Influence of Body Mass Index (BMI) and Waist–Hip Ratio (WHR) on Selected Semen Parameters
Abstract
1. Introduction
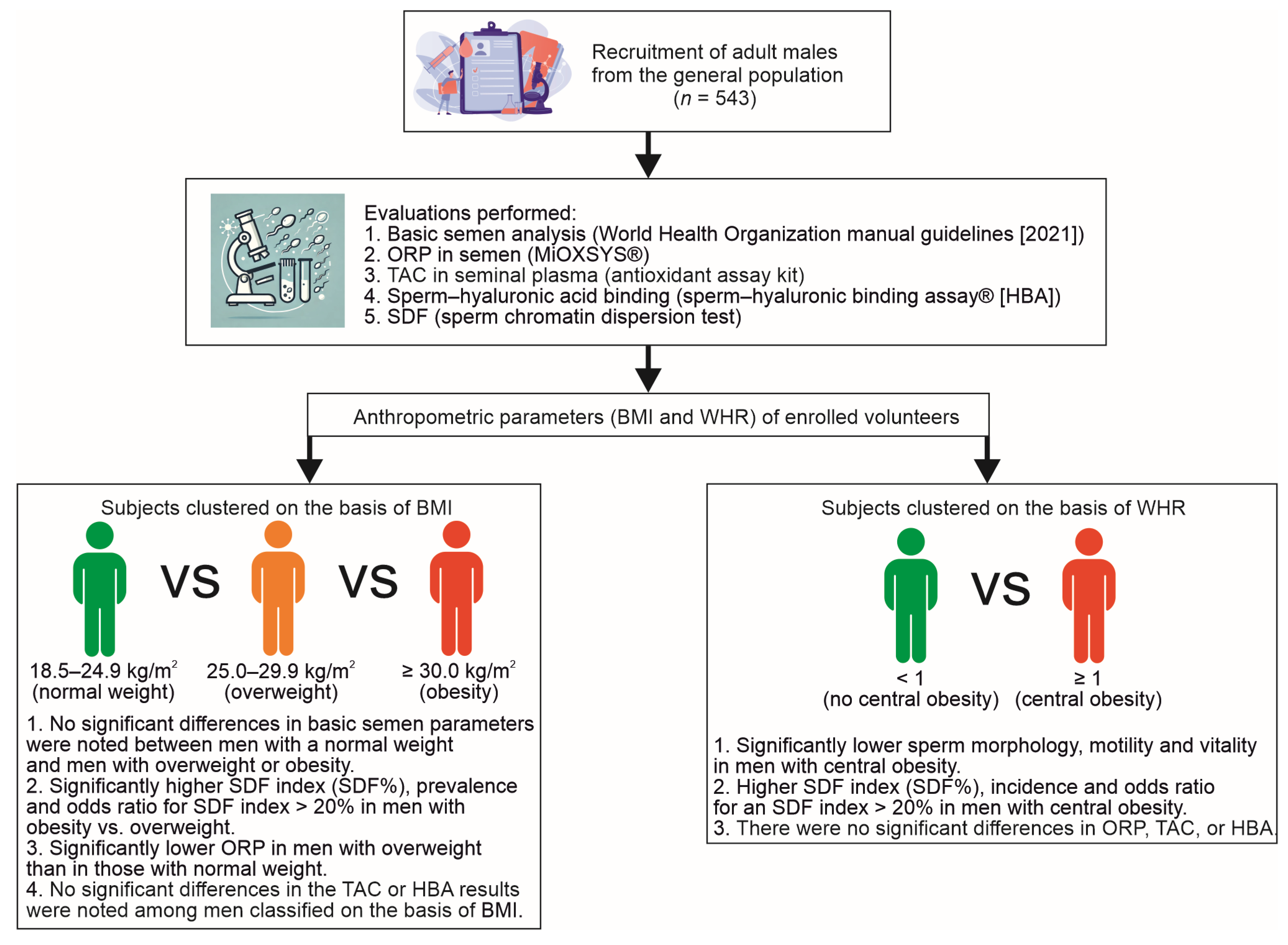
2. Results
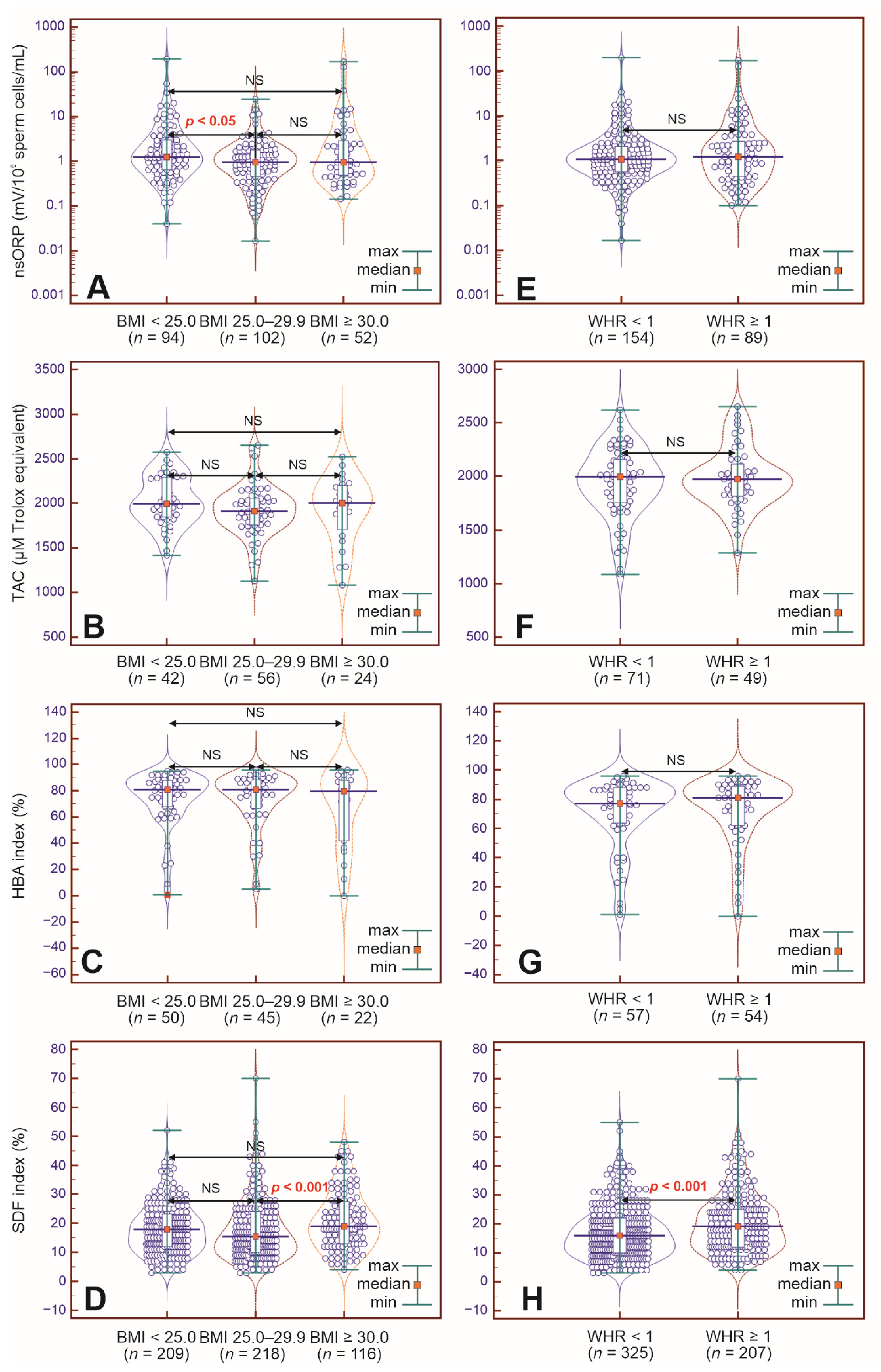
2.1. Study Parameters in BMI-Dependent Groups
2.2. Study Parameters in WHR-Dependent Groups
3. Discussion
3.1. Limited Influence of BMI on Basic Semen Parameters
3.2. Influence of WHR on Basic Semen Parameters
3.3. BMI and WHR Have an Unclear Influence on Nonstandard Semen Test Results
4. Materials and Methods
4.1. Participants
4.2. Body Mass Index
4.3. Waist–Hip Ratio
4.4. Basic Semen Analysis
4.5. Oxidative–Reduction Potential
4.6. Seminal Plasma Total Antioxidant Capacity
4.7. Sperm–Hyaluronan-Binding Assay
4.8. Sperm DNA Fragmentation
4.9. Statistical Analysis
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8OHdG | 8-hydroxy-2′-deoxyguanosine |
| 95% CI | 95% confidence interval |
| ABTS | 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) |
| BMI | body mass index |
| DA | denaturation solution |
| HA | hyaluronic acid/hyaluronan |
| HBA | hyaluronic acid-binding assay |
| HOS test | hypo-osmotic swelling test |
| LS | lysis solution |
| n | number of subjects |
| N | size of group |
| NS | not statistically significant |
| nsORP | normalized static oxidative–reduction potential in semen |
| OR | odds ratio |
| ORP | oxidation–reduction potential |
| PBS | phosphate-buffered saline |
| ROS | reactive oxygen species |
| SCDt | sperm chromatin dispersion test |
| SDF | sperm DNA fragmentation |
| TAC | total antioxidant capacity |
| TZI | teratozoospermia index |
| WHO | World Health Organization |
| WHR | waist–hip ratio |
References
- Alfaro Gómez, M.; Fernández-Santos, M.D.R.; Jurado-Campos, A.; Soria-Meneses, P.J.; Montoro Angulo, V.; Soler, A.J.; Garde, J.J.; Rodríguez-Robledo, V. On Males, Antioxidants and Infertility (MOXI): Certitudes, Uncertainties and Trends. Antioxidants 2023, 12, 1626. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Assidi, M. Infertility in Men: Advances towards a Comprehensive and Integrative Strategy for Precision Theranostics. Cells 2022, 11, 1711. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male Infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Gül, M.; Russo, G.I.; Kandil, H.; Boitrelle, F.; Saleh, R.; Chung, E.; Kavoussi, P.; Mostafa, T.; Shah, R.; Agarwal, A. Male Infertility: New Developments, Current Challenges, and Future Directions. World J. Men’s Health 2024, 42, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, Male Infertility, and the Sperm Epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef]
- Nikolic, A.Z.; Dragojevic-Dikic, S.; Kocic, J.; Babic, U.; Joksimovic, A.; Radakovic-Cosic, J.; Gerginic, V.; Spasic, D.; Dugalic, S.; Petrovic, A.; et al. Influence of Male Body Mass Index on Semen Analysis Parameters and in Vitro Fertilization Outcomes. Medicine 2024, 103, e38949. [Google Scholar] [CrossRef]
- Ameratunga, D.; Gebeh, A.; Amoako, A. Obesity and Male Infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 90, 102393. [Google Scholar] [CrossRef]
- Ma, J.; Wu, L.; Zhou, Y.; Zhang, H.; Xiong, C.; Peng, Z.; Bao, W.; Meng, T.; Liu, Y. Association between BMI and Semen Quality: An Observational Study of 3966 Sperm Donors. Hum. Reprod. 2019, 34, 155–162. [Google Scholar] [CrossRef]
- Abbasihormozi, S.H.; Babapour, V.; Kouhkan, A.; Niasari Naslji, A.; Afraz, K.; Zolfaghary, Z.; Shahverdi, A.H. Stress Hormone and Oxidative Stress Biomarkers Link Obesity and Diabetes with Reduced Fertility Potential. Cell J. 2019, 21, 307–313. [Google Scholar] [CrossRef]
- Esmaeili, V.; Zendehdel, M.; Shahverdi, A.; Alizadeh, A. Relationship between Obesity-Related Markers, Biochemical Metabolic Parameters, Hormonal Profiles and Sperm Parameters among Men Attending an Infertility Clinic. Andrologia 2022, 54, e14524. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Fan, Z.; Xu, R.; Deng, X.; Li, Y.; Liang, S.; Lv, Z.; Huang, S.; Duan, Y.-G.; et al. Association between Central Obesity and Semen Quality: A Cross-Sectional Study in 4513 Chinese Sperm Donation Volunteers. Andrology 2024, 12, 316–326. [Google Scholar] [CrossRef]
- Jing, J.; Peng, Y.; Fan, W.; Han, S.; Peng, Q.; Xue, C.; Qin, X.; Liu, Y.; Ding, Z. Obesity-Induced Oxidative Stress and Mitochondrial Dysfunction Negatively Affect Sperm Quality. FEBS Open Bio 2023, 13, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Cohen, R.V.; le Roux, C.W.; Sumithran, P. Obesity in Adults. Lancet 2024, 404, 972–987. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and Male Infertility: Mechanisms and Management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Crafa, A.; Curto, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Obesity and Male Fertility Disorders. Mol. Asp. Med. 2024, 97, 101273. [Google Scholar] [CrossRef]
- Gilden, A.H.; Catenacci, V.A.; Taormina, J.M. Obesity. Ann. Intern. Med. 2024, 177, ITC65–ITC80. [Google Scholar] [CrossRef]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites 2021, 11, 840. [Google Scholar] [CrossRef]
- Rasouli, M.A.; Dumesic, D.A.; Singhal, V. Male Infertility and Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2024, 31, 203–209. [Google Scholar] [CrossRef]
- Rotimi, D.E.; Acho, M.A.; Falana, B.M.; Olaolu, T.D.; Mgbojikwe, I.; Ojo, O.A.; Adeyemi, O.S. Oxidative Stress-Induced Hormonal Disruption in Male Reproduction. Reprod. Sci. 2024, 31, 2943–2956. [Google Scholar] [CrossRef] [PubMed]
- Alfaiate, M.I.; Tavares, R.S.; Ramalho-Santos, J. A Ripple Effect? The Impact of Obesity on Sperm Quality and Function. Reprod. Fertil. Dev. 2024, 36, RD23215. [Google Scholar] [CrossRef]
- Pearce, K.L.; Hill, A.; Tremellen, K.P. Obesity Related Metabolic Endotoxemia Is Associated with Oxidative Stress and Impaired Sperm DNA Integrity. Basic Clin. Androl. 2019, 29, 6. [Google Scholar] [CrossRef]
- Genchi, V.A.; Rossi, E.; Lauriola, C.; D’Oria, R.; Palma, G.; Borrelli, A.; Caccioppoli, C.; Giorgino, F.; Cignarelli, A. Adipose Tissue Dysfunction and Obesity-Related Male Hypogonadism. Int. J. Mol. Sci. 2022, 23, 8194. [Google Scholar] [CrossRef]
- Peel, A.; Saini, A.; Deluao, J.C.; McPherson, N.O. Sperm DNA Damage: The Possible Link between Obesity and Male Infertility, an Update of the Current Literature. Andrology 2023, 11, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Le, W.; Su, S.-H.; Shi, L.-H.; Zhang, J.-F.; Wu, D.-L. Effect of Male Body Mass Index on Clinical Outcomes Following Assisted Reproductive Technology: A Meta-Analysis. Andrologia 2016, 48, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-C.; Jing, J.; Dai, J.-Y.; Zhao, A.Z.; Yao, Q.; Fan, K.; Wang, G.-H.; Liang, Y.-J.; Chen, L.; Ge, Y.-F.; et al. Body Mass Index, Waist-to-Hip Ratio, Waist Circumference and Waist-to-Height Ratio Cannot Predict Male Semen Quality: A Report of 1231 Subfertile Chinese Men. Andrologia 2015, 47, 1047–1054. [Google Scholar] [CrossRef]
- Keszthelyi, M.; Gyarmathy, V.A.; Kaposi, A.; Kopa, Z. The Potential Role of Central Obesity in Male Infertility: Body Mass Index versus Waist to Hip Ratio as They Relate to Selected Semen Parameters. BMC Public Health 2020, 20, 307. [Google Scholar] [CrossRef]
- Santi, D.; Greco, C.; Barbonetti, A.; Simoni, M.; Maggi, M.; Corona, G. Weight Loss as Therapeutic Option to Restore Fertility in Obese Men: A Meta-Analytic Study. World J. Men’s Health 2024, 42, 333–343. [Google Scholar] [CrossRef]
- Agarwal, A.; Farkouh, A.; Parekh, N.; Zini, A.; Arafa, M.; Kandil, H.; Tadros, N.; Busetto, G.M.; Ambar, R.; Parekattil, S.; et al. Sperm DNA Fragmentation: A Critical Assessment of Clinical Practice Guidelines. World J. Men’s Health 2022, 40, 30–37. [Google Scholar] [CrossRef]
- Esteves, S.C.; Zini, A.; Coward, R.M.; Evenson, D.P.; Gosálvez, J.; Lewis, S.E.M.; Sharma, R.; Humaidan, P. Sperm DNA Fragmentation Testing: Summary Evidence and Clinical Practice Recommendations. Andrologia 2021, 53, e13874. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of Sperm DNA Damage and Its Impact on Male Infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef]
- Farkouh, A.; Salvio, G.; Kuroda, S.; Saleh, R.; Vogiatzi, P.; Agarwal, A. Sperm DNA Integrity and Male Infertility: A Narrative Review and Guide for the Reproductive Physicians. Transl. Androl. Urol. 2022, 11, 1023–1044. [Google Scholar] [CrossRef]
- Muncey, W.; Scott, M.; Lathi, R.B.; Eisenberg, M.L. The Paternal Role in Pregnancy Loss. Andrology 2025, 13, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, N.; Wei, C.; Aili, A.; Ji, X.; Chen, H.; Zhao, H. Effect of Paternal Overweight or Obesity on Semen Parameters, Clinical Pregnancy and Live Birth Outcomes in Men Treated with Intrauterine Insemination (IUI). Transl. Androl. Urol. 2024, 13, 1899–1904. [Google Scholar] [CrossRef]
- Amies-Cull, B.; Wolstenholme, J.; Cobiac, L.; Scarborough, P. Estimating BMI Distributions by Age and Sex for Local Authorities in England: A Small Area Estimation Study. BMJ Open 2022, 12, e060892. [Google Scholar] [CrossRef] [PubMed]
- Minetti, A.E.; Ruggiero, L. Inertial Biometry from Commercial 3D Body Meshes. Biol. Open 2022, 11, bio058927. [Google Scholar] [CrossRef]
- Macek, P.; Terek-Derszniak, M.; Biskup, M.; Krol, H.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. Assessment of Age-Induced Changes in Body Fat Percentage and BMI Aided by Bayesian Modelling: A Cross-Sectional Cohort Study in Middle-Aged and Older Adults. Clin. Interv. Aging 2020, 15, 2301–2311. [Google Scholar] [CrossRef]
- Lundgren, S.; Kuitunen, S.; Pietiläinen, K.H.; Hurme, M.; Kähönen, M.; Männistö, S.; Perola, M.; Lehtimäki, T.; Raitakari, O.; Kaprio, J.; et al. BMI Is Positively Associated with Accelerated Epigenetic Aging in Twin Pairs Discordant for Body Mass Index. J. Intern. Med. 2022, 292, 627–640. [Google Scholar] [CrossRef]
- Wang, S.; Wu, B.; Wang, C.; Ke, Z.; Xiang, P.; Hu, X.; Xiao, J. Influence of Body Mass Index and Waist–Hip Ratio on Male Semen Parameters in Infertile Men in the Real World: A Retrospective Study. Front. Endocrinol. 2023, 14, 1148715. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Kim, S.; Chen, Z.; Sundaram, R.; Schisterman, E.F.; Buck Louis, G.M. The Relationship between Male BMI and Waist Circumference on Semen Quality: Data from the LIFE Study. Hum. Reprod. 2014, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Faure, C.; Sermondade, N.; Boubaya, M.; Eustache, F.; Clément, P.; Briot, P.; Berthaut, I.; Levy, V.; Cedrin-Durnerin, I.; et al. Obesity Leads to Higher Risk of Sperm DNA Damage in Infertile Patients. Asian J. Androl. 2013, 15, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Fariello, R.M.; Pariz, J.R.; Spaine, D.M.; Gozzo, F.C.; Pilau, E.J.; Fraietta, R.; Bertolla, R.P.; Andreoni, C.; Cedenho, A.P. Effect of Smoking on the Functional Aspects of Sperm and Seminal Plasma Protein Profiles in Patients with Varicocele. Hum. Reprod. 2012, 27, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Bakos, H.W.; Tremellen, K. Impact of Body Mass Index on Seminal Oxidative Stress. Andrologia 2011, 43, 121–128. [Google Scholar] [CrossRef]
- Gutin, I. In BMI We Trust: Reframing the Body Mass Index as a Measure of Health. Soc. Theory Health 2018, 16, 256–271. [Google Scholar] [CrossRef]
- Gill, D.; Zuber, V.; Dawson, J.; Pearson-Stuttard, J.; Carter, A.R.; Sanderson, E.; Karhunen, V.; Levin, M.G.; Wootton, R.E.; Klarin, D.; et al. Risk Factors Mediating the Effect of Body Mass Index and Waist-to-Hip Ratio on Cardiovascular Outcomes: Mendelian Randomization Analysis. Int. J. Obes. 2021, 45, 1428–1438. [Google Scholar] [CrossRef]
- Vallet-Buisan, M.; Mecca, R.; Jones, C.; Coward, K.; Yeste, M. Contribution of Semen to Early Embryo Development: Fertilization and Beyond. Hum. Reprod. Update 2023, 29, 395–433. [Google Scholar] [CrossRef]
- Henkel, R.; Morris, A.; Vogiatzi, P.; Saleh, R.; Sallam, H.; Boitrelle, F.; Garrido, N.; Arafa, M.; Gül, M.; Rambhatla, A.; et al. Predictive Value of Seminal Oxidation-Reduction Potential Analysis for Reproductive Outcomes of ICSI. Reprod. Biomed. Online 2022, 45, 1007–1020. [Google Scholar] [CrossRef]
- Pavuluri, H.; Bakhtiary, Z.; Panner Selvam, M.K.; Hellstrom, W.J.G. Oxidative Stress-Associated Male Infertility: Current Diagnostic and Therapeutic Approaches. Medicina 2024, 60, 1008. [Google Scholar] [CrossRef]
- Mottola, F.; Palmieri, I.; Carannante, M.; Barretta, A.; Roychoudhury, S.; Rocco, L. Oxidative Stress Biomarkers in Male Infertility: Established Methodologies and Future Perspectives. Genes 2024, 15, 539. [Google Scholar] [CrossRef]
- Kallinikas, G.; Tsoporis, J.N.; Haronis, G.; Zarkadas, A.; Bozios, D.; Konstantinopoulos, V.; Kozyrakis, D.; Mitiliniou, D.; Rodinos, E.; Filios, A.; et al. The Role of Oral Antioxidants in the Improvement of Sperm Parameters in Infertile Men. World J. Urol. 2024, 42, 71. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R. Antioxidant Paradox. In Biomarkers of Oxidative Stress: Clinical Aspects of Oxidative Stress; Andreescu, S., Henkel, R., Khelfi, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 349–360. ISBN 978-3-031-69962-7. [Google Scholar]
- Dutta, S.; Sengupta, P.; Roychoudhury, S.; Chakravarthi, S.; Wang, C.W.; Slama, P. Antioxidant Paradox in Male Infertility: “A Blind Eye” on Inflammation. Antioxidants 2022, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Oliva, A.; Vergani, E.; Festa, R.; Silvestrini, A. The Dual Role of Oxidants in Male (In)Fertility: Every ROSe Has a Thorn. Int. J. Mol. Sci. 2023, 24, 4994. [Google Scholar] [CrossRef]
- Szucs, M.; Osvath, P.; Jakab, A.; Varga, D.; Varga, B.; Juhasz, B. Hyaluronan Bound Mature Sperm Count (HB-MaSC) Is a More Informative Indicator of Fertility than Conventional Sperm Parameters: Correlations with Body Mass Index (BMI). Reprod. Biol. 2019, 19, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Marchlewska, K.; Erkiert-Kusiak, M.; Walczak-Jędrzejowska, R.; Słowikowska-Hilczer, J. Sperm Migration and Hyaluronic Acid Binding: Implications for Male Fertility Evaluation. Int. J. Mol. Sci. 2024, 25, 9995. [Google Scholar] [CrossRef]
- Sermondade, N.; Dupont, C.; Faure, C.; Boubaya, M.; Cédrin-Durnerin, I.; Chavatte-Palmer, P.; Sifer, C.; Lévy, R. Body Mass Index Is Not Associated with Sperm-Zona Pellucida Binding Ability in Subfertile Males. Asian J. Androl. 2013, 15, 626–629. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Kopecka, V.; Sipek, J.; Rubes, J. Effect of Body Mass Index on Semen Quality, Sperm Chromatin Integrity and Sperm DNA Methylation. Obes. Res. Clin. Pract. 2024, 18, 380–387. [Google Scholar] [CrossRef]
- Li, L.; Ma, Y.; Zhu, C.; Li, Y.; Cao, H.; Wu, Z.; Jin, T.; Wang, Y.; Chen, S.; Dong, W. Paternal Obesity Induces Subfertility in Male Offspring by Modulating the Oxidative Stress-Related Transcriptional Network. Int. J. Obes. 2024, 48, 1318–1331. [Google Scholar] [CrossRef]
- Sruthi, K.G.; John, S.M.; Marconi David, S. Assessment of Obesity in the Indian Setting: A Clinical Review. Clin. Epidemiol. Glob. Health 2023, 23, 101348. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, N.; Sharma, R.S. Waist-hip ratio and waist circumference as simple measures of cardiovascular risk assessment and weight management among medical students. J. Evid. Based Med. Healthc. 2018, 5, 237–242. [Google Scholar] [CrossRef]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://www.who.int/publications-detail-redirect/9789240030787 (accessed on 24 August 2022).
- Roychoudhury, S.; Sharma, R.; Sikka, S.; Agarwal, A. Diagnostic Application of Total Antioxidant Capacity in Seminal Plasma to Assess Oxidative Stress in Male Factor Infertility. J. Assist. Reprod. Genet. 2016, 33, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory Assessment of Oxidative Stress in Semen. Arab. J. Urol. 2018, 16, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rosiak-Gill, A.; Gill, K.; Jakubik, J.; Fraczek, M.; Patorski, L.; Gaczarzewicz, D.; Kurzawa, R.; Kurpisz, M.; Piasecka, M. Age-Related Changes in Human Sperm DNA Integrity. Aging 2019, 11, 5399–5411. [Google Scholar] [CrossRef]
- Gill, K.; Jakubik-Uljasz, J.; Rosiak-Gill, A.; Grabowska, M.; Matuszewski, M.; Piasecka, M. Male Aging as a Causative Factor of Detrimental Changes in Human Conventional Semen Parameters and Sperm DNA Integrity. Aging Male 2020, 23, 1321–1332. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gharagozloo, P. The Assessment of Oxidative Stress in Human Semen: Chaos and Confusion in Pursuit of Diagnostic Precision. Reprod. Biomed. Online 2025, 50, 104488. [Google Scholar] [CrossRef]
- Walczak-Jędrzejowska, R. The Clinical Application Of Oxidation–Reduction Potential (ORP) In Human Semen Using The Male Infertile Oxidative System–MiOXSYS® [Znaczenie Kliniczne Potencjału Oksydacyjno–Redukcyjnego (ORP) W Ludzki Nasieniu Weryfikowanego Za Pomocą Elektrochemicznego Systemu Mioxsys]. Post Androl. Online 2024, 11, 4–33. [Google Scholar] [CrossRef]
- Castleton, P.; Gyawali, P.; Mathews, N.; Mutuku, S.M.; Sharkey, D.J.; McPherson, N.O. MiOXSYS® and OxiSperm® II Assays Appear to Provide No Clinical Utility for Determining Oxidative Stress in Human Sperm-Results from Repeated Semen Collections. Andrology 2023, 11, 1566–1578. [Google Scholar] [CrossRef]
- Castleton, P.E.; Deluao, J.C.; Sharkey, D.J.; McPherson, N.O. Measuring Reactive Oxygen Species in Semen for Male Preconception Care: A Scientist Perspective. Antioxidants 2022, 11, 264. [Google Scholar] [CrossRef]
| Parameters | Total (n = 543) | Men with BMI < 25.0 (kg/m2) Group 1 (n = 209) | Men with BMI 25.0–29.9 (kg/m2) Group 2 (n = 218) | Men with BMI ≥ 30.0 (kg/m2) Group 3 (n = 116) | p Group 1 vs. 2 | p Group 1 vs. 3 | p Group 2 vs. 3 |
|---|---|---|---|---|---|---|---|
| Age (y) | 33.00 18.00–59.00 | 32.00 18.00–59.00 | 33.00 20.00–56.00 | 34.00 22.00–50.00 | 0.000540 | 0.000081 | NS |
| BMI (kg/m2) | 26.12 18.50–50.25 | 23.30 18.50–24.97 | 26.89 25.00–29.90 | 32.25 30.00–50.25 | <0.000001 | <0.000001 | <0.000001 |
| WHR | n = 529 0.980 0.580–1.660 | n = 204 0.94 0.58–1.42 | n = 209 0.99 0.66–1.66 | 1.01 0.88–1.25 | <0.000001 | <0.000001 | 0.000134 |
| Parameters | Total (n = 543) | Men with BMI < 25.0 (kg/m2) Group 1 (n = 209) | Men with BMI 25.0–29.9 (kg/m2) Group 2 (n = 218) | Men with BMI ≥ 30.0 (kg/m2) Group 3 (n = 116) | p Group 1 vs. 2 | p Group 1 vs. 3 | p Group 2 vs. 3 |
|---|---|---|---|---|---|---|---|
| Semen volume (mL) | 3.00 0.20–9.50 | 3.00 0.20–8.00 | 3.50 0.50–9.50 | 3.00 0.50–8.00 | NS | NS | NS |
| Sperm concentration (×106/mL) | 28.00 0.18–360.00 | 25.67 0.34–360. | 31.21 0.40–210.00 | 27.12 0.18–170.00 | NS | NS | NS |
| Total number of spermatozoa (×106) | 84.38 0.27–840.00 | 75.76 0.50–759.37 | 99.52 1.60–840.00 | 78.81 0.27–829.50 | NS | NS | NS |
| Morphologically normal spermatozoa (%) | 1.000 0.00–12.00 | 2.00 0.00–12.00 | 1.50 0.00–12.00 | 1.00 0.00–9.00 | NS | NS | NS |
| Total sperm head defects (%) | n = 541 97.00 78.00–100.00 | 97.00 78.00–100.00 | n = 216 97.00 81.00–100.00 | 97.00 84.00–100.00 | NS | NS | NS |
| Total sperm midpieces defects (%) | n = 541 37.00 9.00–88.00 | 38.00 9.00–85.00 | n = 216 37.00 9.00–88.00 | 35.50 11.00–78.00 | NS | NS | NS |
| Total sperm tail defects (%) | n = 541 29.00 6.00–100.00 | 28.00 6.00–100.00 | n = 216 29.00 7.00–88.00 | 29.00 12.00–73.00 | NS | NS | NS |
| Immature sperm with excess residual cytoplasm (%) | n = 541 4.00 0.00–41.00 | 3.00 0.00–30.00 | n = 216 4.00 0.00–23.00 | 4.00 0.00–41.00 | NS | NS | NS |
| TZI | 1.71 1.15–2.71 | 1.68 1.15–2.71 | 1.71 1.20–2.46 | 1.69 1.25–2.52 | NS | NS | NS |
| Progressive motility (PR) (%) | 51.00 0.00–94.00 | 51.00 0.00–94.00 | 54.00 0.00–87.00 | 48.50 1.00–85.00 | NS | NS | 0.037064 |
| Nonprogressive (NP) motility (%) | 6.00 0.00–34.00 | 6.00 0.00–33.00 | n = 218 5.00 0.00–32.00 | 7.00 0.00–34.00 | NS | 0.020333 | 0.002989 |
| Total sperm motility (PR + NP) (%) | 59.00 0.00–98.00 | 59.00 0.00–98.00 | 62.00 0.00–95.00 | 116 56.00 5.00–96.00 | NS | NS | NS |
| Eosin-negative spermatozoa—live cells (%) | 76.00 10.00–98.00 | 77.00 32.00–98.00 | 77.00 10.00–95.00 | 74.00 33.00–98.00 | NS | NS | NS |
| HOS test-positive spermatozoa—live cells (%) | n = 486 74.00 0.00–98.00 | n = 181 75.00 11.00–98.00 | n = 202 75.00 0.00–91.00 | n = 103 71.00 34.00–96.00 | NS | NS | NS |
| Peroxidase-positive cells (×106/mL) | 0.25 0.00–33.88 | 0.25 0.00–33.88 | 0.25 0.00–12.50 | 0.25 0.00–10.25 | NS | NS | NS |
| Level of Analysed Biomarkers | Total % (n/N) | Group of men with BMI < 25.0 (kg/m2) % (n/N) | Group of men with BMI 25.0–29.9 (kg/m2) % (n/N) | Group of men with BMI ≥ 30.0 (kg/m2) % (n/N) | p Group 1 vs. 2 | p Group 1 vs. 3 | p Group 2 vs. 3 | OR1 (95% CI) p | OR2 (95% CI) p | OR3 (95% CI) p |
|---|---|---|---|---|---|---|---|---|---|---|
| nsORP > 1.37 (mV/106 sperm cells/mL) | 41.93 (104/248) | 47.87 (45/94) | 35.29 (36/102) | 44.23 (23/52) | NS | NS | NS | 0.5939 0.3348–1.0537 NS | 0.8636 0.4372–1.7057 NS | 1.4540 0.7355–2.8743 NS |
| TAC ≤ 1950 (μM Trolox equivalent) | 48.83 (59/122) | 38.09 (16/42) | 53.57 (30/56) | 41.66 (10/24) | NS | NS | NS | 1.8750 0.8302–4.2345 NS | 1.1607 0.4173–3.2284 NS | 0.6190 0.2355–1.6275 NS |
| HBA index < 80% | 47.00 (55/117 | 46.00 (23/50) | 46.66 (21/45) | 50.00 (11/22) | NS | NS | NS | 1.0272 0.4580–2.3036 NS | 1.1739 0.4302–3.2034 NS | 1.1429 0.4119–3.1710 NS |
| SDF index > 20% | 35.91 (195/543) | 35.88 (75/209) | 30.73 (67/218) | 45.68 (53/116) | NS | NS | 0.0068 | 0.7928 0.5296–1.1866 NS | 1.5031 0.9471–2.3855 NS | 1.8960 1.1909–3.0185 0.0070 |
| Parameters | Total (n = 532) | Men with WHR < 1 (n = 325) | Men with WHR ≥ 1 (n = 207) | p |
|---|---|---|---|---|
| Age (y) | 33.00 18.00–59.00 | 32.00 18.00–50.00 | 34.00 23.00–59.00 | 0.000006 |
| BMI (kg/m2) | n = 529 26.10 18.50–50.25 | n =325 25.00 18.50–50.25 | n = 204 27.90 20.25–48.8890 | < 0.000001 |
| WHR | n = 529 0.980 0.580–1.660 | n = 204 0.94 0.58–1.42 | 1.02 1.00–1.66 | < 0.000001 |
| Parameters | Total (n = 532) | Men with WHR < 1 (n = 325) | Men with WHR ≥ 1 (n = 207) | p |
|---|---|---|---|---|
| Semen volume (mL) | 3.00 0.20–9.50 | 3.00 0.20–9.50 | 3.00 0.50–8.50 | NS |
| Sperm concentration (×106/mL) | 28.065 0.18–360.00 | 28.50 0.34–360.00 | 27.13 0.18–143.50 | NS |
| Total number of spermatozoa (×106) | 83.475 0.27–840.00 | 87.60 0.50–840.00 | 81.25 0.27–615.00 | NS |
| Morphologically normal spermatozoa (%) | 1.50 0.00–12.00 | 2.00 0.00–12.00 | 1.00 0.00–12.00 | 0.000742 |
| Total sperm head defects (%) | n = 531 97.00 78.00–100.00 | 96.00 78.00–100.00 | n = 206 98.00 81.00–100.00 | 0.008209 |
| Total sperm midpieces defects (%) | n = 531 37.00 9.00–88.00 | 38.00 9.00–85.00 | n = 206 36.00 9.00–88.00 | NS |
| Total sperm tail defects (%) | n = 531 28.00 6.00–100.00 | 28.00 6.00–100.00 | n = 206 29.00 6.00–88.00 | NS |
| Immature sperm with excess residual cytoplasm (%) | n = 531 4.00 0.00–41.00 | 3.00 0.00–30.00 | n = 206 4.00 0.00–41.00 | NS |
| TZI | 1.71 1.15–2.71 | 1.71 1.21–2.71 | 1.68 1.15–2.52 | NS |
| Progressive motility (PR) (%) | 51.00 0.00–94.00 | 55.00 0.00–94.00 | 48.00 0.00–85.00 | 0.000037 |
| Nonprogressive (NP) motility (%) | 6.00 0.00–34.00 | 6.00 0.00–34.00 | 5.00 0.00–33.00 | NS |
| Total sperm motility (PR + NP) (%) | 59.00 0.00–98.00 | 63.00 0.00–98.00 | 55.00 0.00–96.00 | 0.000010 |
| Eosin-negative spermatozoa—live cells (%) | 76.00 10.00–98.00 | 78.00 15.00–98.00 | 74.00 10.00–98.00 | 0.000039 |
| HOS test-positive spermatozoa—live cells (%) | n = 476 74.00 0.00–98.00 | n = 292 76.00 0.00–98.00 | n = 184 72.00 11.00–91.00 | 0.002440 |
| Peroxidase-positive cells (×106/mL) | 0.25 0.00–33.88 | 0.25 0.00–12.50 | 0.25 0.00–33.88 | NS |
| Level of Analysed Biomarkers | Total % (n/N) | Group of Men with WHR < 1 % (n/N) | Group of Men with WHR ≥ 1 % (n/N) | p Group 1 vs. 2 | OR1 (95% CI) p |
|---|---|---|---|---|---|
| nsORP > 1.37 (mV/106 sperm cells/mL) | 42.38 (103/243) | 40.25 (62/154) | 46.06 (41/89) | NS | 1.2675 0.7485–2.1463 NS |
| TAC ≤ 1950 (μM Trolox equivalent) | 45.83 (55/120) | 46.47 (33/71) | 44.89 (22/49) | NS | 0.9383 0.4517–1.9490 NS |
| HBA index < 80% | 47.74 (53/111) | 50.87 (29/57) | 44.44 (24/54) | NS | 0.7724 0.3660–1.6301 NS |
| SDF index > 20% | 36.09 (192/532) | 30.76 (100/325) | 44.44 (92/207) | 0.0014 | 1.8000 1.2540–2.5838 0.0014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, K.; Fraczek, M.; Kurpisz, M.; Piasecka, M. Influence of Body Mass Index (BMI) and Waist–Hip Ratio (WHR) on Selected Semen Parameters. Int. J. Mol. Sci. 2025, 26, 4089. https://doi.org/10.3390/ijms26094089
Gill K, Fraczek M, Kurpisz M, Piasecka M. Influence of Body Mass Index (BMI) and Waist–Hip Ratio (WHR) on Selected Semen Parameters. International Journal of Molecular Sciences. 2025; 26(9):4089. https://doi.org/10.3390/ijms26094089
Chicago/Turabian StyleGill, Kamil, Monika Fraczek, Maciej Kurpisz, and Małgorzata Piasecka. 2025. "Influence of Body Mass Index (BMI) and Waist–Hip Ratio (WHR) on Selected Semen Parameters" International Journal of Molecular Sciences 26, no. 9: 4089. https://doi.org/10.3390/ijms26094089
APA StyleGill, K., Fraczek, M., Kurpisz, M., & Piasecka, M. (2025). Influence of Body Mass Index (BMI) and Waist–Hip Ratio (WHR) on Selected Semen Parameters. International Journal of Molecular Sciences, 26(9), 4089. https://doi.org/10.3390/ijms26094089








