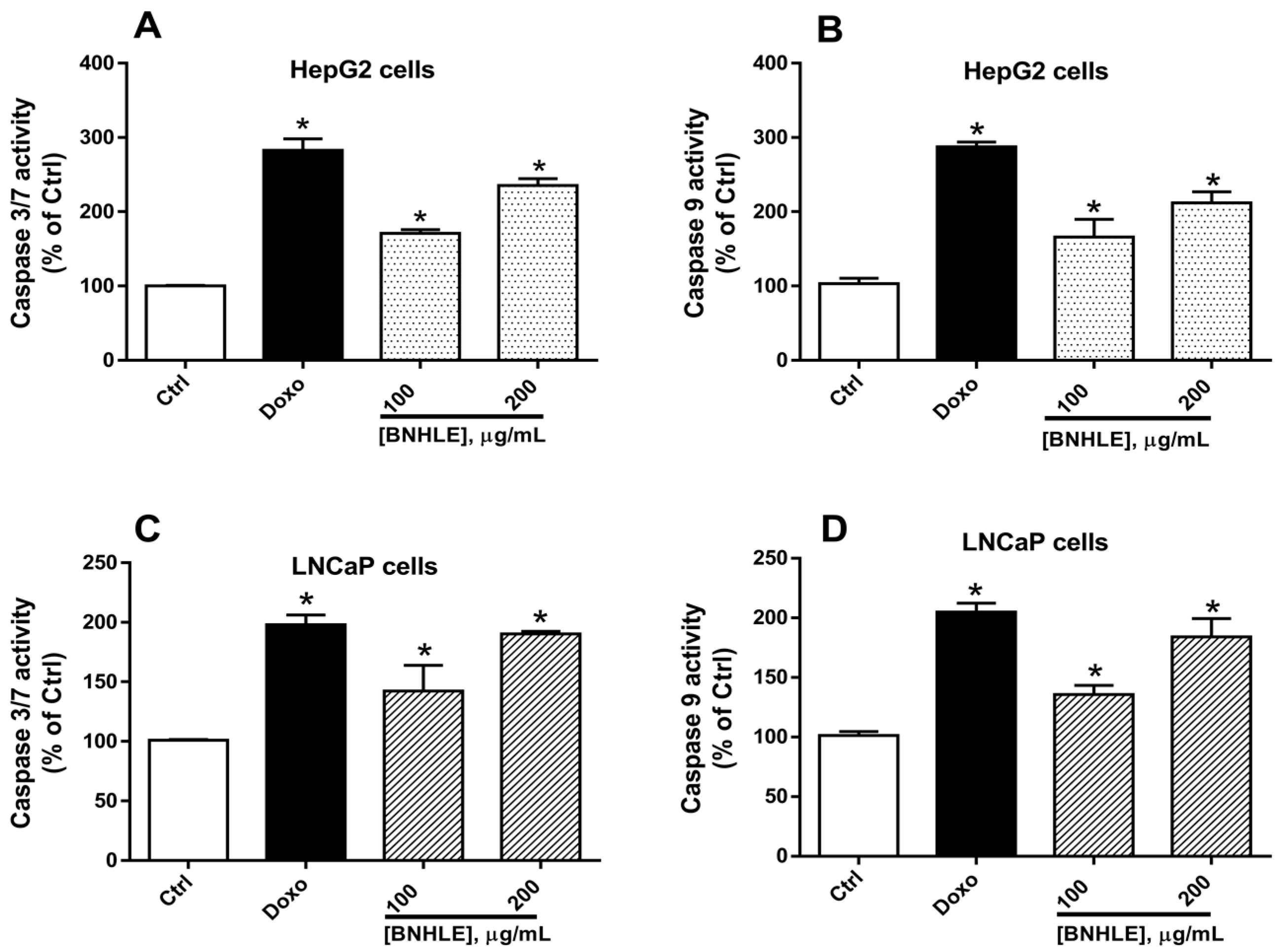
2.3. Buxus natalensis Hydroethanolic Leaf Extract Increases the Caspase Activity in Cancer Cells
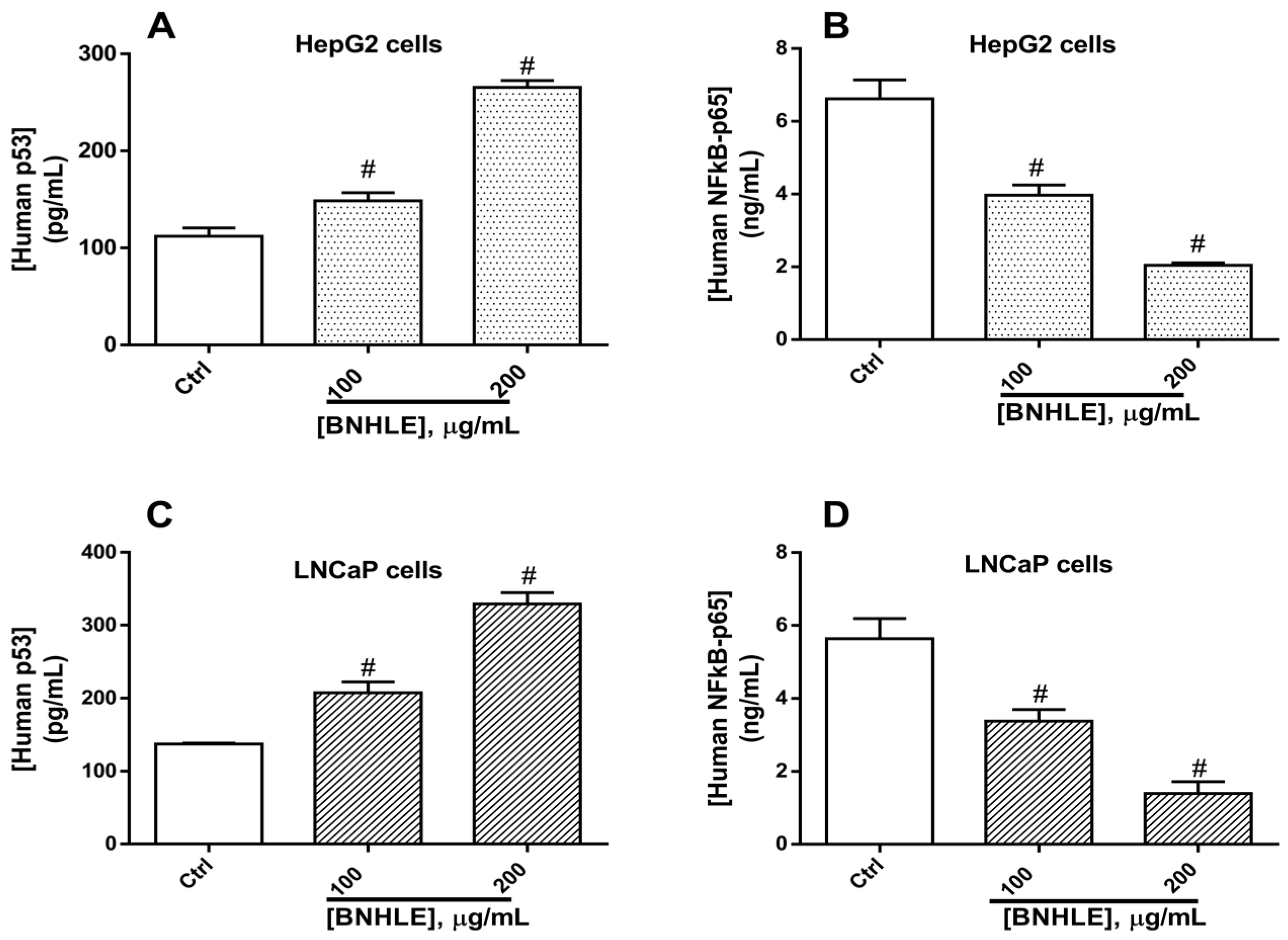
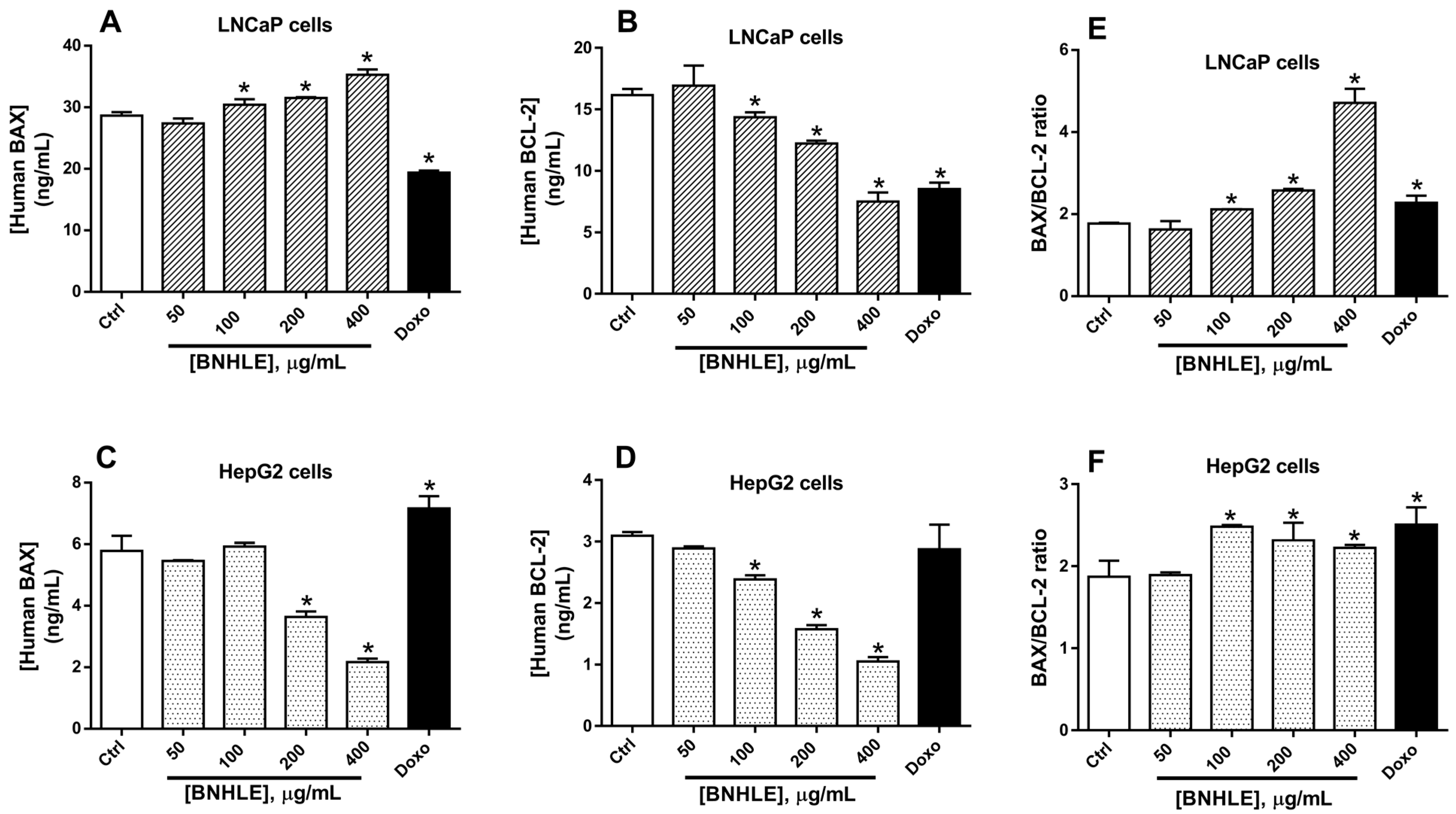
2.4. Buxus natalensis Hydroethanolic Leaf Extract Modulates the Expression Levels of Human p53, BCL2, BAX, and NF-κB-p65 in Cancer Cells
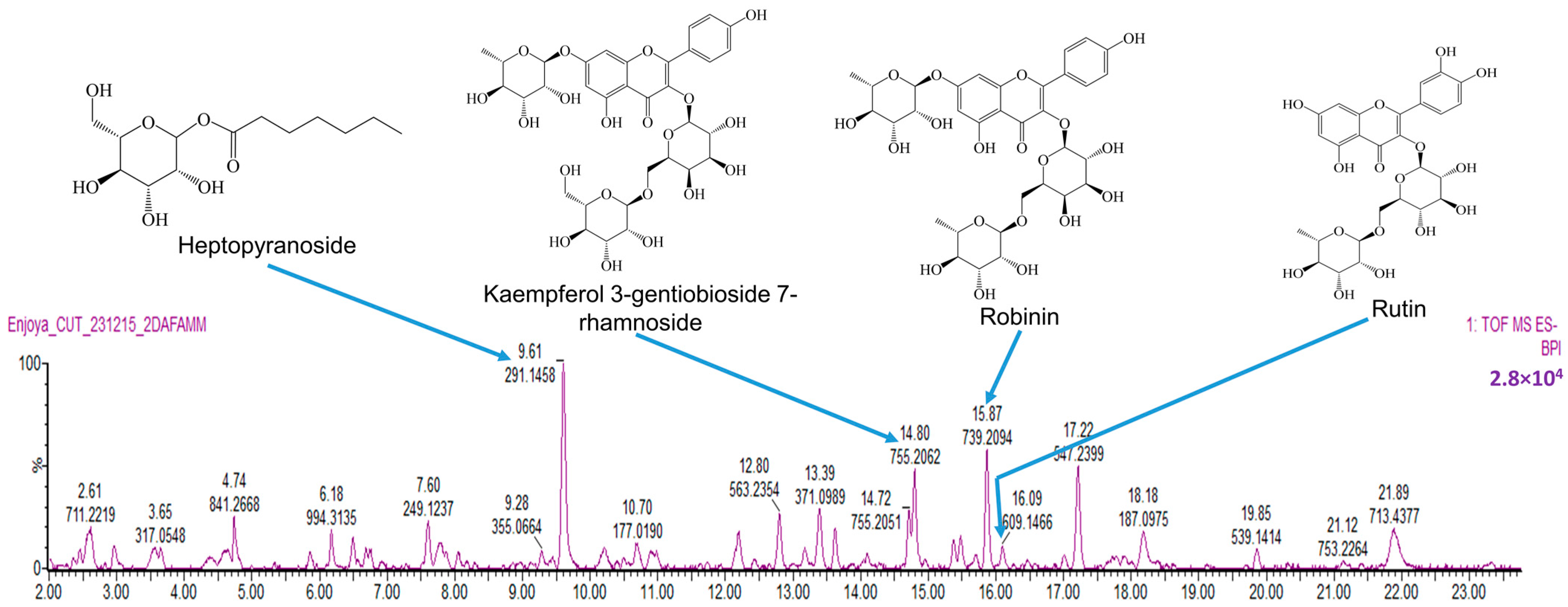
2.5. Phytochemical Analysis of BNHLE Identifies Natural Compounds with Known Anticancer Property
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Cell Proliferation Assay
3.2.1. Cell Culture
3.2.2. Cytotoxicity Assay
3.2.3. Determination of IC50 Values and Selectivity Indexes
3.3. Colony-Formation Assay
3.4. Assessment of Intracellular Reactive Oxygen Species Production
3.5. Assessment of the Caspase Activation in Cancer Cells
3.6. Quantification of the Expression of Human BAX, BCL-2, p53, and NF-κB-p65 in Cancer Cells
3.7. Phytochemical Analysis of B. natalensis Hydroethanolic Leaf Extract (BNHLE)
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| p53 | Tumor suppressor protein 53 |
| BCL-2 | B-cell lymphoma 2 |
| BAX | BCL-2-associated X protein |
| NF-κB-p65 | Nuclear factor-kappa B-p65 subunit |
| BNHLE | Buxus natalensis hydroethanolic leaf extract |
| BNMLE | Buxus natalensis methanolic leaf extract |
| BNALE | Buxus natalensis aqueous leaf extract |
| LC-MS | Liquid chromatography mass spectrometry |
References
- Yu, X.; Zhao, H.; Wang, R.; Chen, Y.; Ouyang, X.; Li, W.; Sun, Y.; Peng, A. Cancer epigenetics: From laboratory studies and clinical trials to precision medicine. Cell Death Discov. 2024, 10, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. MCR 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization: Global Cancer Burden Growing, Amidst Mounting Need for Services; WHO World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- IARC/WHO. International Agency for Research on Cancer, World Health Organization: Cancer Today/Population Fact Sheets: South Africa, Globocan 2022; IARC: Lyon, France; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Finestone, E.; Wishnia, J. Estimating the burden of cancer in South Africa. S. Afr. J. Oncol. 2022, 6, a220. [Google Scholar] [CrossRef]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxidative Med. Cell. Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.; Liu, J.; Aleksandrova, Y.; Klochkov, S.; Fan, R. Therapeutic Influence on Important Targets Associated with Chronic Inflammation and Oxidative Stress in Cancer Treatment. Cancers 2021, 13, 6062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaudhry, G.E.; Md Akim, A.; Sung, Y.Y.; Sifzizul, T.M.T. Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol. 2022, 13, 842376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of reactive oxygen species in inflammation and cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Liu, H.; Wang, L.; Wang, Y.; Zhang, C.; Wang, C.; Yan, Y.; Fan, J.; Xu, G.; Zhang, Q. Amplification of oxidative stress via intracellular ROS production and antioxidant consumption by two natural drug-encapsulated nanoagents for efficient anticancer therapy. Nanoscale Adv. 2020, 2, 3872–3881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- GlobalSurg Collaborative National Institute for Health Research Global Health Research Unit on Global Surgery. Global variation in postoperative mortality and complications after cancer surgery: A multicentre, prospective cohort study in 82 countries. Lancet 2021, 397, 387–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mfotie Njoya, E.; Ndemangou, B.; Akinyelu, J.; Munvera, A.M.; Chukwuma, C.I.; Mkounga, P.; Mashele, S.S.; Makhafola, T.J.; McGaw, L.J. In vitro antiproliferative, anti-inflammatory effects and molecular docking studies of natural compounds isolated from Sarcocephalus pobeguinii (Hua ex Pobeg). Front. Pharmacol. 2023, 14, 1205414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adico, M.D.W.; Bayala, B.; Bunay, J.; Baron, S.; Simpore, J.; Lobaccaro, J.A. Contribution of Sub-Saharan African medicinal plants to cancer research: Scientific basis 2013–2023. Pharmacol. Res. 2024, 202, 107138. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. 2021, 30, e00633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxidative Med. Cell. Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Muhsinah, A.B.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amtaghri, S.; Eddouks, M. Pharmacological and phytochemical properties of the genus Buxus: A review. Fitoterapia 2024, 177, 106081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, X.Y.; Zhang, S.D.; Xu, X.S.; Pei, J.P.; Fu, J.J. Chemical constituents of plants from the genus Buxus. Chem. Biodivers. 2015, 12, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.R.; Carmignani, M.; Cesare, P. Hydroalcoholic extract of Buxus sempervirens shows antiproliferative effect on melanoma, colorectal carcinoma and prostate cancer cells by affecting the autophagic flow. Front. Pharmacol. 2023, 14, 1073338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ait-Mohamed, O.; Battisti, V.; Joliot, V.; Fritsch, L.; Pontis, J.; Medjkane, S.; Redeuilh, C.; Lamouri, A.; Fahy, C.; Rholam, M.; et al. Acetonic extract of Buxus sempervirens induces cell cycle arrest, apoptosis and autophagy in breast cancer cells. PLoS ONE 2011, 6, e24537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, Y.X.; Hu, X.D.; Chen, J.C.; Sun, Y.; Zhang, X.M.; Qing, C.; Qiu, M.H. Cytotoxic triterpenoid alkaloids from Buxus microphylla. J. Nat. Prod. 2009, 72, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.U.; Kaiser, M.; Maser, P.; Schmidt, T.J. Antiprotozoal Nor-Triterpene Alkaloids from Buxus sempervirens L. Antibiotics 2021, 10, 696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Althaus, J.B.; Jerz, G.; Winterhalter, P.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal activity of Buxus sempervirens and activity-guided isolation of O-tigloylcyclovirobuxeine-B as the main constituent active against Plasmodium falciparum. Molecules 2014, 19, 6184–6201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesaik, M.A.; Halim, S.A.; Ul-Haq, Z.; Choudhary, M.I.; Shahnaz, S.; Ayatollahi, S.M.; Murad, S.; Ahmad, A. Immunosuppressive activity of buxidin and E-buxenone from Buxus hyrcana. Chem. Biol. Drug Des. 2010, 75, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Matochko, W.L.; James, A.; Lam, C.W.; Kozera, D.J.; Ata, A.; Gengan, R.M. Triterpenoidal alkaloids from Buxus natalensis and their acetylcholinesterase inhibitory activity. J. Nat. Prod. 2010, 73, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Timm, M.; Saaby, L.; Moesby, L.; Hansen, E.W. Considerations regarding use of solvents in in vitro cell based assays. Cytotechnology 2013, 65, 887–894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Tolcher, A.W.; Rowinsky, E.K. The future of cytotoxic therapy: Selective cytotoxicity based on biology is the key. Breast Cancer Res. 2003, 5, 154–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Radha Abbas Hasoon, M.; Jawad Kadhim, N. Improvement of the Selectivity Index (SI) and Cytotoxicity Activity of Doxorubicin Drug by Panax ginseng Plant Extract. Arch. Razi Inst. 2021, 76, 659–666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moriasi, G.; Ngugi, M.; Mwitari, P.; Omwenga, G. Antioxidant, anti-prostate cancer potential, and phytochemical composition of the ethyl acetate stem bark extract of Boascia coriacea (Pax.). PLoS ONE 2024, 19, e0309258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed] [PubMed Central]
- Mfotie Njoya, E.; Maza, H.L.D.; Mkounga, P.; Koert, U.; Nkengfack, A.E.; McGaw, L.J. Selective cytotoxic activity of isolated compounds from Globimetula dinklagei and Phragmanthera capitata (Loranthaceae). Z. Naturforsch. C J. Biosci. 2020, 75, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Chen, G.L.; Liu, Y.; Zhuang, X.C.; Guo, M.Q. Stimulation of ROS Generation by Extract of Warburgia ugandensis Leading to G(0)/G(1) Cell Cycle Arrest and Antiproliferation in A549 Cells. Antioxidants 2021, 10, 1559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jara-Gutiérrez, C.; Mercado, L.; Paz-Araos, M.; Howard, C.; Parraga, M.; Escobar, C.; Mellado, M.; Madrid, A.; Montenegro, I.; Santana, P.; et al. Oxidative stress promotes cytotoxicity in human cancer cell lines exposed to Escallonia spp. extracts. BMC Complement. Med. Ther. 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, N.H.; Ta, Q.T.H.; Pham, Q.T.; Luong, T.N.H.; Phung, V.T.; Duong, T.H.; Vo, V.G. Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells. Molecules 2020, 25, 2912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marioli-Sapsakou, G.K.; Kourti, M. Targeting Production of Reactive Oxygen Species as an Anticancer Strategy. Anticancer Res. 2021, 41, 5881–5902. [Google Scholar] [CrossRef] [PubMed]
- Jelínek, M.; Balušíková, K.; Schmiedlová, M.; Němcová-Fürstová, V.; Šrámek, J.; Stančíková, J.; Zanardi, I.; Ojima, I.; Kovář, J. The role of individual caspases in cell death induction by taxanes in breast cancer cells. Cancer Cell Int. 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K. Caspase Activation in Cancer Therapy. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2000–2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6027/ (accessed on 16 March 2025).
- Mfotie Njoya, E.; van Dyk, H.; Nambooze, J.; Chukwuma, C.I.; Brink, A.; Makhafola, T.J. Insight into the molecular mechanism of anti-breast cancer therapeutic potential of substituted salicylidene-based compounds using cell-based assays and molecular docking studies. Eur. J. Pharmacol. 2024, 985, 177129. [Google Scholar] [CrossRef] [PubMed]
- Bohuslav, J.; Chen, L.F.; Kwon, H.; Mu, Y.; Greene, W.C. p53 induces NF-kappaB activation by an IkappaB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 2004, 279, 26115–26125. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- McTavish, N.; Copeland, L.A.; Saville, M.K.; Perkins, N.D.; Spruce, B.A. Proenkephalin assists stress-activated apoptosis through transcriptional repression of NF-kappaB- and p53-regulated gene targets. Cell Death Differ. 2007, 14, 1700–1710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavitra, E.; Kancharla, J.; Gupta, V.K.; Prasad, K.; Sung, J.Y.; Kim, J.; Tej, M.B.; Choi, R.; Lee, J.H.; Han, Y.K.; et al. The role of NF-kappaB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed. Pharmacother. 2023, 163, 114822. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hemann, M.T.; Lowe, S.W. The p53-Bcl-2 connection. Cell Death Differ. 2006, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahman, N.; Khan, H.; Zia, A.; Khan, A.; Fakhri, S.; Aschner, M.; Gul, K.; Saso, L. Bcl-2 Modulation in p53 Signaling Pathway by Flavonoids: A Potential Strategy towards the Treatment of Cancer. Int. J. Mol. Sci. 2021, 22, 11315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azimian, H.; Dayyani, M.; Toossi, M.T.B.; Mahmoudi, M. Bax/Bcl-2 expression ratio in prediction of response to breast cancer radiotherapy. Iran. J. Basic. Med. Sci. 2018, 21, 325–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pyo, Y.; Kwon, K.H.; Jung, Y.J. Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits. Foods. 2024, 13, 2253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izzo, S.; Naponelli, V.; Bettuzzi, S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients 2020, 12, 1010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Liu, W.; Hu, X. Robinin inhibits pancreatic cancer cell proliferation, EMT and inflammation via regulating TLR2-PI3k-AKT signaling pathway. Cancer Cell Int. 2023, 23, 328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satari, A.; Amini, S.A.; Raeisi, E.; Lemoigne, Y.; Heidarian, E. Synergetic Impact of Combined 5-Fluorouracil and Rutin on Apoptosis in PC3 Cancer Cells through the Modulation of P53 Gene Expression. Adv. Pharm. Bull. 2019, 9, 462–469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AbouSamra, M.M.; Afifi, S.M.; Galal, A.F.; Kamel, R. Rutin-loaded Phyto-Sterosomes as a potential approach for the treatment of hepatocellular carcinoma: In-vitro and in-vivo studies. J. Drug Deliv. Sci. Technol. 2023, 79, 104015. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mfotie Njoya, E.; McGaw, L.J.; Makhafola, T.J. Investigating the Phytochemical Composition, Antioxidant, and Anti-Inflammatory Potentials of Cassinopsis ilicifolia (Hochst.) Kuntze Extract against Some Oxidative Stress and Inflammation Molecular Markers. Curr. Issues Mol. Biol. 2024, 46, 9639–9658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mfotie Njoya, E.; Tabakam, G.T.; Chukwuma, C.I.; Mashele, S.S.; Makhafola, T.J. Phytoconstituents of Androstachys johnsonii Prain Prevent Reactive Oxygen Species Production and Regulate the Expression of Inflammatory Mediators in LPS-Stimulated RAW 264.7 Macrophages. Antioxidants 2024, 13, 401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| IC50 (µg/mL) of Plant Extracts | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Buxus natalensis | Cancer Cell Lines | Normal Cell | |||||||
| Lung Cancer | Cervical Cancer | Liver Cancer | Breast Cancer | Prostate Cancer | Colorectal Cancer | Chang Liver | |||
| A549 | HeLa | HepG2 | 4T1 | MCF-7 | DU145 | LNCaP | Caco-2 | ||
| Hydroethanolic | >500 | 378.90 ± 3.16 a | 78.01 ± 1.30 a | 105.20 ± 6.40 a | >500 | 258.30 ± 2.47 a | 47.39 ± 1.98 a | >500 | 329.70 ± 2.62 a |
| Aqueous | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Methanolic | >500 | 362.20 ± 5.69 a | 79.07 ± 1.42 a | 98.00 ± 2.89 a | >500 | 219.33 ± 2.40 b | 49.18 ± 2.75 a | >500 | 205.50 ± 9.58 b |
| Doxorubucin (µM) | 1.02 ± 0.50 | 1.00 ± 0.52 | 4.21 ± 1.09 | 1.57 ± 0.25 | 1.96 ± 0.28 | 0.92 ± 0.26 | 1.73 ± 0.12 | 57.33 ± 4.15 | 1.22 ± 0.13 |
| Buxus natalensis | SI Values | |||||||
|---|---|---|---|---|---|---|---|---|
| A549 | HeLa | HepG2 | 4T1 | MCF-7 | DU145 | LNCaP | Caco-2 | |
| Hydroethanolic | nd | 0.87 | 4.22 | 3.13 | nd | 1.28 | 6.96 | nd |
| Aqueous | nd | nd | nd | nd | nd | nd | nd | nd |
| Methanolic | nd | 0.57 | 2.59 | 2.10 | nd | 0.94 | 4.17 | nd |
| Doxorubucin | 1.19 | 1.22 | 0.29 | 0.78 | 0.62 | 1.33 | 0.71 | 0.02 |
| Buxus natalensis | % Yield of Extraction (g Extract/100 g Dry Material) | Phenolic Content (mgGAE/g Extract) | Flavonoid Content (mgQE/g Extract) |
|---|---|---|---|
| Hydroethanolic | 15.78 | 84.64 ± 1.65 | 24.45 ± 1.35 |
| Aqueous | 33.55 | 68.42 ± 1.53 | 16.84 ± 2.26 |
| Methanolic | 13.32 | 95.90 ± 2.10 | 50.88 ± 3.99 |
| Peak N° | Rt (min) | [M − H]− (m/z) | Tentative Assignment (Compound Name) | Ontology | Molecular Formula | Total Score | Peak Height Intensity | Conc. in Extract vs. 3CQA (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.18 | 971.31 | UNPD33759 | Oligosaccharides | C72H120O60 | 4.35 | 6921 | 256 |
| 2 | 9.61 | 291.14 | Heptopyranoside | Fatty acyl glycosides of mono- and disaccharides | C13H24O7 | 4.57 | 25,627 | 947 |
| 3 | 13.18 | 431.19 | MINEs-320976 | Saccharolipids | C20H32O10 | 5.33 | 2471 | 91 |
| 4 | 14.51 | 625.14 | Quercetin 3-glucosyl-(1->2)-galactoside | Flavonoid-3-O-glycosides | C27H29O17 | 7.22 | 1307 | 48 |
| 5 | 14.80 | 755.20 | Kaempferol 3-gentiobioside 7-rhamnoside | Flavonoid-7-O-glycosides | C33H40O20 | 6.75 | 19,443 | 719 |
| 6 | 15.37 | 609.14 | Kaempferol derivative | Flavonoid-3-O-glycosides | C27H30O16 | 6.75 | 3585 | 133 |
| 7 | 15.47 | 581.22 | (7′R)-(+)-Lyoniresinol 9′-glucoside | Lignan glycosides | C28H38O13 | 6.26 | 3964 | 147 |
| 8 | 15.87 | 739.20 | Robinin | Flavonoid-7-O-glycosides | C33H40O19 | 6.64 | 14,525 | 537 |
| 9 | 16.09 | 609.14 | Rutin | Flavonoid-3-O-glycosides | C27H30O16 | 8.09 | 3813 | 141 |
| 10 | 16.46 | 463.08 | Quercetin 3-galactoside | Flavonoid-3-O-glycosides | C21H20O12 | 7.90 | 754 | 28 |
| 11 | 17.22 | 547.23 | UNPD111558 | Terpene glycosides | C25H40O13 | 6.90 | 12,752 | 471 |
| 12 | 17.72 | 593.15 | Astragalin 7-rhamnoside | Flavonoid-7-O-glycosides | C27H30O15 | 8.12 | 1428 | 53 |
| 13 | 17.75 | 457.07 | Unknown | - | - | - | 719 | 27 |
| 14 | 18.08 | 597.24 | Unknown | - | - | - | 434 | 16 |
| 15 | 18.11 | 447.09 | Astragalin | Flavonoid-7-O-glycosides | C21H20O11 | 8.09 | 499 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mfotie Njoya, E.; Tabakam, G.T.; Chukwuma, C.I.; Makhafola, T.J. Buxus natalensis (Oliv.) Hutch (Buxaceae) Exhibits Its Anticancer Potential by Stimulating ROS Production and Caspase-p53-BCL-2-Dependent Apoptosis in Hepatocellular Carcinoma and Prostate Cancer Cell Lines. Int. J. Mol. Sci. 2025, 26, 4173. https://doi.org/10.3390/ijms26094173
Mfotie Njoya E, Tabakam GT, Chukwuma CI, Makhafola TJ. Buxus natalensis (Oliv.) Hutch (Buxaceae) Exhibits Its Anticancer Potential by Stimulating ROS Production and Caspase-p53-BCL-2-Dependent Apoptosis in Hepatocellular Carcinoma and Prostate Cancer Cell Lines. International Journal of Molecular Sciences. 2025; 26(9):4173. https://doi.org/10.3390/ijms26094173
Chicago/Turabian StyleMfotie Njoya, Emmanuel, Gaetan T. Tabakam, Chika I. Chukwuma, and Tshepiso J. Makhafola. 2025. "Buxus natalensis (Oliv.) Hutch (Buxaceae) Exhibits Its Anticancer Potential by Stimulating ROS Production and Caspase-p53-BCL-2-Dependent Apoptosis in Hepatocellular Carcinoma and Prostate Cancer Cell Lines" International Journal of Molecular Sciences 26, no. 9: 4173. https://doi.org/10.3390/ijms26094173
APA StyleMfotie Njoya, E., Tabakam, G. T., Chukwuma, C. I., & Makhafola, T. J. (2025). Buxus natalensis (Oliv.) Hutch (Buxaceae) Exhibits Its Anticancer Potential by Stimulating ROS Production and Caspase-p53-BCL-2-Dependent Apoptosis in Hepatocellular Carcinoma and Prostate Cancer Cell Lines. International Journal of Molecular Sciences, 26(9), 4173. https://doi.org/10.3390/ijms26094173







