Luteolin Induces Nrf2 Activity in C2C12 Cells: Implications for Muscle Health
Abstract
1. Introduction
2. Results
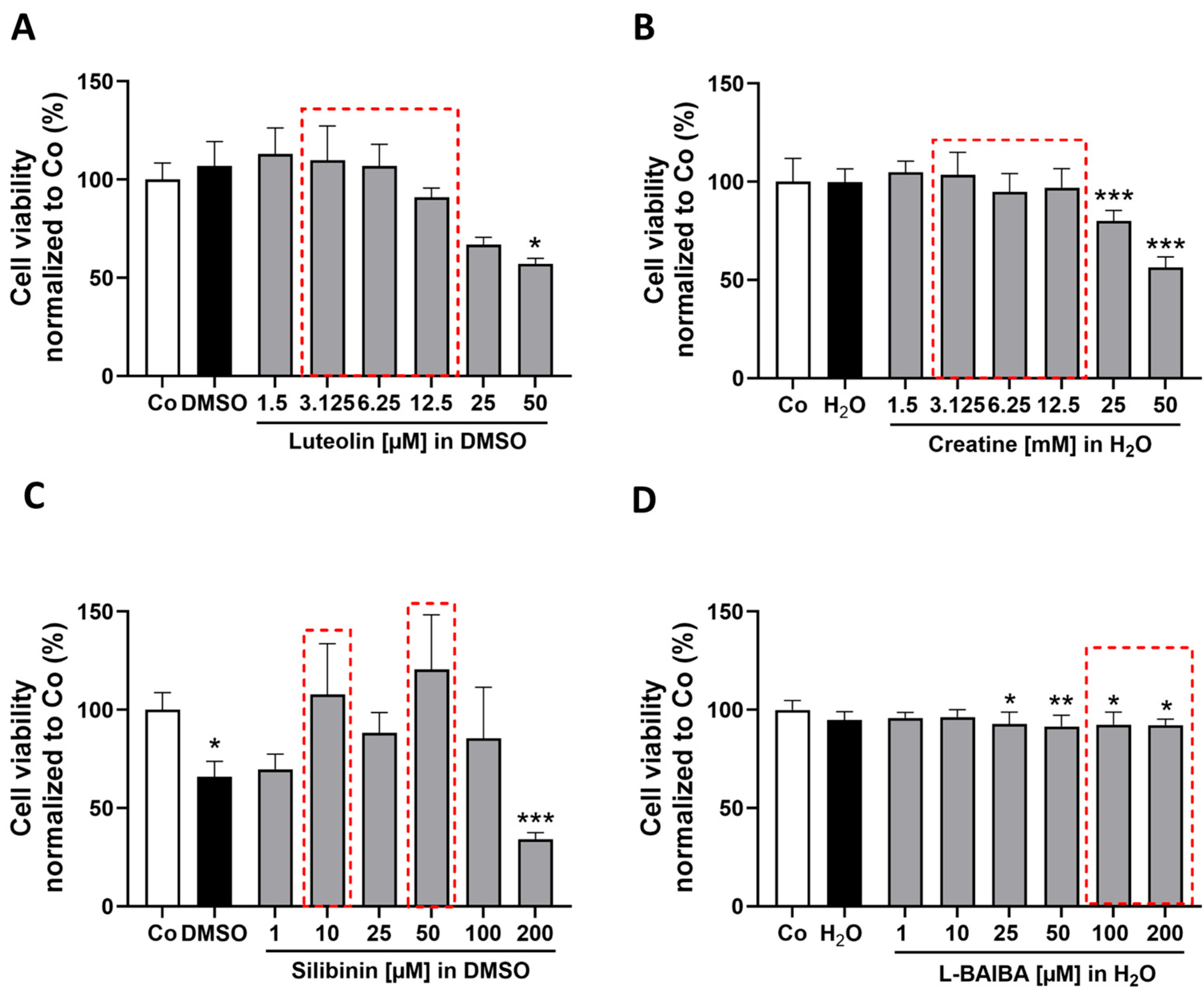
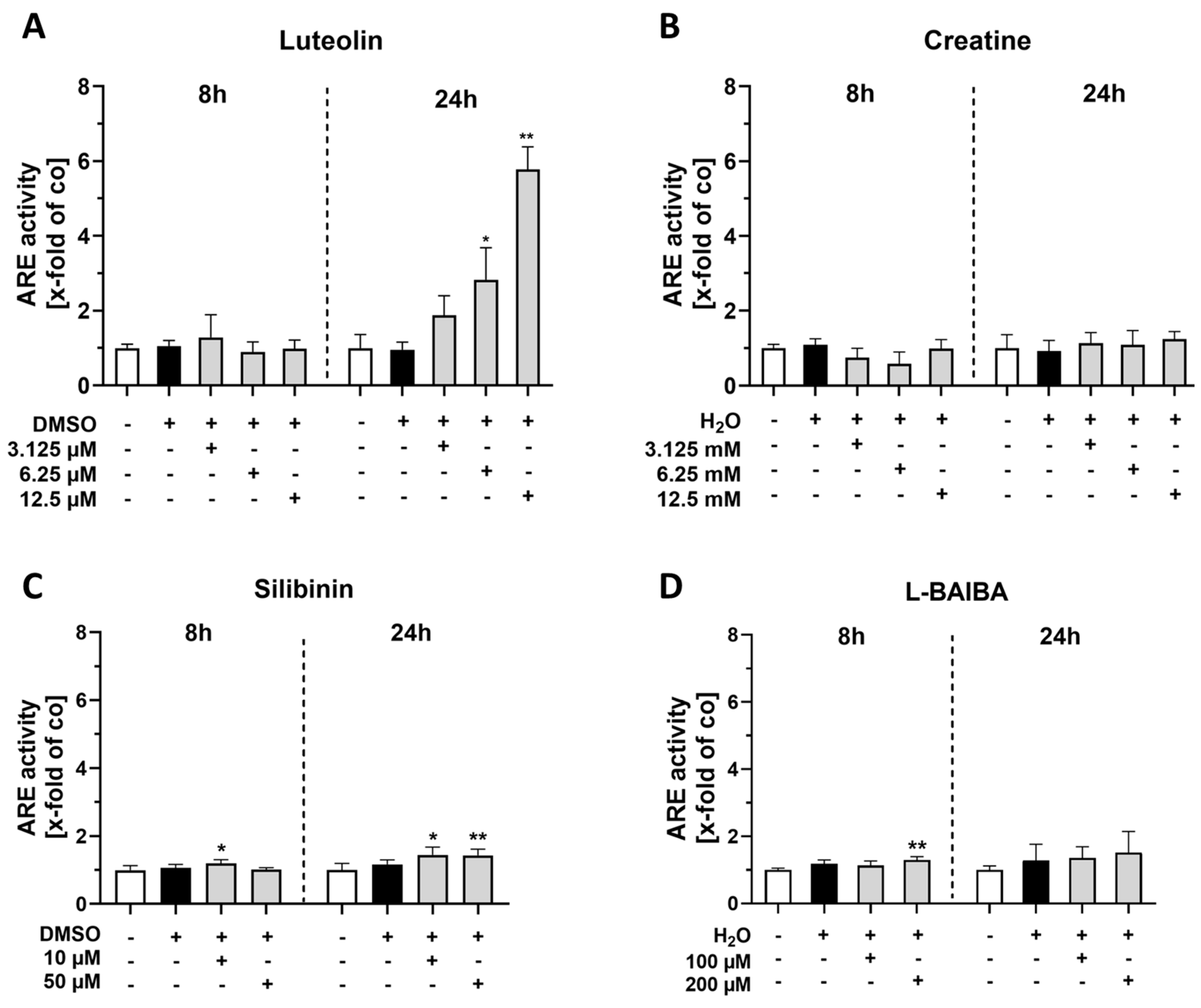
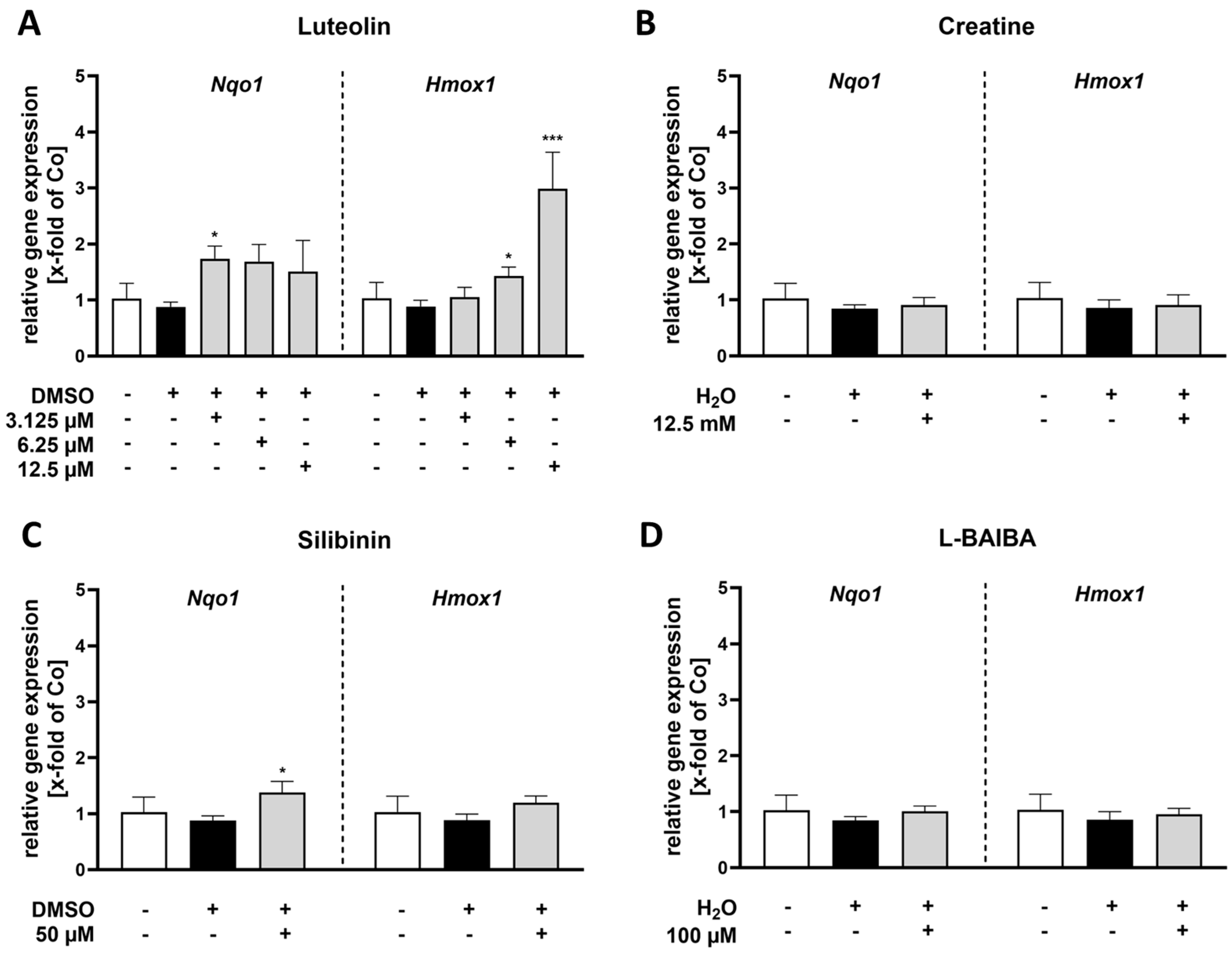
2.1. Luteolin Is the Most Potent of the Tested Nrf2 Activators in Proliferating C2C12 Cells
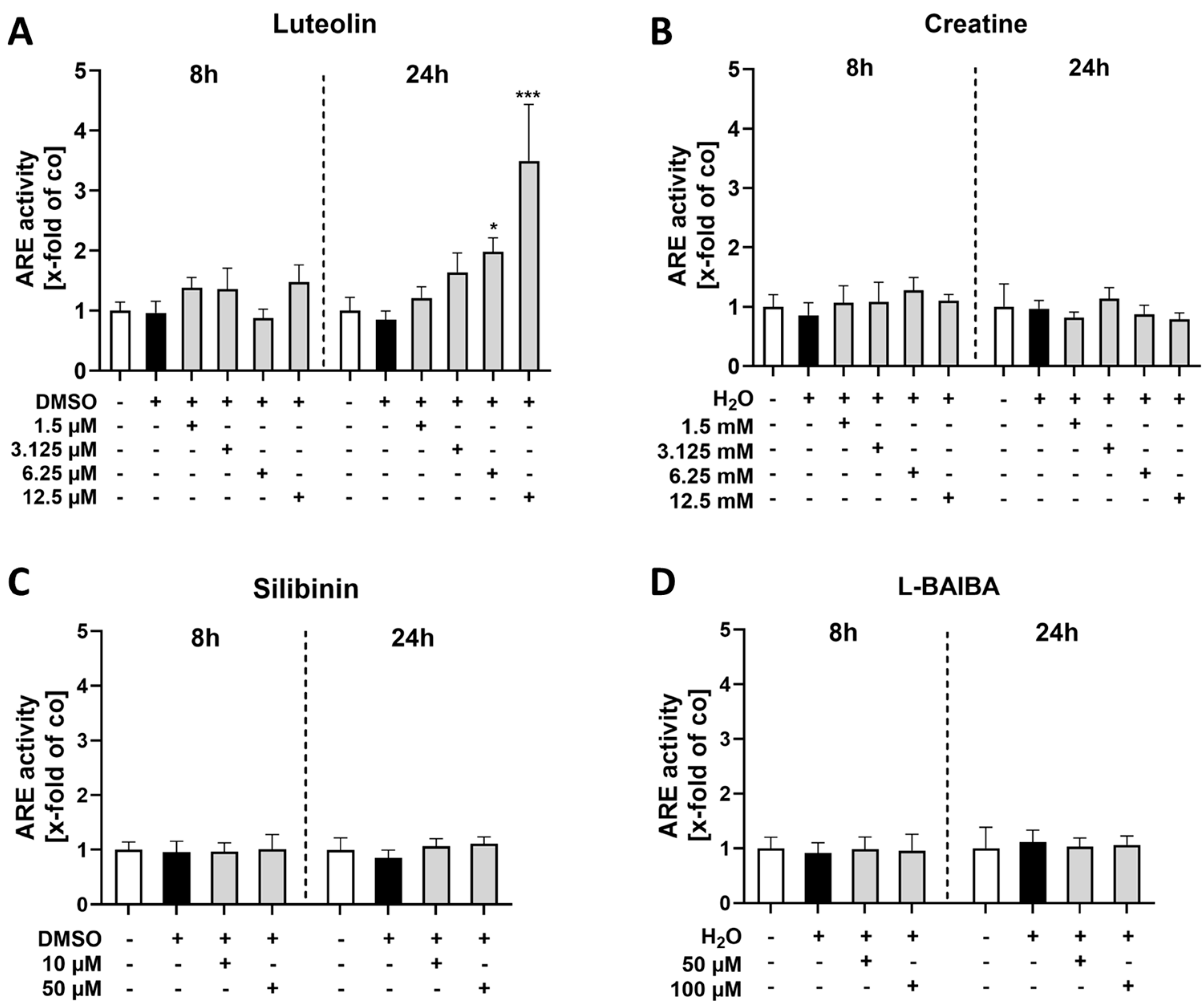
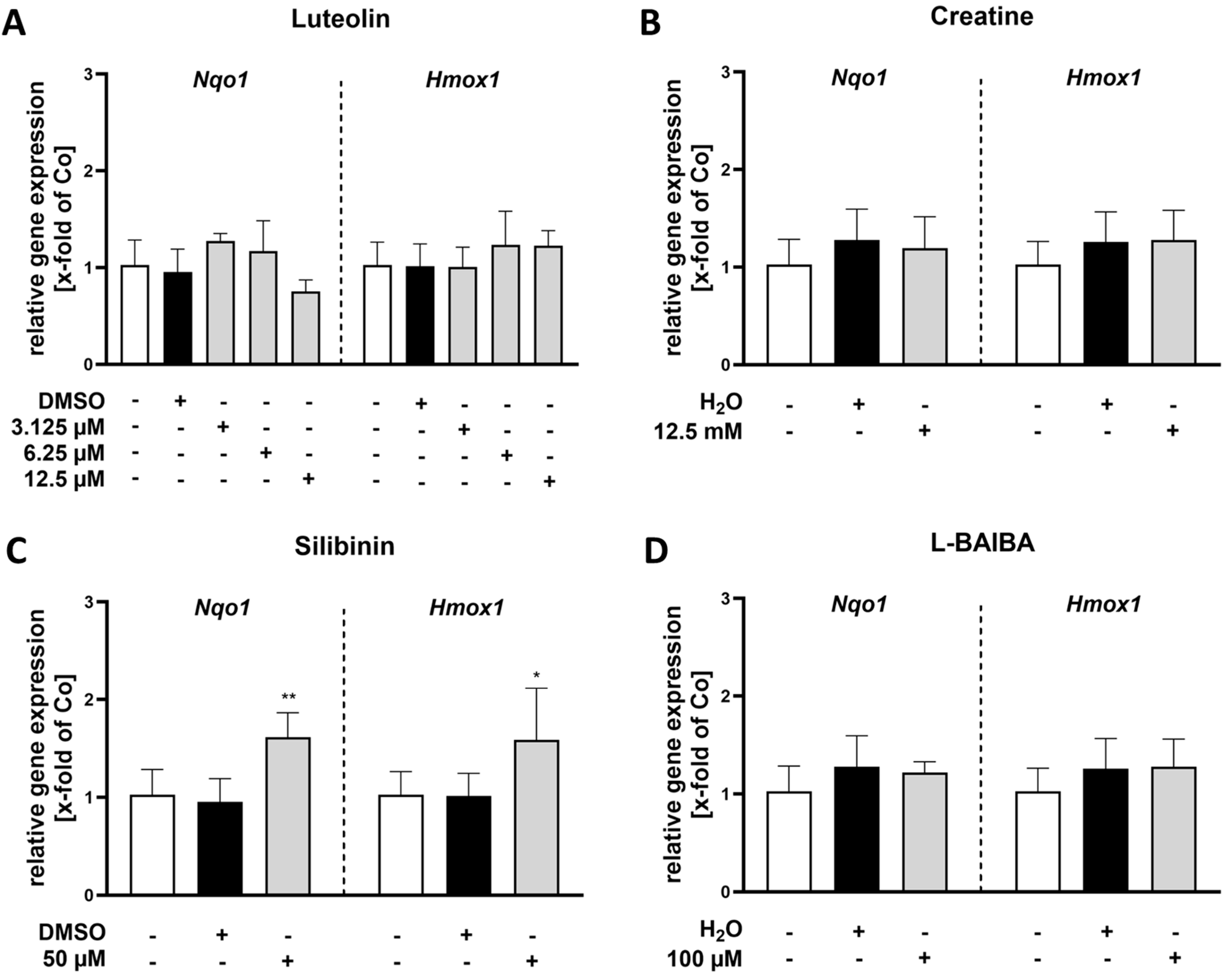
2.2. Luteolin Is the Only Substance Tested That Activates Nrf2 in Differentiated C2C12 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Stimulation
4.3. Cell Viability Assay
4.4. ARE Activity
4.5. RNA Isolation, cDNA Synthesis and qPCR
4.6. Statistics
4.7. Graphic Illustration
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AREs | antioxidant response elements |
| BCCA | branched-chain amino acid |
| Co | control |
| CTB | CellTiter-Blue |
| Cul4a | cullin 4A |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | dimethylsulfoxide |
| FCS | fetal calf serum |
| GPX2 | glutathione peroxidase 2 |
| GSK-3β | glycogen synthase kinase 3 beta |
| HMOX1 | heme oxygenase-1 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| L-BAIBA | L-β-aminoisobutyric acid |
| NF2L2/Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| sMAF | small musculoaponeurotic fibrosarcoma |
| SD | standard deviation |
| Sdha | succinate dehydrogenase complex subunit A |
| SFN | sulforaphane |
| SkM | skeletal muscle |
| β-TrCP | beta-transducin repeat-containing protein |
References
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol. Cell. Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef]
- Uruno, A.; Yagishita, Y.; Katsuoka, F.; Kitajima, Y.; Nunomiya, A.; Nagatomi, R.; Pi, J.; Biswal, S.S.; Yamamoto, M. Nrf2-Mediated Regulation of Skeletal Muscle Glycogen Metabolism. Mol. Cell. Biol. 2016, 36, 1655–1672. [Google Scholar] [CrossRef]
- Ballmann, C.; McGinnis, G.; Peters, B.; Slivka, D.; Cuddy, J.; Hailes, W.; Dumke, C.; Ruby, B.; Quindry, J. Exercise-induced oxidative stress and hypoxic exercise recovery. Eur. J. Appl. Physiol. 2014, 114, 725–733. [Google Scholar] [CrossRef]
- Cobley, J.N.; Sakellariou, G.K.; Owens, D.J.; Murray, S.; Waldron, S.; Gregson, W.; Fraser, W.D.; Burniston, J.G.; Iwanejko, L.A.; McArdle, A.; et al. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014, 70, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; deBeer, J.; Tarnopolsky, M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2010, 49, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Volonte, D.; Liu, Z.; Musille, P.M.; Stoppani, E.; Wakabayashi, N.; Di, Y.P.; Lisanti, M.P.; Kensler, T.W.; Galbiati, F. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol. Biol. Cell 2013, 24, 1852–1862. [Google Scholar] [CrossRef]
- Done, A.J.; Gage, M.J.; Nieto, N.C.; Traustadottir, T. Exercise-induced Nrf2-signaling is impaired in aging. Free Radic. Biol. Med. 2016, 96, 130–138. [Google Scholar] [CrossRef]
- Yamada, M.; Warabi, E.; Oishi, H.; Lira, V.A.; Okutsu, M. Muscle-derived IL-1beta regulates EcSOD expression via the NBR1-p62-Nrf2 pathway in muscle during cancer cachexia. J. Physiol. 2024, 602, 4215–4235. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Okutsu, M.; Yamada, M. Muscle-specific Nrf2 deficiency exacerbates cancer cachexia-induced skeletal muscle atrophy. FASEB J. 2020, 34 (Suppl. S1), 1-1. [Google Scholar] [CrossRef]
- Kobayashi, M.; Li, L.; Iwamoto, N.; Nakajima-Takagi, Y.; Kaneko, H.; Nakayama, Y.; Eguchi, M.; Wada, Y.; Kumagai, Y.; Yamamoto, M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009, 29, 493–502. [Google Scholar] [CrossRef]
- Marcotte, D.; Zeng, W.; Hus, J.C.; McKenzie, A.; Hession, C.; Jin, P.; Bergeron, C.; Lugovskoy, A.; Enyedy, I.; Cuervo, H.; et al. Small molecules inhibit the interaction of Nrf2 and the Keap1 Kelch domain through a non-covalent mechanism. Bioorg. Med. Chem. 2013, 21, 4011–4019. [Google Scholar] [CrossRef]
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef]
- Bello, M. Structural basis of Nrf2 activation by flavonolignans from silymarin. J. Mol. Graph. Model. 2023, 119, 108393. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-Gonzalez, A.; Morales-Martinez, M.; Soriano-Ursua, M.A.; Delgado-Olivares, L.; Sandoval-Gallegos, E.M.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I.; Madrigal-Santillan, E.; Morales-Gonzalez, J.A. Flavolignans from Silymarin as Nrf2 Bioactivators and Their Therapeutic Applications. Biomedicines 2020, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Sun, M.; Chen, H.; Xiao, Y.; Wang, J.; Zhang, Y.; Fang, S.; Kou, J. Silibinin alleviates acute liver failure by modulating AKT/GSK3beta/Nrf2/GPX4 pathway. Naunyn-Schmiedebergs Arch. Pharmacol. [CrossRef]
- Wruck, C.J.; Claussen, M.; Fuhrmann, G.; Romer, L.; Schulz, A.; Pufe, T.; Waetzig, V.; Peipp, M.; Herdegen, T.; Gotz, M.E. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J. Neural Transm. Suppl. 2007, 72, 57–67. [Google Scholar] [CrossRef]
- Kitakaze, T.; Makiyama, A.; Yamashita, Y.; Ashida, H. Low dose of luteolin activates Nrf2 in the liver of mice at start of the active phase but not that of the inactive phase. PLoS ONE 2020, 15, e0231403. [Google Scholar] [CrossRef]
- Duan, F.F.; Guo, Y.; Li, J.W.; Yuan, K. Antifatigue Effect of Luteolin-6-C-Neohesperidoside on Oxidative Stress Injury Induced by Forced Swimming of Rats through Modulation of Nrf2/ARE Signaling Pathways. Oxid. Med. Cell. Longev. 2017, 2017, 3159358. [Google Scholar] [CrossRef]
- Kitakaze, T.; Makiyama, A.; Samukawa, Y.; Jiang, S.; Yamashita, Y.; Ashida, H. A physiological concentration of luteolin induces phase II drug-metabolizing enzymes through the ERK1/2 signaling pathway in HepG2 cells. Arch. Biochem. Biophys. 2019, 663, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Guo, J.; He, B.; Wu, L.; Yang, R.; Li, X.; Fang, D.; Yang, X.; Yang, D.; et al. beta-aminoisobutyrics acid, a metabolite of BCAA, activates the AMPK/Nrf-2 pathway to prevent ferroptosis and ameliorates lung ischemia-reperfusion injury. Mol. Med. 2023, 29, 164. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Taskin, S.; Celik, T.; Demiryurek, S.; Turedi, S.; Taskin, A. Effects of different-intensity exercise and creatine supplementation on mitochondrial biogenesis and redox status in mice. Iran. J. Basic Med. Sci. 2022, 25, 1009–1015. [Google Scholar] [CrossRef]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kim, H.S. Creatine supplementation with exercise reduces alpha-synuclein oligomerization and necroptosis in Parkinson’s disease mouse model. J. Nutr. Biochem. 2024, 126, 109586. [Google Scholar] [CrossRef]
- Tang, X.; Wang, H.; Fan, L.; Wu, X.; Xin, A.; Ren, H.; Wang, X.J. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011, 50, 1599–1609. [Google Scholar] [CrossRef]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Muller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Van Roie, E.; Hiroux, C.; Vanmunster, M.; Coudyzer, W.; Suhr, F.; Bogaerts, S.; Van Thienen, R.; Koppo, K. Omega-3 Supplementation Improves Isometric Strength But Not Muscle Anabolic and Catabolic Signaling in Response to Resistance Exercise in Healthy Older Adults. J. Gerontol. Ser. A 2021, 76, 406–414. [Google Scholar] [CrossRef]
- Picorelli, A.M.; Pereira, L.S.; Pereira, D.S.; Felicio, D.; Sherrington, C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: A systematic review. J. Physiother. 2014, 60, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef]
- Iyanagi, T.; Yamazaki, I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim. Biophys. Acta 1970, 216, 282–294. [Google Scholar] [CrossRef]
- Oh, E.T.; Kim, J.W.; Kim, J.M.; Kim, S.J.; Lee, J.S.; Hong, S.S.; Goodwin, J.; Ruthenborg, R.J.; Jung, M.G.; Lee, H.J.; et al. NQO1 inhibits proteasome-mediated degradation of HIF-1alpha. Nat. Commun. 2016, 7, 13593. [Google Scholar] [CrossRef]
- Asher, G.; Tsvetkov, P.; Kahana, C.; Shaul, Y. A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev. 2005, 19, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.F.; Shen, X.Y.; Lio, C.K.; Dai, Y.; Cheng, C.S.; Liu, J.X.; Yao, Y.D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef]
- Seldon, M.P.; Silva, G.; Pejanovic, N.; Larsen, R.; Gregoire, I.P.; Filipe, J.; Anrather, J.; Soares, M.P. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappaB RelA phosphorylation at serine 276. J. Immunol. 2007, 179, 7840–7851. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Wang, M.; Pu, D.; Zhao, Y.; Chen, J.; Zhu, S.; Lu, A.; Liao, Z.; Sun, Y.; Xiao, Q. Sulforaphane protects against skeletal muscle dysfunction in spontaneous type 2 diabetic db/db mice. Life Sci. 2020, 255, 117823. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, B.; Lu, J.; Li, S.; Baiyun, R.; Lv, Y.; Lu, Q.; Zhang, Z. Dietary luteolin protects against HgCl(2)-induced renal injury via activation of Nrf2-mediated signaling in rat. J. Inorg. Biochem. 2018, 179, 24–31. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.; Xu, J.; Zhang, P.; Deng, R.; Mao, Y.; He, J.; Chen, Y.; Zhang, Y.; Ding, J.; et al. Luteolin Exerts Neuroprotection via Modulation of the p62/Keap1/Nrf2 Pathway in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1551. [Google Scholar] [CrossRef]
- Rajput, S.A.; Shaukat, A.; Wu, K.; Rajput, I.R.; Baloch, D.M.; Akhtar, R.W.; Raza, M.A.; Najda, A.; Rafal, P.; Albrakati, A.; et al. Luteolin Alleviates AflatoxinB(1)-Induced Apoptosis and Oxidative Stress in the Liver of Mice through Activation of Nrf2 Signaling Pathway. Antioxidants 2021, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, C.; Huang, P.; Cheng, Y.; Ma, Y.; Gao, J.; Ding, H. Luteolin alleviates muscle atrophy, mitochondrial dysfunction and abnormal FNDC5 expression in high fat diet-induced obese rats and palmitic acid-treated C2C12 myotubes. J. Nutr. Biochem. 2025, 135, 109780. [Google Scholar] [CrossRef]
- Chian, S.; Thapa, R.; Chi, Z.; Wang, X.J.; Tang, X. Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo. Biochem. Biophys. Res. Commun. 2014, 447, 602–608. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol. Res. 2021, 167, 105526. [Google Scholar] [CrossRef] [PubMed]
- Elmazoglu, Z.; Yar Saglam, A.S.; Sonmez, C.; Karasu, C. Luteolin protects microglia against rotenone-induced toxicity in a hormetic manner through targeting oxidative stress response, genes associated with Parkinson’s disease and inflammatory pathways. Drug Chem. Toxicol. 2020, 43, 96–103. [Google Scholar] [CrossRef]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Theoharides, T.C. Luteolin supplements: All that glitters is not gold. Biofactors 2021, 47, 242–244. [Google Scholar] [CrossRef]
- Tie, F.; Fu, Y.; Hu, N.; Wang, H. Silibinin Protects against H2O2-Induced Oxidative Damage in SH-SY5Y Cells by Improving Mitochondrial Function. Antioxidants 2022, 11, 1101. [Google Scholar] [CrossRef]
- Chi, M.Y.; Zhang, H.; Wang, Y.X.; Sun, X.P.; Yang, Q.J.; Guo, C. Silibinin Alleviates Muscle Atrophy Caused by Oxidative Stress Induced by Cisplatin through ERK/FoxO and JNK/FoxO Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 5694223. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Kress, E.; Fragoulis, A.; Wruck, C.J.; Wolf, R.; Grotzinger, J.; Michalek, M.; Pufe, T.; Tauber, S.C.; Brandenburg, L.O. Psoriasin has divergent effects on the innate immune responses of murine glial cells. J. Neurochem. 2017, 141, 86–99. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Fragoulis, A.; Biller, K.; Fragoulis, S.; Lex, D.; Uhlig, S.; Reiss, L.K. Reference Gene Selection for Gene Expression Analyses in Mouse Models of Acute Lung Injury. Int. J. Mol. Sci. 2021, 22, 7853. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böttcher, N.; Suhr, F.; Pufe, T.; Wruck, C.J.; Fragoulis, A. Luteolin Induces Nrf2 Activity in C2C12 Cells: Implications for Muscle Health. Int. J. Mol. Sci. 2025, 26, 4092. https://doi.org/10.3390/ijms26094092
Böttcher N, Suhr F, Pufe T, Wruck CJ, Fragoulis A. Luteolin Induces Nrf2 Activity in C2C12 Cells: Implications for Muscle Health. International Journal of Molecular Sciences. 2025; 26(9):4092. https://doi.org/10.3390/ijms26094092
Chicago/Turabian StyleBöttcher, Nicole, Frank Suhr, Thomas Pufe, Christoph Jan Wruck, and Athanassios Fragoulis. 2025. "Luteolin Induces Nrf2 Activity in C2C12 Cells: Implications for Muscle Health" International Journal of Molecular Sciences 26, no. 9: 4092. https://doi.org/10.3390/ijms26094092
APA StyleBöttcher, N., Suhr, F., Pufe, T., Wruck, C. J., & Fragoulis, A. (2025). Luteolin Induces Nrf2 Activity in C2C12 Cells: Implications for Muscle Health. International Journal of Molecular Sciences, 26(9), 4092. https://doi.org/10.3390/ijms26094092







