Toll-like Receptor Type 2 and 13 Gene Expression and Immune Cell Profiles in Diploid and Triploid Sterlets (Acipenser ruthenus): Insights into Immune Competence in Polyploid Fish
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
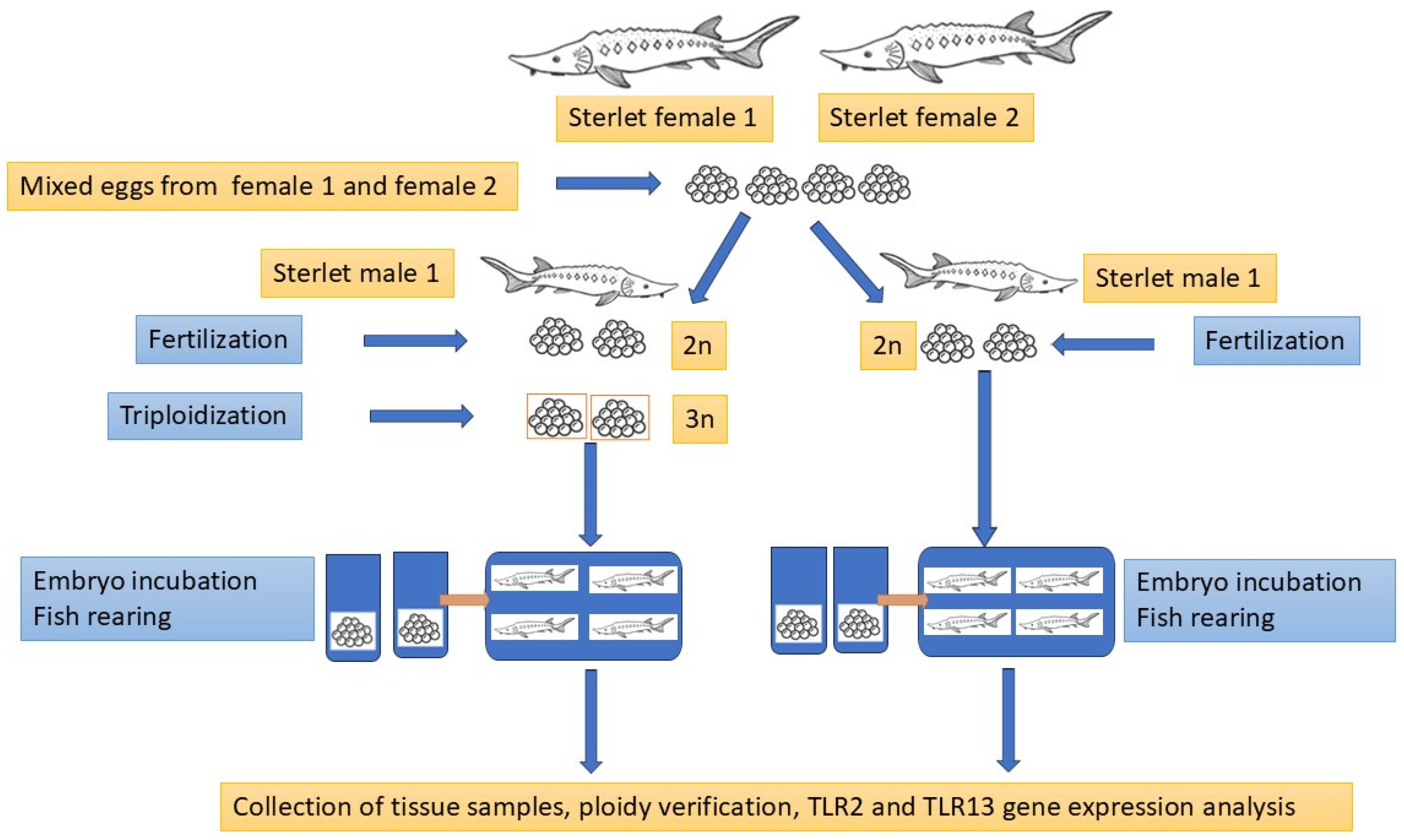
4.1. Fish and Tissue Collection
4.2. Ploidy Verification
4.3. Total RNA Extraction and cDNA Synthesis
4.4. Real-Time PCR (qPCR)
4.5. Blood Smears
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish Shellfish. Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B.M. Innate immunity of fish (overview). Fish Shellfish. Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.O. Fishing for mammalian paradigms in the teleost immune system. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like receptors and the control of immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like receptor recognition of bacteria in fish: Ligand specificity and signal pathways. Fish Shellfish. Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef]
- Mahapatra, S.; Ganguly, B.; Pani, S.; Saha, A.; Samanta, M. A comprehensive review on the dynamic role of toll-like receptors (TLRs) in frontier aquaculture research and as a promising avenue for fish disease management. Int. J. Biol. Macromol. 2023, 253, 126541. [Google Scholar] [CrossRef]
- Palti, Y. Toll-like receptors in bony fish: From genomics to function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar] [CrossRef]
- Ozinsky, A.; Underhill, D.M.; Fontenot, J.D.; Hajjar, A.M.; Smith, K.D.; Wilson, C.B.; Schroeder, L.; Aderem, A. The Repertoire for Pattern Recognition of Pathogens by the Innate Immune System Is Defined by Cooperation between Toll-like Receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 13766–13771. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Zhou, Y.; Fang, H.; Lin, S.; Wang, P.-F.; Xiong, R.-P.; Chen, J.; Xiong, X.-Y.; Lv, F.-L.; Liang, Q.-L.; et al. Toll-like Receptor 2/4 Heterodimer Mediates Inflammatory Injury in Intracerebral Hemorrhage. Ann. Neurol. 2014, 75, 876–889. [Google Scholar] [CrossRef]
- Su, S.-B.; Tao, L.; Deng, Z.-P.; Chen, W.; Qin, S.-Y.; Jiang, H.-X. TLR10: Insights, Controversies and Potential Utility as a Therapeutic Target. Scand. J. Immunol. 2021, 93, e12988. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Fumia, A.; D’Angelo, R.; Mangano, A.; Lombardo, G.P.; Giliberti, A.; Messina, E.; Alesci, A.; Lauriano, E.R. Expression and function of toll-like receptor 2 in vertebrate. Acta Histochem. 2023, 125, 152028. [Google Scholar] [CrossRef] [PubMed]
- Colleselli, K.; Stierschneider, A.; Wiesner, C. An Update on Toll-like Receptor 2, Its Function and Dimerization in Pro- and Anti-Inflammatory Processes. Int. J. Mol. Sci. 2023, 24, 12464. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Hirono, I.; Takami, M.; Miyata, M.; Miyazaki, T.; Han, H.-J.; Takano, T.; Endo, M.; Aoki, T. Characterization of gene structure and expression of two toll-like receptors from Japanese flounder, Paralichthys olivaceus. Immunogenetics 2004, 56, 38–46. [Google Scholar] [CrossRef]
- Jault, C.; Pichon, L.; Chluba, J. Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol. Immunol. 2004, 40, 759–771. [Google Scholar] [CrossRef]
- Baoprasertkul, P.; Peatman, E.; Abernathy, J.; Liu, Z. Structural characterization and expression analysis of Toll-like receptor 2 gene from catfish. Fish Shellfish. Immunol. 2007, 22, 418–426. [Google Scholar] [CrossRef]
- Wei, Y.C.; Pan, T.S.; Chang, M.X.; Huang, B.; Xu, Z.; Luo, T.R.; Nie, P. Cloning and expression of Toll-like receptors 1 and 2 from a teleost fish, the orange-spotted grouper Epinephelus coioides. Vet. Immunol. Immunopathol. 2011, 141, 173–182. [Google Scholar] [CrossRef]
- Samanta, M.; Swain, B.; Basu, M.; Panda, P.; Mohapatra, G.B.; Sahoo, B.R.; Maiti, N.K. Molecular characterization of toll-like receptor 2 (TLR2), analysis of its inductive expression and associated down-stream signaling molecules following ligands exposure and bacterial infection in the Indian major carp, rohu (Labeo rohita). Fish Shellfish. Immunol. 2012, 32, 411–425. [Google Scholar] [CrossRef]
- Fan, Z.-J.; Jia, Q.-J.; Yao, C.-L. Characterization and expression analysis of Toll-like receptor 2 gene in large yellow croaker, Larimichthys crocea. Fish Shellfish. Immunol. 2015, 44, 129–137. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, G.; Liu, Q.; Zhang, S. Cloning and expression study of a Toll-like receptor 2 (tlr2) gene from turbot, Scophthalmus maximus. Fish Shellfish. Immunol. 2016, 59, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Fink, I.R.; Pietretti, D.; Voogdt, C.G.P.; Westphal, A.H.; Savelkoul, H.F.J.; Forlenza, M.; Wiegertjes, G.F. Molecular and functional characterization of Toll-like receptor (Tlr)1 and Tlr2 in common carp (Cyprinus carpio). Fish Shellfish. Immunol. 2016, 56, 70–83. [Google Scholar] [CrossRef] [PubMed]
- He, L.B.; Wang, H.; Luo, L.F.; Jiang, S.H.; Liu, L.Y.; Li, Y.M.; Huang, R.; Liao, L.J.; Zhu, Z.Y.; Wang, Y.P. Characterization, expression analysis and localization pattern of toll-like receptor 1 (tlr1) and toll-like receptor 2 (tlr2) genes in grass carp Ctenopharyngodon Idella. J. Fish. Biol. 2016, 89, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, L.; Zhu, K.C.; Guo, H.Y.; Liu, B.; Jiang, S.G.; Zhang, D.C. Genomic structure and molecular characterization of Toll-like receptors 1 and 2 from golden pompano Trachinotus ovatus (Linnaeus, 1758) and their expression response to three types of pathogen-associated molecular patterns. Dev. Comp. Immunol. 2018, 86, 34–40. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Su, B.; Xue, T.; Cao, M.; Li, C. Genome-wide identification, characterization, and expression of the Toll-like receptors in Japanese flounder (Paralichthys olivaceus). Aquaculture 2021, 545, 737127. [Google Scholar] [CrossRef]
- Tang, R.; Wang, S.; Han, P.; Zhang, Q.; Zhang, S.; Xing, X.; Shao, R.; Xu, W.; Xu, Q.; Wei, Q.; et al. Toll-like receptor (TLR) 2 and TLR13 from the endangered primitive-ray finned fish Dabry’s sturgeon (Acipenser dabryanus) and their expression profiling upon immune stimulation. Aquac. Rep. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Oldenburg, M.; Krüger, A.; Ferstl, R.; Kaufmann, A.; Nees, G.; Sigmund, A.; Bathke, B.; Lauterbach, H.; Suter, M.; Dreher, S.; et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 2012, 337, 1111–1115. [Google Scholar] [CrossRef]
- Shi, Z.; Cai, Z.; Sanchez, A.; Zhang, T.; Wen, S.; Wang, J.; Yang, J.; Fu, S.; Zhang, D. A Novel Toll-like Receptor That Recognizes Vesicular Stomatitis Virus. J. Biol. Chem. 2011, 286, 4517–4524. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, X.; Chu, Q.; Xu, T. Discovery of toll-like receptor 13 exists in the teleost fish: Miiuy croaker (Perciformes, Sciaenidae). Dev. Comp. Immunol. 2016, 61, 25–33. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, X.; Yu, X.; Wang, Y.; Zhou, Y.; He, J.; Shi, Y.; Zhang, Y.; Lin, H.; Lu, D. Identification and functional characterization of Toll-like receptor 13 from orange-spotted grouper (Epinephelus coioides). Fish Shellfish. Immunol. 2018, 74, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xu, Y.; Wang, X.; Wang, S.; Zhang, Q.; Wang, Z.; Gao, Q. TLR13, TLR22, TRAF6, and TAK1 in the soiny mullet (Liza haematocheila): Molecular characterization and expression profiling analysis. Dev. Comp. Immunol. 2020, 112, 103774. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Pei, L.; Wang, P.; Liu, L.; Li, G.; Liu, B.; Lǚ, Z.; Hiromasa, T.; Pan, H.; Ogura, A. Molecular Characterization and Evolution Analysis of Two Forms of TLR5 and TLR13 Genes Base on Larimichthys crocea Genome Data. Int. J. Genom. 2020, 2020, 4895037. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Chen, S.N.; Huo, H.J.; Nie, P. Identification and expression analysis of sixteen Toll-like receptor genes, TLR1, TLR2a, TLR2b, TLR3, TLR5M, TLR5S, TLR7−9, TLR13a−c, TLR14, TLR21−23 in mandarin fish Siniperca chuatsi. Dev. Comp. Immunol. 2021, 121, 104100. [Google Scholar] [CrossRef]
- Feng-ying, G.; Ji-cai, P.; Miao, W.; Mai-xin, L.; Zhi-gang, L.; Jian-meng, C.; Xiao-li, K.; Meng-meng, Y. Structurally diverse genes encode TLR13 in Nile tilapia: The two receptors can recognize Streptococcus 23S RNA and conduct signal transduction through MyD88. Mol. Immunol. 2021, 132, 60–78. [Google Scholar] [CrossRef]
- Yu, X.; Liang, Y.; Zhou, Y.; He, L.; Liu, Y.; Fu, L.; Lin, H.; Zhang, Y.; Lu, D. 23S rRNA from Vibrio parahaemolyticus regulates the innate immune response via recognition by TLR13 in orange-spotted grouper (Epinephelus coioides). Dev. Comp. Immunol. 2021, 114, 103837. [Google Scholar] [CrossRef]
- Da, F.; Tan, H.; Wan, X.; Lin, G.; Jian, J.; Wen, Z.; Cai, S. Molecular characterization, expression and response to immune challenges of 3 members of the toll-like receptor superfamily 11 in the golden pompano (Trachinotus ovatus). Aquac. Rep. 2022, 25, 101268. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Tang, X.; Xu, D.; Chi, C.; Lv, Z.; Liu, H. Characterization of a novel Toll-like receptor 13 homologue from a marine fish Nibea albiflora, revealing its immunologic function as PRRs. Dev. Comp. Immunol. 2023, 139, 104563. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Sakai, M.; Hikima, J.; Kono, T. Fish cytokines: Current research and applications. Fish. Sci. 2021, 87, 1–9. [Google Scholar] [CrossRef]
- Netea, M.G.; van der Graaf, C.; Van der Meer, J.W.; Kullberg, B.J. Toll-like receptors and the host defense against microbial pathogens: Bringing specificity to the innate-immune system. J. Leukoc. Biol. 2004, 75, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Birstein, V.J.; Doukakis, P.; Sorkin, B.; DeSalle, R. Population Aggregation Analysis of Three Caviar-Producing Species of Sturgeons and Implications for the Species Identification of Black Caviar. Conserv. Biol. 1998, 12, 766–775. [Google Scholar] [CrossRef]
- Chandra, G.; Fopp-Bayat, D. Trends in aquaculture and conservation of sturgeons: A review of molecular and cytogenetic tools. Rev. Aquac. 2021, 13, 119–137. [Google Scholar] [CrossRef]
- Radosavljević, V.; Milićević, V.; Maksimović-Zorić, J.; Veljović, L.; Nešić, K.; Pavlović, M.; Ljubojević-Pelić, D.; Marković, Z. Sturgeon diseases in aquaculture. Arh. Vet. Med. 2019, 12, 5–20. [Google Scholar] [CrossRef]
- Christensen, K.A.; Sakhrani, D.; Rondeau, E.B.; Richards, J.; Koop, B.F.; Devlin, R.H. Effect of triploidy on liver gene expression in coho salmon (Oncorhynchus kisutch) under different metabolic states. BMC Genom. 2019, 20, 336. [Google Scholar] [CrossRef]
- Maxime, V. The physiology of triploid fish: Current knowledge and comparison with diploid fish. Fish Fish. 2008, 9, 67–78. [Google Scholar] [CrossRef]
- Ortiz, M.; Esteban, M.A. Biology and functions of fish thrombocytes: A review. Fish Shellfish. Immunol. 2024, 148, 109509. [Google Scholar] [CrossRef]
- Wlasow, T.; Fopp-Bayat, D. The effect of thermal shock on morphological characteristics of blood cells in Siberian sturgeon (Acipenser baerii) triploids. Acta Vet. Brno 2011, 80, 215–218. [Google Scholar] [CrossRef]
- Rożyński, M.; Demska-Zakęś, K.; Fopp-Bayat, D. Hematological and blood gas profiles of triploid Siberian sturgeon (Acipenser baerii Brandt). Arch. Pol. Fish. 2015, 23, 197–203. [Google Scholar] [CrossRef]
- Salkova, E.; Gela, D.; Pecherkova, P.; Flajshans, M. Examination of white blood cell indicators for three different ploidy level sturgeon species reared in an indoor recirculation aquaculture system for one year. Vet. Med. 2022, 67, 138–149. [Google Scholar] [CrossRef]
- Zapata, A.G. Lympho-Hematopoietic Microenvironments and Fish Immune System. Biology 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Levy-Pereira, N.; Carriero, M.M.; Yasui, G.S.; Meira, C.M.; de Sousa, R.L.M.; Maia, A.A.M.; Senhorini, J.A.; Pilarski, F. Effects of triploid induction on innate immunity and hematology in Astyanax altiparanae. Fish Shellfish. Immunol. 2021, 116, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cadonic, I.G.; Heath, J.W.; Dixon, B.; Craig, P.M. Diploid and triploid Chinook salmon (Oncorhynchus tshawytscha) have altered microRNA responses in immune tissues after infection with Vibrio anguillarum. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2023, 48, 101121. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, W.; Abbas, K.; Zhou, X.; Yang, Y.; Diana, J.S.; Wang, H.; Wang, H.; Li, Y.; Sun, Y. Haematological characterization of loach Misgurnus anguillicaudatus: Comparison among diploid, triploid and tetraploid specimens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 1001–1008. [Google Scholar] [CrossRef]
- Fopp-Bayat, D.; Kolman, R.; Woznicki, P. Induction of meiotic gynogenesis in sterlet (Acipenser ruthenus) using UV-irradiated bester sperm. Aquaculture 2007, 264, 54–58. [Google Scholar] [CrossRef]
- Fopp-Bayat, D.; Kujawa, R. Protective Right for a Utility Model—Device for Thermal Shock and Incubation of Sturgeon Fish Eggs. Patent Number 72570, 1 May 2022. [Google Scholar]
- Fopp-Bayat, D.; Nitkiewicz, A.; Gomułka, P. The Effect of Cryopreserved Sperm on the Early Development, Survival, and Growth of Intergeneric Sterbel Hybrids (Acipenser ruthenus × Huso huso). Int. J. Mol. Sci. 2024, 25, 5784. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, H.; Tian, Z.; Sun, A.; Dong, Y.; Dong, T.; Hu, H. Effects of 11-ketotestosterone on development of the previtellogenic ovary in the sterlet, Acipenser ruthenus. Front. Endocrinol. 2020, 11, 115. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum Information for publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Svobodová, Z.; Pravda, D.; Paláčková, J. Unified Methods of Haematological Examination of Fish; Edition Methods; Research Institute of Fish Culture and Hydrobiology: Vodňany, Czech Republic, 1991; Volume 22, 31p. [Google Scholar]

| Immune Nucleated Cells Percentage (%) | Diploid Sturgeon | Triploid Sturgeon | p |
|---|---|---|---|
| WBC | 7.78 ± 0.42 | 4.73 ± 0.66 | <0.001 |
| Lymphocytes | 46.13 ± 2.55 | 20.78 ± 2.57 | <0.001 |
| Monocytes | 3.33 ± 0.55 | 4.83 ± 1.02 | N |
| Neutrophils | 17.35 ± 0.23 | 26.23 ± 0.94 | <0.0001 |
| Eosinophils | 2.5 ± 0.46 | 3.15 ± 0.3 | N |
| Thrombocytes | 30.68 ± 1.69 | 45.10 ± 4.33 | <0.05 |
| Gene Name | Primer Sequences (F: Sense, R: Antisense) | Amplicon Length (bp) | Concentration of Primers (nM): Sense/Antisense |
|---|---|---|---|
| TLR2 | F: CTCTCGGAGCACTTTGTTCG R: ACTGCCCTCTGTCCTTCATC | 212 | 200/200 |
| TLR13 | F: ATACAACACGCACGATGAGC R: TAGTGGTGGCTGATGATGCA | 180 | 400/200 |
| EF1α | F:GGACTCCACTGAGCCACCT R: GGGTTGTAGCCGATCTTCTTG | 89 | 200/400 |
| RPL13 | F:GGACGTGGTTTCACCCTTG R: GGGAAGAGGATGAGTTTGGA | 166 | 200/400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonska, O.; Duda, S.; Gajowniczek, S.; Nitkiewicz, A.; Fopp-Bayat, D. Toll-like Receptor Type 2 and 13 Gene Expression and Immune Cell Profiles in Diploid and Triploid Sterlets (Acipenser ruthenus): Insights into Immune Competence in Polyploid Fish. Int. J. Mol. Sci. 2025, 26, 3986. https://doi.org/10.3390/ijms26093986
Jablonska O, Duda S, Gajowniczek S, Nitkiewicz A, Fopp-Bayat D. Toll-like Receptor Type 2 and 13 Gene Expression and Immune Cell Profiles in Diploid and Triploid Sterlets (Acipenser ruthenus): Insights into Immune Competence in Polyploid Fish. International Journal of Molecular Sciences. 2025; 26(9):3986. https://doi.org/10.3390/ijms26093986
Chicago/Turabian StyleJablonska, Olga, Sara Duda, Szczepan Gajowniczek, Anna Nitkiewicz, and Dorota Fopp-Bayat. 2025. "Toll-like Receptor Type 2 and 13 Gene Expression and Immune Cell Profiles in Diploid and Triploid Sterlets (Acipenser ruthenus): Insights into Immune Competence in Polyploid Fish" International Journal of Molecular Sciences 26, no. 9: 3986. https://doi.org/10.3390/ijms26093986
APA StyleJablonska, O., Duda, S., Gajowniczek, S., Nitkiewicz, A., & Fopp-Bayat, D. (2025). Toll-like Receptor Type 2 and 13 Gene Expression and Immune Cell Profiles in Diploid and Triploid Sterlets (Acipenser ruthenus): Insights into Immune Competence in Polyploid Fish. International Journal of Molecular Sciences, 26(9), 3986. https://doi.org/10.3390/ijms26093986






