Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Selection of Studies
2.4. Risk of Bias in Individual Studies
2.5. Quality Assessment
2.6. Data Extraction
3. Results
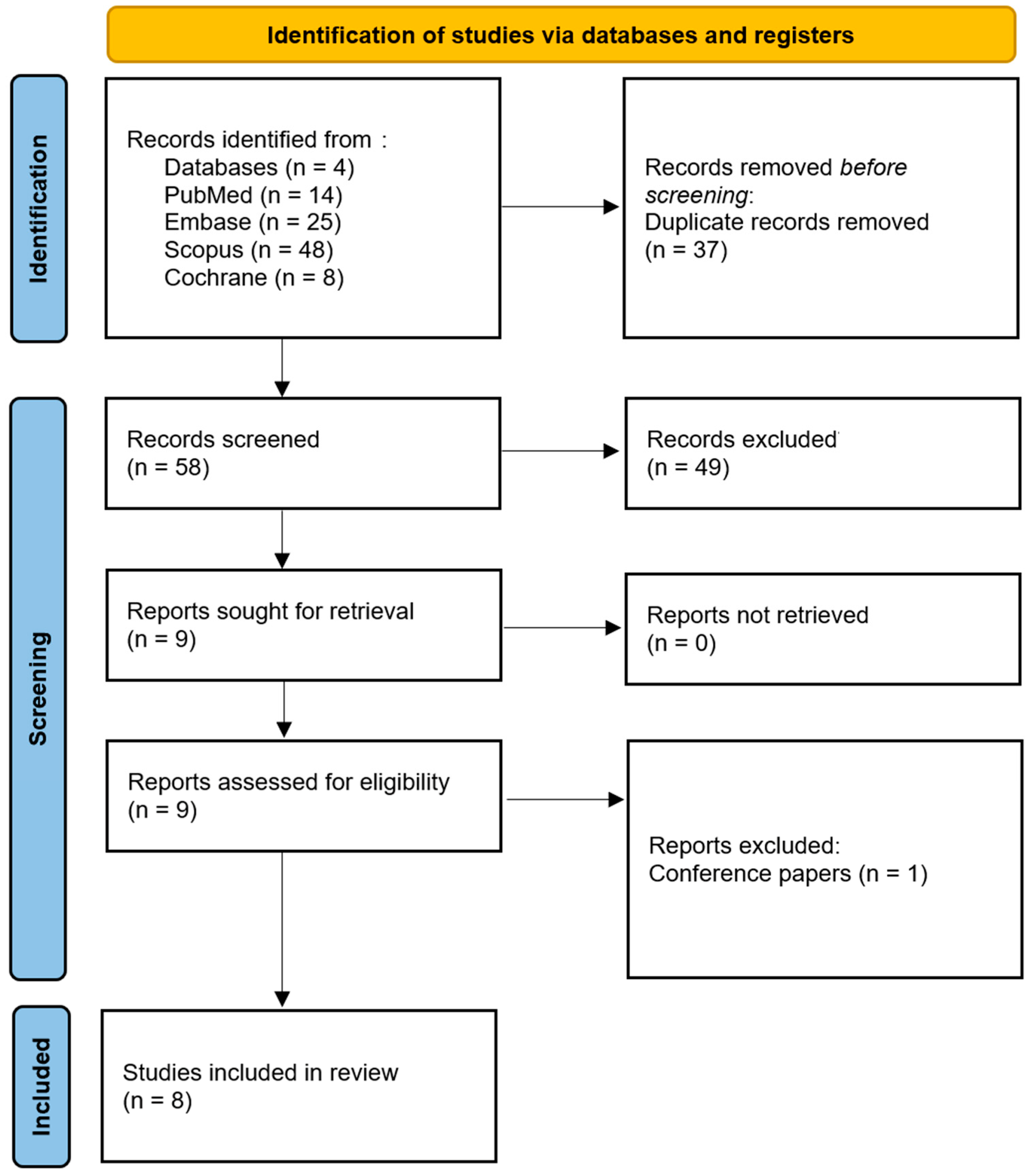
3.1. Study Selection
3.2. Data Presentation
3.3. Main Study Outcomes
4. Discussion
4.1. Results in the Context of Other Evidence
4.2. Limitations of the Evidence
4.3. Limitations of the Review Process
4.4. Implications for Practice, Policy, and Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, 104550. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Zeng, X.; Hu, X.; Hua, H. The global prevalence of oral leukoplakia: A systematic review and meta-analysis from 1996 to 2022. BMC Oral Health 2023, 23, 645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- IARC. Oral cancer prevention. IARC Handb. Cancer Prev. 2023, 19, 1–358. Available online: https://publications.iarc.fr/ (accessed on 20 December 2024).
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Saman Warnakulasuriya, J.S.G. Epidemiology of Oral and Oropharyngeal Cancers. In Book: Textbook of Oral Cancer; Springer: Cham, Switzerland, 2020; pp. 5–21. [Google Scholar] [CrossRef]
- Kalavrezos, N.; Scully, C. Mouth cancer for clinicians part 14: Cancer prevention. Dent. Update 2016, 43, 772–784. [Google Scholar] [CrossRef]
- Lodi, G.; Carrozzo, M.; Furness, S.; Thongprasom, K. Interventions for treating oral lichen planus: A systematic review. Br. J. Dermatol. 2012, 166, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talreja, L.; Goyal, R.; Yadav, D.; Singh, N.; Kalita, S.; Jaiswal, S.B. Evaluation of Clinical Outcomes and Recurrence After Surgical Excision of Oral Leukoplakia: A Prospective Cohort Study. Cureus 2024, 16, e71593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thongprasom, K.; Dhanuthai, K. Steriods in the treatment of lichen planus: A review. J. Oral Sci. 2008, 50, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Glatstein, E. Clinical applications of photodynamic therapy. Ann. Med. 1994, 26, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Jerjes, W.; Hamdoon, Z.; Hopper, C. Photodynamic therapy in the management of potentially malignant and malignant oral disorders. Head Neck Oncol. 2012, 4, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and Hypericin-Mediated Antimicrobial Photodynamic Therapy as Alternative Treatments for Oral Candidiasis: A Systematic Review. Pharmaceutics 2024, 17, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Zięba, N.; Turski, R.; Misiołek, M.; Wiench, R. Hypericin-Mediated Photodynamic Therapy for Head and Neck Cancers: A Systematic Review. Biomedicines 2025, 13, 181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Łopaciński, M.; Los, A.; Skaba, D.; Wiench, R. Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes. Pharmaceutics 2025, 17, 217. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The use of photodynamic therapy in medical practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waingade, M.; Medikeri, R.S.; Rathod, P. Effectiveness of methylene blue photosensitizers compared to that of corticosteroids in the management of oral lichen planus: A systematic review and meta-analysis. J. Dent. Anesth Pain Med. 2022, 22, 175–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhanya, M.; Umamaheswari, T.N.; Eeswaramoorthy, R.; Eswaramoorthy, R. In Vitro Evaluation of Light-Induced Cytotoxic Property: Synergistic Effects of Anthocyanin/Curcumin as a Photosensitizer. Cureus 2023, 15, e48537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibbotson, S.H.; Wong, T.H.; Morton, C.A.; Collier, N.J.; Haylett, A.; McKenna, K.E.; Mallipeddi, R.; Moseley, H.; Rhodes, L.E.; Seukeran, D.C.; et al. Adverse effects of topical photodynamic therapy: A consensus review and approach to management. Br. J. Dermatol. 2019, 180, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M. Welch Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; Cochrane: London, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 15 January 2025).
- Jajarm, H.H.; Falaki, F.; Sanatkhani, M.; Ahmadzadeh, M.; Ahrari, F.; Shafaee, H. A comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: A randomized clinical controlled trial. Lasers Med. Sci. 2015, 30, 1475–1480. [Google Scholar] [CrossRef]
- Lavaee, F.; Shadmanpour, M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019, 25, 1954–1963. [Google Scholar] [CrossRef]
- Mirza, S.; Rehman, N.; Alrahlah, A.; Alamri, W.R.; Vohra, F. Efficacy of photodynamic therapy or low level laser therapy against steroid therapy in the treatment of erosive-atrophic oral lichen planus. Photodiagnosis Photodyn. Ther. 2018, 21, 404–408. [Google Scholar] [CrossRef]
- Romano, A.; Contaldo, M.; Della Vella, F.; Russo, D.; Lajolo, C.; Serpico, R.; Di Stasio, D. Topical toluidine blue-mediated photodynamic therapy for the treatment of oral lichen planus. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. 1), 27–33. [Google Scholar]
- Muhaxheri, G.; Boras, V.V.; Gabrić, D.; Dabić, D.T.; Kvesić, A.J. No Efficacy of Photodynamic Therapy with Toluidine Blue in Oral Lichen Planus. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 132. [Google Scholar]
- Pietruska, M.; Sobaniec, S.; Bernaczyk, P.; Cholewa, M.; Pietruski, J.K.; Dolińska, E.; Skurska, A.; Duraj, E.; Tokajuk, G. Clinical evaluation of photodynamic therapy efficacy in the treatment of oral leukoplakia. Photodiagnosis Photodyn. Ther. 2014, 11, 34–40. [Google Scholar] [CrossRef]
- Sobaniec, S.; Bernaczyk, P.; Pietruski, J.; Cholewa, M.; Skurska, A.; Dolińska, E.; Duraj, E.; Tokajuk, G.; Paniczko, A.; Olszewska, E.; et al. Clinical assessment of the efficacy of photodynamic therapy in the treatment of oral lichen planus. Lasers Med. Sci. 2013, 28, 311–316. [Google Scholar] [CrossRef]
- Istomin, Y.P.; Artemyeva, T.P.; Tzerkovsky, D.A. Photodynamic therapy with photosensitizer photolon for oral leukoplakia. Biomed. Photonics 2016, 5, 13–20. [Google Scholar] [CrossRef]
- Zhukavets, A.; Istomin, Y.; Belatsarkouski, I.; Trizna, N.; Artemieva, T.; Tzerkovsky, D. Photodynamic therapy of the oral leukoplakia. In Proceedings of the 5th World Congress of the International Academy of Oral Oncology (IAOO), Sao Paolo, Brazil, 8–11 July 2015; Volume: Poster PP102. [Google Scholar]
- Gulzar, M.A.; Gul, N.; Alvi, F.D.; Khattak, Y.R.; Hasan, U.S.; Haneef, M.B.; Ahmad, I. Comparison of photodynamic therapy and corticosteroid therapy in management of oral lichen planus: A systematic review of randomized controlled trials. Photodiagnosis Photodyn. Ther. 2023, 44, 103747. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, A.; Arduino, P.G.; Massolini, G.; Romagnoli, E.; Broccoletti, R. Clinical evaluation of the efficiency of low-level laser therapy for oral lichen planus: A prospective case series. Lasers Med. Sci. 2014, 29, 185–190. [Google Scholar] [CrossRef][Green Version]
- Jajarm, H.H.; Falaki, F.; Mahdavi, O. A comparative pilot study of low intensity laser versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus. Photomed. Laser Surg. 2011, 29, 421–425. [Google Scholar] [CrossRef]
- Kazancioglu, H.O.; Erisen, M. Comparison of Low-level laser therapy versus ozone therapy in the treatment of Oral Lichen planus. Ann. Dermatol. 2015, 27, 485–491. [Google Scholar] [CrossRef]
- Dillenburg, C.S.; Martins, M.A.; Munerato, M.C.; Marques, M.M.; Carrard, V.C.; Filho, M.S.; Castilho, R.M.; Martins, M.D. Efficacy of laser phototherapy in comparison to topical clobetasol for the treatment of oral lichen planus: A randomized controlled trial. J. Biomed. Opt. 2014, 19, 068002. [Google Scholar] [CrossRef]
- Hoseinpour Jajarm, H.; Asadi, R.; Bardideh, E.; Shafaee, H.; Khazaei, Y.; Emadzadeh, M. The effects of photodynamic and low-level laser therapy for treatment of oral lichen planus-A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2018, 23, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, N.H.; Clint, J.B.; Reddy, S.S.; Nagi, R.; Chauhan, P.; Sharma, S.; Sharma, P.; Kaur, A.; Shetty, B.; Ashwini, S.; et al. Clinical evaluation of photodynamic therapy for the treatment of refractory oral Lichen planus-A case series. Photodiagnosis Photodyn. Ther. 2018, 24, 280–285. [Google Scholar] [CrossRef]
- Peralta-Mamani, M.; Silva, B.M.D.; Honório, H.M.; Rubira-Bullen, I.R.F.; Hanna, R.; Silva, P.S.S.D. Clinical efficacy of photodynamic therapy in management of oral potentially malignant disorders: A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2024, 24, 101899. [Google Scholar] [CrossRef] [PubMed]
- Jerjes, W.; Upile, T.; Hamdoon, Z.; Mosse, C.A.; Akram, S.; Hopper, C. Photodynamic therapy outcome for oral dysplasia. Lasers Surg. Med. 2011, 43, 192–199. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Wang, K.; Zhao, Y.; Xu, J.; Fan, Y. Clinical evaluation of photodynamic therapy for oral leukoplakia: A retrospective study of 50 patients. BMC Oral Health 2024, 24, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akram, Z.; Javed, F.; Hosein, M.; Al-Qahtani, M.A.; Alshehri, F.; Alzahrani, A.I.; Vohra, F. Photodynamic therapy in the treatment of symptomatic oral lichen planus: A systematic review. Photodermatol. Photoimmunol. Photomed. 2018, 34, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Maweri, S.A.; Ashraf, S.; Kalakonda, B.; Halboub, E.; Petro, W.; AlAizari, N.A. Efficacy of photodynamic therapy in the treatment of symptomatic oral lichen planus: A systematic review. J. Oral Pathol. Med. 2018, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Reddy, S.S.; Nagi, R.; Nagaraju, R.; Kunjumon, S.P.; Sen, R. The Effect of Photodynamic Therapy on Oral-Premalignant Lesions: A Systematic Review. J. Clin. Exp. Dent. 2022, 14, e285–e292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vohra, F.; Al-Kheraif, A.A.; Qadri, T.; Hassan, M.I.; Ahmed, A.; Warnakulasuriya, S.; Javed, F. Efficacy of photodynamic therapy in the management of oral premalignant lesions. A systematic review. Photodiagnosis Photodyn. Ther. 2015, 12, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.R.; Lerman, M.A.; Patel, N.; Grecco, C.; Costa, C.A.; Amiji, M.M.; Bagnato, V.S.; Soukos, N.S. Safety assessment of oral photodynamic therapy in rats. Lasers Med. Sci. 2013, 28, 479–486. [Google Scholar] [CrossRef] [PubMed]
| Source | Search Term | Number of Results |
|---|---|---|
| PubMed | ((“Toluidine Blue” OR “Chlorin-e6” OR “Chlorine-e6” OR “dimethyl sulfoxide” OR “Photolon”) AND ((“Photodynamic Therapy”) OR “PDT” OR “Photochemotherapy” OR “aPDT” OR “Antimicrobial Photodynamic Therapy”) AND ((“Lichen Planus, Oral”) OR “Oral Lichen Planus” OR “ORL” OR “Leukoplakia, Oral” OR “Oral Leukoplakia” OR “Erythroplakia” OR “Erythroleukoplakia” OR “Erythroplasia” OR “Proliferative verrucous leukoplakia” OR “Oral verrucous leukoplakia” OR “Oral Submucous Fibrosis” OR “Smokers palate” OR “Reverse smoking” OR “Oral lupus erythematosus” OR “Discoid Lupus Erythematosus” OR “Actinic Cheilitis” OR “Actinic Keratosis” OR “dyskeratosis congenita” OR “Oral Graft versus Host Disease” OR “OGVHD” OR “Oral GVHD” OR “Oral Lichenoid Lesions” OR ”Oral potentially malignant disorders” OR “OPMD” OR “Oral Premalignant Lesions” OR “Oral potentially malignant lesions” OR “Oral precancerous lesions” OR “PMD”)) | 14 |
| Embase | (‘Toluidine Blue’ OR ‘Chlorin-e6′ OR ‘Chlorine-e6′ OR ‘dimethyl sulfoxide’ OR ‘Photolon’ AND ((‘Photodynamic Therapy’ OR ‘PDT’ OR ‘photochemotherapy’ OR ‘aPDT’ OR ‘Antimicrobial Photodynamic Therapy’):ti,ab) AND ((‘Lichen Planus, Oral’ OR ‘Oral Lichen Planus’ OR ‘ORL’ OR ‘Leukoplakia, Oral’ OR ‘Oral Leukoplakia’ OR ‘Erythroplakia’ OR ‘Erythroleukoplakia’ OR ‘Erythroplasia’ OR ‘Proliferative verrucous leukoplakia’ OR ‘Oral verrucous leukoplakia’ OR ‘Oral Submucous Fibrosis’ OR ‘Smokers palate’ OR ‘Reverse smoking’ OR ‘Oral lupus erythematosus’ OR ‘Discoid Lupus Erythematosus’ OR ‘Actinic Cheilitis’ OR ‘Actinic Keratosis’ OR ‘dyskeratosis congenita’ OR ‘Oral Graft versus Host Disease’ OR ‘OGVHD’ OR ‘Oral GVHD’ OR ‘Oral Lichenoid Lesions’ OR ‘Oral potentially malignant disorders’ OR ‘OPMD’ OR ‘Oral Premalignant Lesions’ OR ‘Oral potentially malignant lesions’ OR ‘Oral precancerous lesions’ OR ‘PMD’)):ti,ab) | 25 |
| Scopus | TITLE-ABS-KEY ((“Toluidine Blue” OR “Chlorin-e6” OR “Chlorine-e6” OR “dimethyl sulfoxide” OR “Photolon”) AND (“Photodynamic Therapy” OR “PDT” OR “Photochemotherapy” OR “aPDT” OR “acrobial Photodynamic Therapy”) AND (“Lichen Planus, Oral” OR “Oral Lichen Planus” OR “ORL” OR “Leukoplakia, Oral” OR “Oral Leukoplakia” OR “Erythroplakia” OR “Erythroleukoplakia” OR “Erythroplasia” OR “Proliferative verrucous leukoplakia” OR “Oral verrucous leukoplakia” OR “Oral Submucous Fibrosis” OR “Smokers palate” OR “Reverse smoking” OR “Oral lupus erythematosus” OR “Discoid Lupus Erythematosus” OR “Actinic Cheilitis” OR “Actinic Keratosis” OR “dyskeratosis congenita” OR “Oral Graft versus Host Disease” OR “OGVHD” OR “Oral GVHD” OR “Oral Lichenoid Lesions” OR “Oral potentially malignant disorders” OR “OPMD” OR “Oral Premalignant Lesions” OR “Oral potentially malignant lesions” OR “Oral precancerous lesions” OR “PMD”)) | 48 |
| Cochrane | ((“toluidine blue” OR chlorine-e6 OR chlorin-e6 OR “dimethyl-sulfoxide” OR photolon):ti,ab,kw) and ((“photodynamic therapy” OR “PDT” OR “photochemotherapy” OR “antimicrobial photodynamic therapy” OR “aPDT”):ti,ab,kw) AND ((“oral lichen planus” OR “oral leukoplakia” OR “oral erythroplakia” OR “oral erythroplasia” OR “erythroleukoplakia” OR “proliferative verrucous leukoplakia” OR “oral verrucous leukoplakia” OR “oral submucous fibrosis” OR “smokers palate” OR “reverse smoking” OR “oral lupus erythematosus” OR “discoid lupus erythematosus” OR “actinic cheilitis” OR “actinic keratosis” OR “dyskeratosis congenita” OR “oral graft versus host disease” OR “OGVHD” OR “Oral GVHD” OR “oral lichenoid lesion” OR “oral potentially malignant disorders” OR “OPMD” OR “oral premalignant lesions” OR “oral potentially malignant lesions” OR “oral precancerous lesions” OR “PMD”):ti,ab,kw) | 8 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Study | ||||||||
|---|---|---|---|---|---|---|---|---|
| Evaluation Criteria | Jajarm et al. (2015) [26] | Lavaee et al. (2019) [27] | Mirza et al. (2018) [28] | Romano, Contaldo et al. (2019) [29] | Muhaxheri et al. (2017) [30] | Pietruska et al. (2014) [31] | Sobaniec et al. (2013) [32] | Istomin et al. (2016) [33] |
| 1. Concentration of the photosensitizer mentioned/provided by stating the photosensitizer trade name | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 2. The form of photosensitizer administration as well as the incubation time mentioned | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. All significant parameters of the light source such as type of laser, wavelength, energy fluence, and power density mentioned | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 4. Information about the frequency of the irradiation as well as the number of sessions included | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 5. Adequate OPMD diagnosis histopathologically confirmed | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 6. Site of the OMPD detailed | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7. Inclusion/exclusion criteria defined | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 |
| 8. No missing outcome data | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 9. Statistical analysis implied | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Total score | 9 | 9 | 9 | 5 | 4 | 8 | 7 | 7 |
| Risk of Bias | Low | Low | Low | Moderate | Moderate | Low | Low | Low |
| First Author(s) and Reference | Year | Country | Model of the Study | Photosensitizer | Type of OPMD |
|---|---|---|---|---|---|
| Jajarm et al. [26] | 2015 | Iran | in vivo | toluidine blue | oral lichen planus |
| Lavaee et al. [27] | 2019 | Iran | in vivo | toluidine blue | oral lichen planus |
| Mirza et al. [28] | 2018 | Pakistan | in vivo | toluidine blue | oral lichen planus |
| Romano, Contaldo et al. [29] | 2019 | Italy | in vivo | toluidine blue | oral lichen planus |
| Muhaxheri et al. [30] | 2017 | Croatia | in vivo | toluidine blue | oral lichen planus |
| Pietruska et al. [31] | 2014 | Poland | in vivo | chlorin-e6/Photolon® | oral leukoplakia |
| Sobaniec et al. [32] | 2013 | Poland | in vivo | chlorin-e6/Photolon® | oral lichen planus |
| Istomin et al. [33] | 2016 | Belarus | in vivo | chlorin-e6/photolon | oral leukoplakia |
| Study and Reference | Type of the Laser | Wavelength [nm] | Energy Fluence [J/cm2] | Power Density [mW/cm2] /Power [mW] | Irradiation Time [min] | Frequency | Number of Sessions | Interval Time | Spot Surface [cm2]/Diameter [cm]/Tip |
|---|---|---|---|---|---|---|---|---|---|
| Jajarm et al. [26] | GaAlAs diode laser (Mustang 2000, Russia) | 630 | 1.5 | 10 mW/cm2 | 2.5 | continuous | - | 2 × weekly for 1 month | 1 cm2 |
| Lavaee et al. [27] | InGaAlP diode laser (Azor-2k, Russia) | 660 | 19.23 | 25 mW | 10 | spot | 3 | 1 week | 0.78 cm2 |
| Mirza et al. [28] | GaAlAs diode laser | 630 | 1.5 | 10 mW/cm2 | 2.5 | continuous | - | 2 × weekly for 1 month | 1 cm2 |
| Romano, Contaldo et al. [29] | FotoSan® | 630 | - | - | 2.5 | - | 2–5 | 14 days minimum | - |
| Muhaxheri et al. [30] | GaAlAs diode laser | 685 | 2.00 | 30 mW | - | continuous | 6 | 2–3 days (irradiation on days: 1, 3, 5, 8, 10, 12) | 1 cm2 |
| Pietruska et al. [31] | semiconductor laser (Haemato, Poland) | 660 | 90 | 300 mW | - | - | 10 | 2 weeks | diffuser tip |
| Sobaniec et al. [32] | semiconductor laser (Haemato Poland) | 660 | 90 | 300 mW | - | - | 10 | 2 weeks | diffuser tip |
| Istomin et al. [33] | semiconductor laser UPL PDT (LEMT, Belarus) | 660 | 25–100 | 0.07–0.32 W/cm2 | 2.5–13 | - | 1–3 | - | 1 cm |
| Study and Reference | Photosensitizer | Administration | Concentration/Dose | Application Protocol | Incubation Time [min] | Trade Name of the PS |
|---|---|---|---|---|---|---|
| Jajarm et al. [26] | TB | topical | 1 mg/mL | micropipette (50 μL in total) | 10 | - |
| Lavaee et al. [27] | TB | topical | 1 mg/mL | sterile swab | 10 | - |
| Mirza et al. [28] | TB | topical | 1 mg/mL | micropipette (50 μL in total) | 10 | - |
| Romano, Contaldo et al. [29] | TB | topical | - | 2% AA (1 min) → drying with gauze → TB → 1% AA (1 min) | - | - |
| Muhaxheri et al. [30] | TB | topical | - | cotton stick | 10 | - |
| Pietruska et al. [31] | C-e6 | topical | 20% C-e6, 10% DS | occlusive dressing on a dried mucosa: C-e6 on a nonwoven fabric, covered with a polyethylene sheet, and a sterile gauze | 60 | Photolon® (Haemato, Poland) |
| Sobaniec et al. [32] | C-e6 | topical | 20% C-e6, 10% DS | occlusive dressing on a dried mucosa: C-e6 on a nonwoven fabric, covered with a polyethylene sheet, and a sterile gauze | 60 | Photolon® (Haemato, Poland) |
| Istomin et al. [33] | C-e6 | i.v. | 1.7–2.5 mg/kg | intravenously in the darkened room | 150–180 | photolon |
| Study and Reference | Patients | M:F | Age/Mean Age + SD [yr] | Site of the Lesions | Type of the Lesions (Number of Affected Patients) | Study Design/Treatment Protocol | Results |
|---|---|---|---|---|---|---|---|
| Jajarm et al. [26] | 25 randomly allocated: 11 experimental group, 14 control group | 8:17 3:8 5:9 | - | T or BM | OLP; atrophic-erosive | RCT; comparing the effect of TB-PDT with topical CS in the treatment of OLP; experimental group: TB-PDT for 1 month; control group: topical corticosteroids (dexamethasone, 0.5 mg/5 mL water, in mouthwash), four times a day for 1 month. Sign scores were assessed using the Thongprasom sign scoring. Improvement of the lesions was measured by applying efficacy indices (EI). Evaluation of experienced pain was assessed using the 1–10 VAS scale. During the treatment, effects were evaluated weekly, and after completion of the treatment, a 2-, 3-, and 4-week follow-up was included. | The study demonstrated that TB-PDT was effective in the management of OLP. Sign scores of changes significantly decreased after the treatment in both groups: experimental (p = 0.021), control (p = 0.002); however, there was no significant difference (p= 0.72) between the groups. The intensity of lesions significantly reduced after treatment in both groups, experimental (p = 0.005), and control (p= 0.001), and a significant difference between the groups was observed (p = 0.001). The mean amount of improvement in pain was significantly higher in the control group (p < 0.001) (α = 0.05). In the follow-up statistically significant difference was observed (p = 0.042), as a relapse did not show in 100% of patients in the control group, compared to 72.7% of patients in the experimental group. |
| Lavaee et al. [27] | 11 (22 sides) randomly, double- blind allocated: intervention side (11), control side (11) | 2:9 | - | bilateral OM | OLP; symptomatic atrophic/ erosive/ulcerative | BRCT; comparing the effect of TB-PDT with topical CS in the treatment of OLP; intervention side: all patients underwent TB-PDT in 3 sessions in one-week intervals—session 0, 1, and 2, respectively; alongside sham laser irradiation for the control side. On the third-week follow-up (session 3), an effect of the TB-PDT was evaluated and topical CS (triamcinolone acetonide 0.1%) was prescribed for 4 weeks in all patients. On the seventh week, follow-up (session 4) outcome assessment was held. Sign scores were assessed using the Thongprasom sign scoring (TH); the symptoms (pain) were evaluated with the 1–10 VAS scale. Moreover, clinical severity index (SI) and efficacy index (EI) were determined. | The major conclusion was that TB-PDT can be used as an alternative therapy besides standard methods, and can be considered as a new approach for refractory OLP. In the final assessment, the results of 8 patients were evaluated (3 patients were excluded). For the intervention side, statistically significant improvement between sessions 0 and 4 in all scores (VAS, TH, SI, and EI) was observed (p value < 0.05). Except for the TH score in the control side (p value = 0.056), differences between the changes in all indices were statistically significant between session 0 and 4 (p value < 0.05). |
| Mirza et al. [28] | 45 randomly allocated: 15-PDT group, 15-LLLT group, 15-GS group | 8:37 3:12 1:14 4:11 | 52.6 ± 11.4 50.8 ± 14.7 49.2 ± 10.6 | T or BM | OLP; atrophic-erosive | RCT; comparing the effects of TB-PDT, LLLT, or topical GS in the treatment of OLP; group 1: TB-PDT in two sessions, two times weekly for 1 month; group 2: LLLT in maximum of 10 sessions, two times weekly (three-day intervals), group 3: topical CS (dexamethasone, 0.5 mg/5 mL water) four times a day for 1 month. Sign scores were evaluated using the Thongprasom sign scoring; moreover, an efficacy index (EI) was determined. 1–10 VAS scale was applied to evaluate the symptoms (pain). | The study proved that TB-PDT and LLLT were effective in the management of erosive-atrophic OLP. A significant difference in sign score change before and after the treatment in TB-PDT group (p = 0.03), LLLT group (p = 0.04), and CS group (p = 0.02) was observed. Moreover, statistically significant difference was noted between TB-PDT group (p = 0.001) and LLLT group (p = 0.001) before and after the treatment. This tendency did not show in CS group. Mean improvement in pain was significantly higher in the GS group (p < 0.001). The EI improvement in the TB-PDT group was significantly greater compared to LLLT and GS groups. |
| Romano, Contaldo et al. [29] | 5 | 2:3 | 62–71 | upper adherent G | OLP; multifocal, homogenous | Study assessed the feasibility of TB-PDT in the treatment of OLP. All patients were treated with TB-PDT. The efficacy was assessed using the authors’ own method, which included measuring the lesion (in mm) on each session. | Outcomes of the study indicate that TB-PDT is a valuable treatment option for OLP. Complete response (total disappearance of clinically visible lesions) was noted in four patients. One patient presented with a partial response—54% reduction in size (T0 = 22 mm; Tf = 12 mm). |
| Muhaxheri et al. [30] | 5 | 1:4 | 50–80 | AR (2); G (1); BM + AR (1); T (1) | OLP; refractory | The study aimed to evaluate the efficacy of TB-PDT for the treatment of refractory OPL. All patients were treated with TB-mediated PDT. | The study found that PDT with toluidine blue was not effective in five patients with refractory OLP. |
| Pietruska et al. [31] | 23 (44 lesions) | 7:16 | 21–79 | BM + L (38); G + T (6) | OL; homogenous flat | The study evaluated the efficacy of C-e6-PDT in the treatment of OL. All patients were treated with C-e6-PDT. Efficacy was assessed by measuring the lesion (in mm) at 1, 2, 5, and 10 sessions. To evaluate the efficacy, the authors’ own method was applied, in which lesions were divided into 5 groups. Additionally, gender- and age-related data, and smoking as a factor were analyzed. | Researchers proved that C-e6-PDT results in a significant size reduction in OL lesions. A size reduction was noted in 34 of 44 sites; 12 sites presented with a complete regression. The mean size reduction of OL lesions was statistically significant (by 53.8%). In all groups (M, F, smokers, and nonsmokers) a statistically significant reduction in the lesion size on the buccal and lip mucosa was observed. The differences between age groups were insignificant. |
| Sobaniec et al. [32] | 23 (48 lesions) | 6:17 | 31–82 | BM + L (40); G + T (8) | OLP | The study assessed the efficacy of C-e6-PDT in the treatment of OLP. All patients were treated with C-e6-PDT. Efficacy was assessed by measuring the lesion (in mm) at 1, 2, 5, and 10 sessions. The efficacy was evaluated according to the authors’ own method, in which lesions were classified into 5 size groups. Additionally, gender- and age-related data, and smoking as a factor were analyzed. | C-e6-PDT turned out to be an efficient and non-invasive treatment for OLP. The size reduction was noted in 39 of 48 sites, including 14 with a complete regression. The mean size reduction in OLP lesions was statistically significant (by 55%). The greatest, statistically significant effects were noted for the lesions on the buccal and lip mucosa (reduction by 57.6%) for all the groups (M, F, smokers, nonsmokers). C-e6-PDT was statistically significantly less effective for the lesions on the gingiva and tongue (30.0% reduction). Regarding age, the best effects were observed for the lesions on the buccal and lip mucosa in patients over 75 years (by 66.9%). |
| Istomin et al. [33] | 40 (109 lesions) | 7:33 | 55 ± 14 | BM (17); T (15); G (5); OF (3) | OL; flat (38), verrucous (2) | The study evaluated the tolerability, safety, and immediate results of photon-mediated PDT for the treatment of OL. All patients underwent photolon-mediated PDT, which was administered intravenously; 15–20 min before the treatment, local anesthesia was carried out. Additionally, fluorescence spectrophotometry was used to plan, monitor, and optimize the PDT. After completion of the PDT, a 7-day, and 1-, 3-, and 6-month follow-up was included. The evaluation of immediate results was carried out according to the WHO criteria: complete regression, partial regression (by 50% in size or more), and no effect (less than 50% or no reduction in size). The final evaluation was held 1–2 months after completion of the PDT. | Researchers proved that PDT may be considered an effective and well-tolerated option for the treatment of circumscribed or widespread OL. On the 1–2-month follow-up, 38 of 40 patients (95%) presented with a complete regression. In 2 cases, all with verrucous type, partial regression was observed. There were no complications regarding the photolon administration. During the PDT, patients reported local pain sensation, which was well managed with local anaesthesia (ketorolac, i.m. or 2% lidocaine, topically). On days 2–6 after the completion of PDT, hemorrhagic necrosis was observed in the area of treated lesions. All the affected spots presented with full epithelialization after 3–6 weeks. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. https://doi.org/10.3390/ijms26062528
Kruczek-Kazibudzka A, Lipka B, Fiegler-Rudol J, Tkaczyk M, Skaba D, Wiench R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(6):2528. https://doi.org/10.3390/ijms26062528
Chicago/Turabian StyleKruczek-Kazibudzka, Anna, Barbara Lipka, Jakub Fiegler-Rudol, Marcin Tkaczyk, Dariusz Skaba, and Rafał Wiench. 2025. "Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review" International Journal of Molecular Sciences 26, no. 6: 2528. https://doi.org/10.3390/ijms26062528
APA StyleKruczek-Kazibudzka, A., Lipka, B., Fiegler-Rudol, J., Tkaczyk, M., Skaba, D., & Wiench, R. (2025). Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. International Journal of Molecular Sciences, 26(6), 2528. https://doi.org/10.3390/ijms26062528






