Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies—A Narrative Review
Abstract
1. Overview of Peripheral Nerve Injuries
1.1. Introduction
1.2. Classification of Peripheral Nerve Injuries
1.3. Physiopathology of Peripheral Nerve Lesions
2. Methodology
3. Discussion and Literature Review on Therapeutic Management of Peripheral Nerve Injuries
3.1. Direct Nerve Repair
3.2. Autologous Nerve Grafts
3.3. Nerve Transfers
3.4. End-to-Side Coaptation
3.5. Nerve Allograft Transplantation
3.6. Nerve Conduits
3.6.1. Autograft-Based Conduits
3.6.2. Synthetic Nerve Conduits
Natural Polymers
Synthetic Polymers
Composite Materials
Ceramics and Other Materials
4. Emerging Trends and Future Directions in Nerve Regeneration
4.1. Three-Dimensional (3D) Bioprinting and Personalized Conduits
4.2. Nanotechnology- and Nanofiber-Based Conduits
4.3. Growth Factor-Based Therapeutic Strategies and the Role of Gene Therapy for Peripheral Nerve Regeneration
4.4. The Role of Stem Cells in Peripheral Nerve Regeneration
4.5. Pharmacological and Bioactive Compound Interventions for Peripheral Nerve Repair
4.5.1. Tacrolimus
4.5.2. Calcium Channel Blockers
4.5.3. Statins
4.5.4. Lipoic Acid
4.5.5. Vitamin B
4.5.6. Erythropoietin
4.5.7. Melatonin
4.5.8. Hyaluronic Acid
4.5.9. Curcumin
4.6. The Role of Electrical Stimulation in Nerve Regeneration
5. Clinical Algorithm for Selecting Reconstructive Methods for Peripheral Nerve Transections
6. Examples of Nerve Repair Strategies in Clinical Application
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, T.C.; Leung, Y.Y. Innovations in Peripheral Nerve Regeneration. Bioengineering 2024, 11, 444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matos Cruz, A.J.; De Jesus, O. Neurotmesis. [Updated 2023 August 23]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559108/ (accessed on 14 February 2025).
- Sonawane, K.; Dixit, H.; Thota, N.; Mistry, T.; Balavenkatasubramanian, J. “Knowing It Before Blocking It”, the ABCD of the Peripheral Nerves: Part B (Nerve Injury Types, Mechanisms, and Pathogenesis). Cureus 2023, 15, e43143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Althagafi, A.; Nadi, M. Acute Nerve Injury. [Updated 2023 August 7]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549848/ (accessed on 14 February 2025).
- Martins, R.S.; Bastos, D.; Siqueira, M.G.; Heise, C.O.; Teixeira, M.J. Traumatic injuries of peripheral nerves: A review with emphasis on surgical indication. Arq. Neuropsiquiatr. 2013, 71, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, S.; Malviya, R.; Sundram, S. Management of peripheral nerve injuries using natural based biomaterials and their derivatives: Advances and prospective. MedComm.—Biomater. Appl. 2024, 3, e72. [Google Scholar] [CrossRef]
- Siemionow, M.; Brzezicki, G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int. Rev. Neurobiol. 2009, 87, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Biso, G.M.N.R.; Munakomi, S. Neuroanatomy, Neurapraxia. [Updated 2022 October 24]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024; [Figure, Seddon and Sunderland Classification of Nerve Injury Contributed by GMN Biso, MD]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557746/figure/article-25766.image.f1/ (accessed on 15 December 2024).
- Landers, M.R.; Altenburger, P. Peripheral nerve injury. Adv. Physiother. 2003, 5, 67–82. [Google Scholar] [CrossRef]
- Ditty, B.J.; Omar, N.B.; Rozzelle, C.J. Surgery for peripheral nerve trauma. In Nerves and Nerve Injuries. Vol. 2: Pain, Treatment, Injury, Disease, and Future Directions, 1st ed.; Tubbs, R.S., Rizk, E., Shoja, M.M., Barbaro, N., Spinner, R.J., Eds.; Elsevier: Amsterdam, The Netherland, 2015; pp. 373–381. [Google Scholar] [CrossRef]
- Radić, B.; Radić, P.; Duraković, D. Peripheral nerve injury in sports. Acta Clin. Croat. 2018, 57, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, A.; Aruta, G.; De Marco, R.; Zeppa, P.; Titolo, P.; Colonna, M.R.; Galeano, M.; Costa, A.L.; Vincitorio, F.; Garbossa, D.; et al. Traumatic peripheral nerve injuries: A classification proposal. J. Orthop. Traumatol. 2023, 24, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamble, N.; Shukla, D.; Bhat, D. Peripheral Nerve Injuries: Electrophysiology for the Neurosurgeon. Neurol. India 2019, 67, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2022, 66, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, M.; Tang, Q.; Zhu, L.; Koleini, M.; Zou, D. The effects of different tensile parameters for the neurodynamic mobilization technique on tricipital muscle wet weight and MuRf-1 expression in rabbits with sciatic nerve injury. J. Neuroeng. Rehabil. 2015, 12, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wieringa, P.A.; Gonçalves de Pinho, A.R.; Micera, S.; van Wezel, R.J.A.; Moroni, L. Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies. Adv. Healthc. Mater. 2018, 7, e1701164. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, E.A.; Tuszynski, M.H.; Lu, P.; Dulin, J.N. Molecular and Cellular Mechanisms of Axonal Regeneration After Spinal Cord Injury. Mol. Cell. Proteom. 2016, 15, 394–408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Z.; Jin, Y. Intrinsic Control of Axon Regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Horn, K.P.; Busch, S.A.; Hawthorne, A.L.; van Rooijen, N.; Silver, J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 2008, 28, 9330–9341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sulaiman, W.; Gordon, T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: From bench top research to bedside application. Ochsner. J. 2013, 13, 100–108. [Google Scholar] [PubMed] [PubMed Central]
- Waller, A. Experiments on the Section of the Glosso-Pharyngeal and Hypoglossal Nerves of the Frog, and Observations of the Alterations Produced Thereby in the Structure of Their Primitive Fibres. Edinb. Med. Surg. J. 1851, 76, 369–376. [Google Scholar] [PubMed] [PubMed Central]
- Beirowski, B.; Adalbert, R.; Wagner, D.; Grumme, D.S.; Addicks, K.; Ribchester, R.R.; Coleman, M.P. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005, 6, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Burrell, J.C.; Zeng, J.; Motiwala, F.I.; Shi, S.; Cullen, D.K.; Le, A.D. Implantation of a nerve protector embedded with human GMSC-derived Schwann-like cells accelerates regeneration of crush-injured rat sciatic nerves. Stem. Cell Res. Ther. 2022, 13, 263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burn. Trauma 2020, 8, tkaa002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, L.; He, J.; Shen, N.; Chen, S. Molecular and cellular mechanisms underlying peripheral nerve injury-induced cellular ecological shifts: Implications for neuroregeneration. IBRO Neurosci. Rep. 2024, 18, 120–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boivin, A.; Pineau, I.; Barrette, B.; Filali, M.; Vallières, N.; Rivest, S.; Lacroix, S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 2007, 27, 12565–12576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thakur, K.K.; Saini, J.; Mahajan, K.; Singh, D.; Jayswal, D.P.; Mishra, S.; Bishayee, A.; Sethi, G.; Kunnumakkara, A.B. Therapeutic implications of toll-like receptors in peripheral neuropathic pain. Pharmacol. Res. 2017, 115, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Sano, M.; Omura, T.; Omura, K.; Hasegawa, T.; Funahashi, S.; Nagano, A. Spatiotemporal quantification of tumor necrosis factor-alpha and interleukin-10 after crush injury in rat sciatic nerve utilizing immunohistochemistry. Neurosci. Lett. 2007, 417, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monk, K.R.; Feltri, M.L.; Taveggia, C. New insights on Schwann cell development. Glia 2015, 63, 1376–1393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayashi, A.; Koob, J.W.; Liu, D.Z.; Tong, A.Y.; Hunter, D.A.; Parsadanian, A.; Mackinnon, S.E.; Myckatyn, T.M. A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves. Exp. Neurol. 2007, 207, 128–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro-Resende, V.T.; Koenig, B.; Nichterwitz, S.; Oberhoffner, S.; Schlosshauer, B. Strategies for inducing the formation of bands of Büngner in peripheral nerve regeneration. Biomaterials 2009, 30, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Panzer, K.V.; Burrell, J.C.; Helm, K.V.T.; Purvis, E.M.; Zhang, Q.; Le, A.D.; O’Donnell, J.C.; Cullen, D.K. Tissue Engineered Bands of Büngner for Accelerated Motor and Sensory Axonal Outgrowth. Front. Bioeng. Biotechnol. 2020, 8, 580654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuentes-Flores, A.; Geronimo-Olvera, C.; Girardi, K.; Necuñir-Ibarra, D.; Patel, S.K.; Bons, J.; Wright, M.C.; Geschwind, D.; Hoke, A.; Gomez-Sanchez, J.A.; et al. Senescent Schwann cells induced by aging and chronic denervation impair axonal regeneration following peripheral nerve injury. EMBO Mol. Med. 2023, 15, e17907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J. Commun. Disord. 2010, 43, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.; Gordon, T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery 2009, 65 (Suppl. S4), A105–A114. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Matsumura, K. Biological role of dystroglycan in Schwann cell function and its implications in peripheral nervous system diseases. J. Biomed. Biotechnol. 2010, 2010, 740403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, H.; Shah, W.; Holland, P.; Carbonetto, S. Integrins and dystroglycan regulate astrocyte wound healing: The integrin beta1 subunit is necessary for process extension and orienting the microtubular network. Dev. Neurobiol. 2008, 68, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Toy, D.; Namgung, U. Role of glial cells in axonal regeneration. Exp. Neurobiol. 2013, 22, 68–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markus, A.; Patel, T.D.; Snider, W.D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 2002, 12, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003, 27, 277–324. [Google Scholar] [CrossRef] [PubMed]
- Tannemaat, M.R.; Eggers, R.; Hendriks, W.T.; de Ruiter, G.C.; van Heerikhuize, J.J.; Pool, C.W.; Malessy, M.J.; Boer, G.J.; Verhaagen, J. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur. J. Neurosci. 2008, 28, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Mackinnon, S.E. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp. Neurol. 2015, 265, 171–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madison, R.D.; Sofroniew, M.V.; Robinson, G.A. Schwann cell influence on motor neuron regeneration accuracy. Neuroscience 2009, 163, 213–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Qudah, M.A.; Al-Dwairi, A. Mechanisms and regulation of neurotrophin synthesis and secretion. Neurosciences 2016, 21, 306–313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657–10688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wysokiński, A. Serum levels of brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) in depressed patients with schizophrenia. Nord. J. Psychiatry 2016, 70, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Kumar, P.; Raza, K.; Prasoon, P.; Dantham, S.; Mochan, S. Regulatory role of NGFs in neurocognitive functions. Rev. Neurosci. 2017, 28, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Malahias, M.; Hindocha, S.; Khan, W.S. Peripheral nerve injury: Principles for repair and regeneration. Open Orthop. J. 2014, 8, 199–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Supra, R.; Agrawal, D.K. Peripheral Nerve Regeneration: Opportunities and Challenges. J. Spine Res. Surg. 2023, 5, 10–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hohman, M.H.; De Jesus, O. Facial Nerve Repair. [Updated 2023 August 23]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560623/ (accessed on 20 February 2025).
- Pereira, C.T.; Hill, E.E.; Stasyuk, A.; Parikh, N.; Dhillon, J.; Wang, A.; Li, A. Molecular Basis of Surgical Coaptation Techniques in Peripheral Nerve Injuries. J. Clin. Med. 2023, 12, 1555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braga Silva, J.; Becker, A.S.; Leal, B.L.M.; Busnello, C.V. Advances of Direct Peripheral Nerve Repair Techniques: Do We Already Have Enough Scientific Evidence? Indian J. Orthop. 2022, 57, 189–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zimmermann, K.S.; Aman, M.; Harhaus, L.; Boecker, A.H. Improving outcomes in traumatic peripheral nerve injuries to the upper extremity. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Philipeaux, J.M.; Vulpian, A. Note sur des essais de greffe d’un troncon du nerf lingual entre les deux bouts du nerf hypoglosse, apres excision d’un segment de ce dernier nerf. Arch. Physiol. Norm. Pathol. 1870, 3, 618–620. [Google Scholar]
- Bunnell, S.; Boyes, J.H. Nerve grafts. Am. J. Surg. 1939, 44, 64–75. [Google Scholar] [CrossRef]
- D’Arpa, S.; Claes, K.E.Y.; Stillaert, F.; Colebunders, B.; Monstrey, S.; Blondeel, P. Vascularized nerve “grafts”: Just a graft or a worthwhile procedure? Plast. Aesthet. Res. 2015, 2, 183–194. [Google Scholar] [CrossRef]
- Xu, G.; Zou, X.; Dong, Y.; Alhaskawi, A.; Zhou, H.; Ezzi, S.H.A.; Kota, V.G.; Abdulla, M.H.A.H.; Alenikova, O.; Abdalbary, S.A.; et al. Advancements in autologous peripheral nerve transplantation care: A review of strategies and practices to facilitate recovery. Front. Neurol. 2024, 15, 1330224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baek, A.; Isaacs, J. Management of “Long” Nerve Gaps. J. Hand Surg. Glob. Online 2024, 6, 685–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meena, P.; Kakkar, A.; Kumar, M.; Khatri, N.; Nagar, R.K.; Singh, A.; Malhotra, P.; Shukla, M.; Saraswat, S.K.; Srivastava, S.; et al. Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 2021, 383, 617–644. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Saffari, T.M.; Bedar, M.; Hundepool, C.A.; Bishop, A.T.; Shin, A.Y. The role of vascularization in nerve regeneration of nerve graft. Neural Regen. Res. 2020, 15, 1573–1579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terzis, J.K.; Kostopoulos, V.K. Vascularized nerve grafts and vascularized fascia for upper extremity nerve reconstruction. Hand 2010, 5, 19–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toia, F.; Matta, D.; De Michele, F.; Pirrello, R.; Cordova, A. Animal models of vascularized nerve grafts: A systematic review. Neural Regen. Res. 2023, 18, 2615–2618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strange, F.G. An operation for nerve pedicle grafting; preliminary communication. Br. J. Surg. 1947, 34, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I.; Ham, F.J. The free vascularized nerve graft. A further experimental and clinical application of microvascular techniques. Plast. Reconstr. Surg. 1976, 57, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.T.; Wu, J.J.; Ma, J.; Xing, X.X.; Zhang, J.P.; Hua, X.Y.; Zheng, M.X.; Xu, J.G. Peripheral nerve transfers for dysfunctions in central nervous system injuries: A systematic review. Int. J. Surg. 2024, 110, 3814–3826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tung, T.H.; Mackinnon, S.E. Nerve transfers: Indications, techniques, and outcomes. J. Hand Surg. Am. 2010, 35, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Moucharafieh, R.C.; Badra, M.I.; Boulos, K.A.; Mansour, J.I.; Daher, J.C.; Wardani, H.M.; Nour, H.G.A.E.; Sayde, E.G.; Nehme, A.H. Nerve transfers in the upper extremity: A review. Injury 2020, 51, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Mackinnon, S.E. Clinical outcomes following median to radial nerve transfers. J. Hand Surg. Am. 2011, 36, 201–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duraku, L.S.; Chaudhry, T.; George, S.; Madura, T.; Zuidam, J.M.; Hundepool, C.A.; Teunis, T.; Baas, M.; Ramadan, S.; Burahee, A.S.; et al. Motor nerve transfers for reconstruction of traumatic upper extremity nerve injuries: A scoping review. JPRAS Open 2024, 43, 581–594. [Google Scholar] [CrossRef]
- Baltzer, H.; Woo, A.; Oh, C.; Moran, S.L. Comparison of Ulnar Intrinsic Function following Supercharge End-to-Side Anterior Interosseous-to-Ulnar Motor Nerve Transfer: A Matched Cohort Study of Proximal Ulnar Nerve Injury Patients. Plast. Reconstr. Plast. Reconstr. Surg. 2016, 138, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.; Englbrecht, M.; Koban, K.C.; Cotofana, S.C.; Stewart, J.K.; Giunta, R.E.; Schenck, T.L. Nerve transfer of the anterior interosseous nerve to the thenar branch of the median nerve—An anatomical and histological analysis. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, F.; Trindade, J.C.; Hoshino, K.; Mazzoni Neto, A. End-to-side neurorrhaphy with removal of the epineurial sheath: An experimental study in rats. Plast. Reconstr. Plast. Reconstr. Surg. 1994, 94, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Geuna, S.; Papalia, I.; Ronchi, G.; d’Alcontres, F.S.; Natsis, K.; Papadopulos, N.A.; Colonna, M.R. The reasons for end-to-side coaptation: How does lateral axon sprouting work? Neural Regen. Res. 2017, 12, 529–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayashi, A.; Yanai, A.; Komuro, Y.; Nishida, M.; Inoue, M.; Seki, T. Collateral sprouting occurs following end-to-side neurorrhaphy. Plast. Reconstr. Plast. Reconstr. Surg. 2004, 114, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Pannucci, C.; Moradzadeh, A.; Kawamura, D.; Magill, C.; Hunter, D.A.; Tong, A.Y.; Parsadanian, A.; Mackinnon, S.E.; Myckatyn, T.M. Axotomy or compression is required for axonal sprouting following end-to-side neurorrhaphy. Exp. Neurol. 2008, 211, 539–550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dvali, L.T.; Myckatyn, T.M. End-to-side nerve repair: Review of the literature and clinical indications. Hand Clin. 2008, 24, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.K.; Mackinnon, S.E. Experience with nerve allograft transplantation. Semin. Plast. Surg. 2007, 21, 242–249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bittner, G.D.; Bushman, J.S.; Ghergherehchi, C.L.; Roballo, K.C.S.; Shores, J.T.; Smith, T.A. Typical and atypical properties of peripheral nerve allografts enable novel strategies to repair segmental-loss injuries. J. Neuroinflammation 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartzell, T.L.; Benhaim, P.; Imbriglia, J.E.; Shores, J.T.; Goitz, R.J.; Balk, M.; Mitchell, S.; Rubinstein, R.; Gorantla, V.S.; Schneeberger, S.; et al. Surgical and technical aspects of hand transplantation: Is it just another replant? Hand Clin. 2011, 27, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Elkwood, A.I.; Holland, N.R.; Arbes, S.M.; Rose, M.I.; Kaufman, M.R.; Ashinoff, R.L.; Parikh, M.A.; Patel, T.R. Nerve allograft transplantation for functional restoration of the upper extremity: Case series. J. Spinal Cord Med. 2011, 34, 241–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siemionow, M.; Sonmez, E. Nerve allograft transplantation: A review. J. Reconstr. Microsurg. 2007, 23, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Unadkat, J.; Sacks, J.M.; Schneeberger, S.; Lee, W.P.A. Relative antigenicity of allograft components and differential rejection. In Transplantation of Composite Tissue Allografts; Hewitt, C.W., Lee, W.P.A., Gordon, C.R., Eds.; Springer: Boston, MA, USA, 2008; pp. 55–69. [Google Scholar] [CrossRef]
- Nakamoto, J.C.; Wataya, E.Y.; Nakamoto, H.A.; Santos, G.B.; Ribaric, I.; Herrera, A.K.A.; Faria, J.C.M. Evaluation of the Use of Nerve Allograft Preserved in Glycerol. Plast. Reconstr. Surg. Glob. Open. 2021, 9, e3514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daeschler, S.C.; Feinberg, K.; Harhaus, L.; Kneser, U.; Gordon, T.; Borschel, G.H. Advancing Nerve Regeneration: Translational Perspectives of Tacrolimus (FK506). Int. J. Mol. Sci. 2023, 24, 12771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zuo, K.J.; Saffari, T.M.; Chan, K.; Shin, A.Y.; Borschel, G.H. Systemic and Local FK506 (Tacrolimus) and its Application in Peripheral Nerve Surgery. J. Hand Surg. Am. 2020, 45, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Kasukurthi, R.; Kale, S.S.; Santosa, K.B.; Hunter, D.A.; Johnson, P.; Yan, Y.; Mohanakumar, T.; Mackinnon, S.E.; Tung, T.H. Costimulation blockade inhibits the indirect pathway of allorecognition in nerve allograft rejection. Muscle Nerve 2011, 43, 120–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Giannis, D.; Moris, D.; Cendales, L.C. Costimulation Blockade in Vascularized Composite Allotransplantation. Front. Immunol. 2020, 11, 544186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ray, W.Z.; Kale, S.S.; Kasukurthi, R.; Papp, E.M.; Johnson, P.J.; Santosa, K.B.; Yan, Y.; Hunter, D.A.; Mackinnon, S.E.; Tung, T.H. Effect of cold nerve allograft preservation on antigen presentation and rejection. J. Neurosurg. 2011, 114, 256–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, S.; Wei, H.; Cheng, H.; Qi, Y.; Gu, Y.; Ma, X.; Sun, J.; Ye, F.; Guo, F.; Cheng, C. Advances in nerve guidance conduits for peripheral nerve repair and regeneration. Am. J. Stem. Cells 2023, 12, 112–123. [Google Scholar] [PubMed] [PubMed Central]
- de Ruiter, G.C.; Malessy, M.J.; Yaszemski, M.J.; Windebank, A.J.; Spinner, R.J. Designing ideal conduits for peripheral nerve repair. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braga-Silva, J. The use of silicone tubing in the late repair of the median and ulnar nerves in the forearm. J. Hand Surg. 1999, 24, 703–706. [Google Scholar] [CrossRef]
- Merle, M.; Dellon, A.L.; Campbell, J.N.; Chang, P.S. Complications from silicon-polymer intubulation of nerves. Microsurgery 1989, 10, 130–133. [Google Scholar] [CrossRef]
- Zhao, Q.; Dahlin, L.B.; Kanje, M.; Lundborg, G. Repair of the transected rat sciatic nerve: Matrix formation within implanted silicone tubes. Restor. Neurol. Neurosci. 1993, 5, 197–204. [Google Scholar] [CrossRef]
- Muheremu, A.; Ao, Q. Past, Present, and Future of Nerve Conduits in the Treatment of Peripheral Nerve Injury. Biomed. Res. Int. 2015, 2015, 237507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belkas, J.S.; Munro, C.A.; Shoichet, M.S.; Johnston, M.; Midha, R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials 2005, 26, 1741–1749. [Google Scholar] [CrossRef]
- Jacobs, T.; Patil, D.; Ziccardi, V.B. Both Type I Bovine Collagen Conduits and Porcine Small Intestine Submucosa Conduits Result in Functional Sensory Recovery Following Peripheral Nerve Microsurgery: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2024, 82, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Thangadurai, M.; Sundaramurthi, D.; Sethuraman, S. Additive manufacturing of peripheral nerve conduits—Fabrication methods, design considerations and clinical challenges. SLAS Technol. 2023, 28, 102–126. [Google Scholar] [CrossRef] [PubMed]
- Mankavi, F.; Ibrahim, R.; Wang, H. Advances in Biomimetic Nerve Guidance Conduits for Peripheral Nerve Regeneration. Nanomaterials 2023, 13, 2528. [Google Scholar] [CrossRef]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Liang, R.; Lin, J.; Chen, J.; Zhang, Q.; Li, J.; Wang, M.; Hui, X.; Tan, H.; Fu, Q. Biodegradable polyurethane nerve guide conduits with different moduli influence axon regeneration in transected peripheral nerve injury. J. Mater. Chem. B 2021, 9, 7979–7990. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, W.; Yang, Y.; Yang, Y.; Liu, W. Material advancement in tissue-engineered nerve conduit. Nanotechnol. Rev. 2021, 10, 488–503. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef] [PubMed]
- Schlosshauer, B.; Muller, E.; Schroder, B.; Planck, H.; Muller, H.-W.; Lietz, M. Nerve Guide. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G.E., Bowlin, G.L., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1901–1924. [Google Scholar]
- Bozkurt, A.; Lassner, F.; O’Dey, D.; Deumens, R.; Böcker, A.; Schwendt, T.; Janzen, C.; Suschek, C.V.; Tolba, R.; Kobayashi, E.; et al. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials 2012, 33, 1363–1375. [Google Scholar] [CrossRef]
- Heinzel, J.C.; Quyen Nguyen, M.; Kefalianakis, L.; Prahm, C.; Daigeler, A.; Hercher, D.; Kolbenschlag, J. A systematic review and meta-analysis of studies comparing muscle-in-vein conduits with autologous nerve grafts for nerve reconstruction. Sci. Rep. 2021, 11, 11691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Alina, S. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci.—PROG. POLYM. SCI. 2011, 36, 1254–1276. [Google Scholar]
- Zhang, M.; Li, C.; Zhou, L.P.; Pi, W.; Zhang, P.X. Polymer Scaffolds for Biomedical Applications in Peripheral Nerve Reconstruction. Molecules 2021, 26, 2712. [Google Scholar] [CrossRef] [PubMed]
- Toba, T.; Nakamura, T.; Shimizu, Y.; Matsumoto, K.; Ohnishi, K.; Fukuda, S.; Yoshitani, M.; Ueda, H.; Hori, Y.; Endo, K. Regeneration of canine peroneal nerve with the use of a polyglycolic acid-collagen tube filled with laminin-soaked collagen sponge: A comparative study of collagen sponge and collagen fibers as filling materials for nerve conduits. J. Biomed. Mater. Res. 2001, 58, 622–630. [Google Scholar] [CrossRef]
- Yoshii, S.; Oka, M.; Shima, M.; Taniguchi, A.; Akagi, M. Bridging a 30-mm nerve defect using collagen filaments. J. Biomed. Mater. Res. Part A 2003, 67, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yang, W.; Chen, J.; Zhang, J.; Lu, X.; Zhao, X.; Huang, K.; Li, H.; Chang, P.; Wang, Z.; et al. A silk sericin/silicone nerve guidance conduit promotes regeneration of a transected sciatic nerve. Adv. Healthc. Mater. 2015, 4, 2195–2205. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, F.; Cheng, W.; Gao, X.; Ding, Z.; Zhang, X.; Lu, Q.; Kaplan, D.L. Nerve Guidance Conduits with Hierarchical Anisotropic Architecture for Peripheral Nerve Regeneration. Adv. Healthc. Mater. 2021, 10, 2100427. [Google Scholar] [CrossRef]
- Magaz, A.; Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive Silk-Based Nerve Guidance Conduits for Augmenting Peripheral Nerve Repair. Adv. Healthc. Mater. 2018, 7, 1800308. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Cao, Y.; Yao, J.; Wu, J.; Gu, X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 2005, 128, 1897–1910. [Google Scholar] [CrossRef]
- Fiddes, P.S.; Tartare-Deckert, S.; Brenner, E.R. 3D Printed Conductive Multiscale Nerve Guidance Conduit with Hierarchical Fibers for Peripheral Nerve Regeneration. Adv. Sci. 2023, 10, 2205744. [Google Scholar] [CrossRef]
- Talebi, A.R.; Labbaf, S.; Rahmati, S. Biofabrication of a flexible and conductive 3D polymeric scaffold for neural tissue engineering applications; physical, chemical, mechanical, and biological evaluations. Polym. Adv. Technol. 2022, 34, 134–144. [Google Scholar] [CrossRef]
- Escobar, A.; Serafin, A.; Carvalho, M.; Culebras, M.; Cantarero, A.; Beaucamp, A.; Reis, R.L.; Oliveira, J.M.; Collins, M.N. Electroconductive poly(3,4-ethylenedioxythiophene) (PEDOT) nanoparticle-loaded silk fibroin biocomposite conduits for peripheral nerve regeneration. Adv. Compos. Hybrid Mater. 2023, 6, 118. [Google Scholar] [CrossRef]
- Dellinger, M. Nerve Repair Conduits Incorporating Silica Fibers. US Patent 12,005,154, 11 June 2024. [Google Scholar]
- Xuan, H.V.; Wu, S.; Jin, Y.; Wei, S.; Xiong, F.; Xue, Y.; Li, B.; Yang, Y.; Yuan, H. A Bioinspired Self-Healing Conductive Hydrogel Promoting Peripheral Nerve Regeneration. Adv. Sci. 2023, 10, 2302519. [Google Scholar] [CrossRef]
- Li, R.; Li, D.H.; Zhang, H.Y.; Wang, J.; Li, X.K.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crabtree, J.R.; Mulenga, C.M.; Tran, K.; Feinberg, K.; Santerre, J.P.; Borschel, G.H. Biohacking Nerve Repair: Novel Biomaterials, Local Drug Delivery, Electrical Stimulation, and Allografts to Aid Surgical Repair. Bioengineering 2024, 11, 776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Yan, L.; Li, R.; Song, Z.; Ding, J.; Liu, B.; Chen, X. 3D Printed Personalized Nerve Guide Conduits for Precision Repair of Peripheral Nerve Defects. Adv. Sci. 2022, 9, e2103875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hsu, S.H. Biomaterials and neural regeneration. Neural Regen. Res. 2020, 15, 1243–1244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Wu, Y.; Gou, Z.; Tao, J.; Zhang, J.; Liu, Q.; Kang, T.; Jiang, S.; Huang, S.; He, J.; et al. 3D-engineering of Cellularized Conduits for Peripheral Nerve Regeneration. Sci. Sci. Rep. 2016, 6, 32184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwai, T.; Ikeguchi, R.; Aoyama, T.; Noguchi, T.; Yoshimoto, K.; Sakamoto, D.; Fujita, K.; Miyazaki, Y.; Akieda, S.; Nagamura-Inoue, T.; et al. Nerve regeneration using a Bio 3D conduit derived from umbilical cord-Derived mesenchymal stem cells in a rat sciatic nerve defect model. PLoS ONE 2024, 19, e0310711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Das, S.; Thimukonda Jegadeesan, J.; Basu, B. Advancing Peripheral Nerve Regeneration: 3D Bioprinting of GelMA-Based Cell-Laden Electroactive Bioinks for Nerve Conduits. ACS Biomater. Sci. Eng. 2024, 10, 1620–1645. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Wang, J. Development of 3D-Printed, Biodegradable, Conductive PGSA Composites for Nerve Tissue Regeneration. Macromol. Biosci. 2023, 23, e2200470. [Google Scholar] [CrossRef] [PubMed]
- Convertino, D.; Trincavelli, M.L.; Giacomelli, C.; Marchetti, L.; Coletti, C. Graphene-based nanomaterials for peripheral nerve regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1306184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, R.; Rauti, R.; Scaini, D.; Antman-Passig, M.; Meshulam, O.; Naveh, D.; Ballerini, L.; Shefi, O. Graphene-based nanomaterials for neuroengineering: Recent advances and future prospective. Adv. Funct. Mater. 2021, 31, 2104887. [Google Scholar] [CrossRef]
- Hui, Y.; Yan, Z.; Yang, H.; Xu, X.; Yuan, W.E.; Qian, Y. Graphene Family Nanomaterials for Stem Cell Neurogenic Differentiation and Peripheral Nerve Regeneration. ACS Appl. Bio. Mater. 2022, 5, 4741–4759. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science. 2004 Oct Balandin AA. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing Graphene-Based Materials with Neural Cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 2011, 32, 9374–9382. [Google Scholar] [CrossRef] [PubMed]
- Uz, M.; Donta, M.S.; Mededovic, M.; Sakaguchi, D.S.; Mallapragada, S.K. Development of gelatin and graphene-based nerve regeneration conduits using three-dimensional (3D) printing strategies for electrical transdifferentiation of mesenchymal stem cells. Ind. Eng. Chem. Res. 2019, 58, 7421–7427. [Google Scholar] [CrossRef]
- Bahremandi Tolou, N.; Salimi Jazi, H.R.; Kharaziha, M.; Lisi, N.; Faggio, G.; Tamburrano, A. Fabrication of nerve guide conduit based on 3D graphene/polymer for nerve tissue engineering. J. Adv. Mater. Eng. 2022, 39, 61–73. [Google Scholar] [CrossRef]
- Dresvyanina, E.N.; Tagandurdyyeva, N.A.; Kodolova-Chukhontseva, V.V.; Dobrovol’skaya, I.P.; Kamalov, A.M.; Nashchekina, Y.A.; Nashchekin, A.V.; Ivanov, A.G.; Yukina, G.Y.; Yudin, V.E. Structure and Properties of Composite Fibers Based on Chitosan and Single-Walled Carbon Nanotubes for Peripheral Nerve Regeneration. Polymers 2023, 15, 2860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, B.; Wu, T.; He, L.; Zhang, J.; Yuan, Y.; Huang, X.; El-Hamshary, H.; Al-Deyab, S.S.; Xu, T.; Mo, X. Development of Dual Neurotrophins-Encapsulated Electrosupun Nanofibrous Scaffolds for Peripheral Nerve Regeneration. J. Biomed. Nanotechnol. 2016, 12, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yin, J.B.; Hao, H.P.; Zhu, C.; Zhang, T.; Lu, Y.C.; Wang, L.Y.; Wang, Z.; Li, Y.Q. Tissue engineering of nanosilver-embedded peripheral nerve scaffold to repair nerve defects under contamination conditions. Int. J. Artif. Organs. 2015, 38, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhang, F.S.; Qin, M.Y.; Jiang, H.R.; Zhang, M.; Qu, Y.; Wang, Y.L.; Zhang, P.X. Growth factors: Bioactive macromolecular drugs for peripheral nerve injury treatment—Molecular mechanisms and delivery platforms. Biomed. Pharmacother. 2024, 170, 116024. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Gander, B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J. Control Release 2012, 161, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.N.; Coulson, E.J. High-affinity TrkA and p75 neurotrophin receptor complexes: A twisted affair. J. Biol. Chem. 2022, 298, 101568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cintron-Colon, A.F.; Almeida-Alves, G.; VanGyseghem, J.M.; Spitsbergen, J.M. GDNF to the rescue: GDNF delivery effects on motor neurons and nerves, and muscle re-innervation after peripheral nerve injuries. Neural Regen. Res. 2022, 17, 748–753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spezia, M.C.; Dy, C.J.; Brogan, D.M. The Physiologic Basis of Molecular Therapeutics for Peripheral Nerve Injury: A Primer. J. Hand Surg. Glob. Online 2024, 6, 676–680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eggers, R.; de Winter, F.; Tannemaat, M.R.; Malessy, M.J.A.; Verhaagen, J. GDNF Gene Therapy to Repair the Injured Peripheral Nerve. Front. Bioeng. Biotechnol. 2020, 8, 583184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marin, A.; Herlea, V.; Bancu, A.; Giuglea, C.; Țăpoi, D.A.; Ciongariu, A.M.; Marin, G.G.; Marinescu, S.A.; Dobrete, N.A.; Dumitru, A.V.; et al. Correlation Between the Clinical and Histopathological Results in Experimental Sciatic Nerve Defect Surgery. Medicina 2025, 61, 317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Liu, X.; Wang, Y. Evaluation of Platelet-Rich Plasma Therapy for Peripheral Nerve Regeneration: A Critical Review of Literature. Front. Front. Bioeng. Biotechnol. 2022, 10, 808248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.L.; Liu, X.L.; Kang, Z.C.; Wang, Y.S. Platelet-rich plasma promotes peripheral nerve regeneration after sciatic nerve injury. Neural Regen. Res. 2023, 18, 375–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kemp, S.W.P.; Walsh, S.K.; Midha, R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol. Res. 2008, 30, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Shakhbazau, A.; Mohanty, C.; Shcharbin, D.; Bryszewska, M.; Caminade, A.M.; Majoral, J.P.; Alant, J.; Midha, R. Doxycycline-regulated GDNF expression promotes axonal regeneration and functional recovery in transected peripheral nerve. J. Control Release 2013, 172, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Hoyng, S.A.; Gnavi, S.; de Winter, F.; Eggers, R.; Ozawa, T.; Zaldumbide, A.; Hoeben, R.C.; Malessy, M.J.; Verhaagen, J. Developing a potentially immunologically inert tetracycline-regulatable viral vector for gene therapy in the peripheral nerve. Gene Ther. 2014, 21, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, L.M.; Ee, X.; Iyer, N.; Hunter, D.; Mackinnon, S.E.; Wood, M.D.; Sakiyama-Elbert, S.E. Finely Tuned Temporal and Spatial Delivery of GDNF Promotes Enhanced Nerve Regeneration in a Long Nerve Defect Model. Tissue Eng. Part A 2015, 21, 2852–2864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.-F. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Hsu, M.N.; Liao, H.T.; Truong, V.A.; Huang, K.L.; Yu, F.J.; Chen, H.H.; Nguyen, T.K.N.; Makarevich, P.; Parfyonova, Y.; Hu, Y.C. CRISPR-based Activation of Endogenous Neurotrophic Genes in Adipose Stem Cell Sheets to Stimulate Peripheral Nerve Regeneration. Theranostics 2019, 9, 6099–6111. Available online: https://www.thno.org/v09p6099.htm (accessed on 20 February 2025). [CrossRef] [PubMed]
- Lanier, S.T.; Hill, J.R.; Dy, C.J.; Brogan, D.M. Evolving Techniques in Peripheral Nerve Regeneration. J. Hand Surg. Am. 2021, 46, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Huang, S.X.; Strickland, A.; Doan, R.A.; Summers, D.W.; Mao, X.; Park, J.; DiAntonio, A.; Milbrandt, J. Gene therapy targeting SARM1 blocks pathological axon degeneration in mice. J. Exp. Med. 2019, 216, 294–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hughes, R.O.; Bosanac, T.; Mao, X.; Engber, T.M.; DiAntonio, A.; Milbrandt, J.; Devraj, R.; Krauss, R. Small Molecule SARM1 Inhibitors Recapitulate the SARM1-/- Phenotype and Allow Recovery of a Metastable Pool of Axons Fated to Degenerate. Cell Rep. 2021, 34, 108588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Annacontini, L.; Carbone, A.; Beccia, E.; Cecchino, L.R.; Parisi, D.; Di Gioia, S.; Lembo, F.; Angiolillo, A.; Mastrangelo, F.; et al. The Role of Adipose-Derived Stem Cells, Dermal Regenerative Templates, and Platelet-Rich Plasma in Tissue Engineering-Based Treatments of Chronic Skin Wounds. Stem. Cells Int. 2020, 2020, 7056261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.; Ikemoto, T.; Tokunaga, T.; Okikawa, S.; Miyazaki, K.; Yamada, S.; Saito, Y.; Morine, Y.; Shimada, M. Newly Generated 3D Schwann-Like Cell Spheroids From Human Adipose-Derived Stem Cells Using a Modified Protocol. Cell Transpl. 2022, 31, 9636897221093312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masgutov, R.; Masgutova, G.; Mullakhmetova, A.; Zhuravleva, M.; Shulman, A.; Rogozhin, A.; Syromiatnikova, V.; Andreeva, D.; Zeinalova, A.; Idrisova, K.; et al. Adipose-Derived Mesenchymal Stem Cells Applied in Fibrin Glue Stimulate Peripheral Nerve Regeneration. Front. Med. 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathot, F.; Rbia, N.; Bishop, A.T.; Hovius, S.E.R.; Shin, A.Y. Adipose derived mesenchymal stem cells seeded onto a decellularized nerve allograft enhances angiogenesis in a rat sciatic nerve defect model. Microsurgery 2020, 40, 585–592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathot, F.; Saffari, T.M.; Rbia, N.; Nijhuis, T.H.J.; Bishop, A.T.; Hovius, S.E.R.; Shin, A.Y. Functional Outcomes of Nerve Allografts Seeded with Undifferentiated and Differentiated Mesenchymal Stem Cells in a Rat Sciatic Nerve Defect Model. Plast. Reconstr. Plast. Reconstr. Surg. 2021, 148, 354–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rbia, N.; Bulstra, L.F.; Thaler, R.; Hovius, S.E.R.; van Wijnen, A.J.; Shin, A.Y. In Vivo Survival of Mesenchymal Stromal Cell-Enhanced Decellularized Nerve Grafts for Segmental Peripheral Nerve Reconstruction. J. Hand Surg. Am. 2019, 44, e1–e514. [Google Scholar] [CrossRef] [PubMed]
- Saffari, T.M.; Mathot, F.; Thaler, R.; van Wijnen, A.J.; Bishop, A.T.; Shin, A.Y. Microcomputed analysis of nerve angioarchitecture after combined stem cell delivery and surgical angiogenesis to nerve allograft. J. Plast. Reconstr. Aesthet Surg. 2021, 74, 1919–1930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koplay, T.G.; Yildiran, G.; Dursunoglu, D.; Aktan, M.; Duman, S.; Akdag, O.; Karamese, M.; Tosun, Z. The Effects of Adipose-Derived Mesenchymal Stem Cells and Adipose-Derived Mesenchymal Stem Cell-Originating Exosomes on Nerve Allograft Regeneration: An Experimental Study in Rats. Ann. Plast. Surg. 2023, 90, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, G.; Huang, T.C.T.; Li, J.; Long, Z.; Reisdorf, R.; Shin, A.Y.; Amadio, P.; Behfar, A.; Zhao, C.; et al. Enhancing Functional Recovery after Segmental Nerve Defect Using Nerve Allograft Treated with Plasma-Derived Exosome. Plast. Reconstr. Plast. Reconstr. Surg. 2023, 152, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Mutlu, E.C.; Ahmed, Z.; Ben-Nissan, B.; Stamboulis, A. Applications of Stem Cell-Derived Extracellular Vesicles in Nerve Regeneration. Int. J. Mol. Sci. 2024, 25, 5863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salehpour, A.; Karimi, Z.; Ghasemi Zadeh, M.; Afshar, M.; Kameli, A.; Mooseli, F.; Zare, M.; Afshar, A. Therapeutic potential of mesenchymal stem cell-derived exosomes and miRNAs in neuronal regeneration and rejuvenation in neurological disorders: A mini review. Front. Cell Neurosci. 2024, 18, 1427525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Zhang, F.; Fu, X.; Han, N. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury. Int. J. Mol. Sci. 2024, 25, 7882. [Google Scholar] [CrossRef]
- Namini, M.S.; Daneshimehr, F.; Beheshtizadeh, N.; Mansouri, V.; Ai, J.; Jahromi, H.K.; Ebrahimi-Barough, S. Cell-free therapy based on extracellular vesicles: A promising therapeutic strategy for peripheral nerve injury. Stem. Cell Res. Ther. 2023, 14, 254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dogny, C.; André-Lévigne, D.; Kalbermatten, D.F.; Madduri, S. Therapeutic Potential and Challenges of Mesenchymal Stem Cell-Derived Exosomes for Peripheral Nerve Regeneration: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 6489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerout, N.; Paviot, A.; Bon-Mardion, N.; Honoré, A.; Obongo, R.; Duclos, C.; Marie, J.P. Transplantation of olfactory ensheathing cells to evaluate functional recovery after peripheral nerve injury. J. Vis. Exp. 2014, 84, e50590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.P.; Liao, J.X.; Liu, Y.Y.; Luo, H.L.; Zhang, W.J. Potential therapeutic effect of olfactory ensheathing cells in neurological diseases: Neurodegenerative diseases and peripheral nerve injuries. Front. Immunol. 2023, 14, 1280186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delarue, Q.; Guérout, N. Transplantation of Olfactory Ensheathing Cells: Properties and Therapeutic Effects after Transplantation into the Lesioned Nervous System. Neuroglia 2022, 3, 1–22. [Google Scholar] [CrossRef]
- Yang, H.; He, B.R.; Hao, D.J. Biological roles of olfactory ensheathing cells in facilitating neural regeneration: A systematic review. Mol. Neurobiol. 2015, 51, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Radtke, C.; Wewetzer, K.; Reimers, K.; Vogt, P.M. Transplantation of olfactory ensheathing cells as adjunct cell therapy for peripheral nerve injury. Cell Transpl. 2011, 20, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Radtke, C.; Aizer, A.A.; Agulian, S.K.; Lankford, K.L.; Vogt, P.M.; Kocsis, J.D. Transplantation of olfactory ensheathing cells enhances peripheral nerve regeneration after microsurgical nerve repair. Brain Res. 2009, 1254, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.H.; Kim, B.Y.; Jang, D.H.; Choi, S.W.; Joen, S.H.; Kim, H.; Lee, S.U. Peripheral Nerve Regeneration Using a Nerve Conduit with Olfactory Ensheathing Cells in a Rat Model. Tissue Eng. Regen. Med. 2021, 18, 453–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wang, W.T.; Gong, C.R.; Li, C.; Shi, M. Combination of olfactory ensheathing cells and human umbilical cord mesenchymal stem cell-derived exosomes promotes sciatic nerve regeneration. Neural Regen. Res. 2020, 15, 1903–1911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bota, O.; Fodor, L. The influence of drugs on peripheral nerve regeneration. Drug Metab. Rev. 2019, 51, 266–292. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving peripheral nerve regeneration: From molecular mechanisms to potential therapeutic targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, G.N.; Megaloikonomos, P.D.; Mavrogenis, A.F. The Present and Future for Peripheral Nerve Regeneration. Orthopedics 2017, 40, e141–e156. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; English, A.W. Strategies to promote peripheral nerve regeneration: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2016, 43, 336–350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abushukur, Y.; Knackstedt, R. The Impact of Supplements on Recovery After Peripheral Nerve Injury: A Review of the Literature. Cureus 2022, 14, e25135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agon, M.; Ymer, M. The Role of Pharmacological Agents in Nerve Regeneration after Peripheral Nerve Repair. Front. Immunol. 2023, 13, 1084101. [Google Scholar] [CrossRef]
- Poshekhontseva, V.Y.; Fokina, V.V.; Tarlachkov, S.V.; Machulin, A.V.; Shutov, A.A.; Donova, M.V. Streptomyces tsukubensis VKM Ac-2618D-an Effective Producer of Tacrolimus. Appl. Biochem. Microbiol. 2021, 57, 939–948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyons, W.E.; George, E.B.; Dawson, T.M.; Steiner, J.P.; Snyder, S.H. Immunosuppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc. Natl. Acad. Sci. USA 1994, 91, 3191–3195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gold, B.G.; Katoh, K.; Storm-Dickerson, T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J. Neurosci. 1995, 15, 7509–7516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeh, C.; Bowers, D.; Hadlock, T.A. Effect of FK506 on functional recovery after facial nerve injury in the rat. Arch. Facial. Plast. Surg. 2007, 9, 333–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shim, S.; Yuan, J.P.; Kim, J.Y.; Zeng, W.; Huang, G.; Milshteyn, A.; Kern, D.; Muallem, S.; Ming, G.L.; Worley, P.F. Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of neuronal growth cones via regulation of TRPC1 channel opening. Neuron 2009, 64, 471–483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toll, E.C.; Seifalian, A.M.; Birchall, M.A. The role of immunophilin ligands in nerve regeneration. Regen. Med. 2011, 6, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Konofaos, P.; Terzis, J.K. FK506 and nerve regeneration: Past, present, and future. J. Reconstr. Microsurg. 2013, 29, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Cao, Q.; Sui, T.; Du, S.; Kong, D.; Cao, X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 2013, 4, e526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arun, A.; Abt, N.B.; Tuffaha, S.; Brandacher, G.; Barone, A.A.L. Nerve regeneration in vascularized composite allotransplantation: Current strategies and future directions. Plast. Aesthet. Res. 2015, 2, 226–235. [Google Scholar] [CrossRef]
- Xue, J.W.; Jiao, J.B.; Liu, X.F.; Jiang, Y.T.; Yang, G.; Li, C.Y.; Yin, W.T.; Ling, L. Inhibition of Peripheral Nerve Scarring by Calcium Antagonists, Also Known as Calcium Channel Blockers. Artif. Organs. 2016, 40, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.G.; Zhang, L.L.; Agresti, M.; Logiudice, J.; Yan, Y.H.; Wang, Z.; Sanger, J.R.; Matloub, H.S. The effect of calcium modulating agents on peripheral nerve recovery after crush. J. Neurosci. Methods 2013, 217, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Rishal, I.; Fainzilber, M. Retrograde signaling in axonal regeneration. Exp. Neurol. 2010, 223, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Huang, C.Y. The effect of combined steroid and calcium channel blocker injection on human hypertrophic scars in animal model: A new strategy for the treatment of hypertrophic scars. Dermatol. Surg. 2010, 36, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P. Calcium transport and buffering in neurons. Trends Neurosci. 1988, 11, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Siesjö, B.K. Pathophysiology and treatment of focal cerebral ischemia. Part II: Mechanisms of damage and treatment. J. Neurosurg. 1992, 77, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Tymianski, M.; Tator, C.H. Normal and abnormal calcium homeostasis in neurons: A basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery 1996, 38, 1176–1195. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Lewis, J.M.; Siman, R. Spectrin proteolysis in the hippocampus: A biochemical marker for neuronal injury and neuroprotection. Ann. N. Y. Acad. Sci. 1993, 679, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A. Excitatory amino acid receptors, neural membrane phospholipid metabolism and neurological disorders. Brain Res. Brain Res. Rev. 1991, 16, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.J.; Brorson, J.R.; Bleakman, D.; Chard, P.S.; Miller, R.J. Calcium influx and neurodegeneration. Ann. N. Y. Acad. Sci. 1993, 679, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.G.; Zhang, L.L.; Agresti, M.A.; Shen, F.; Matloub, H.S.; Yan, Y.; Li, J.; Gu, Y.D.; Logiudice, J.A.; Havlik, R. Effect of calcitonin on cultured schwann cells. Muscle Nerve 2017, 56, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, C.; Hai, B.; Ma, T.; Zhang, W.; Tan, J.; Fu, X.; Wang, H.; Xu, Y.; Song, C. Chitosan conduits filled with simvastatin/Pluronic F-127 hydrogel promote peripheral nerve regeneration in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A.; Zahri, S.; Bayrami, A. Rosuvastatin enhanced functional recovery after sciatic nerve injury in the rat. Eur. J. Pharmacol. 2020, 882, 173260. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, N.; Xu, Y.; Zhu, J.; Chen, Z.; Liu, Z.; Dang, G.; Song, C. Simvastatin treatment improves functional recovery after experimental spinal cord injury by upregulating the expression of BDNF and GDNF. Neurosci. Lett. 2011, 487, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Yang, D.Y.; Ou, Y.C.; Ho, S.P.; Cheng, F.C.; Chen, C.J. Neuroprotective effect of atorvastatin in an experimental model of nerve crush injury. Neurosurgery 2010, 67, 376–388; discussion 388-9. [Google Scholar] [CrossRef] [PubMed]
- Dincer, U.; Verim, A.; Becerik, Ç.; Gürsan, N.; Tepe Karaca, Ç.; Toros, S.Z. The Effect of Rosuvastatin on Facial Nerve Regeneration After Facial Nerve Injury: An Experimental Animal Study. Ann. Otol. Rhinol. Laryngol. 2025, 134, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ghayour, M.B.; Abdolmaleki, A.; Rassouli, M.B. Neuroprotective effect of Lovastatin on motor deficit induced by sciatic nerve crush in the rat. Eur. J. Pharmacol. 2017, 812, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Yoon, G.H.; Chung, S.S.; Abid, M.N.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol. Psychiatry 2017, 22, 407–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tekdemir, E.; Tatlipinar, A.; Özbeyli, D.; Tekdemir, Ö.; Kınal, E. The effects of lipoic acid and methylprednisolone on nerve healing in rats with facial paralysis. Acta Otolaryngol. 2018, 138, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Guido, H.; Aalt, B. Lipoic Acid: A Multifunctional Nutraceutical; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar] [CrossRef]
- Azizi, S.; Heshmatian, B.; Amini, K.; Raisi, A.; Azimzadeh, M. Alpha-lipoic acid loaded in chitosan conduit enhances sciatic nerve regeneration in rat. Iran J. Basic Med. Sci. 2015, 18, 228–233. [Google Scholar] [PubMed] [PubMed Central]
- Horasanli, B.; Hasturk, A.E.; Arikan, M.; Togral, G.; Helvacioglu, F.; Dagdeviren, A.; Mut, S.; Harman, F.; Argun, G. Comparative evaluation of the electrophysiological, functional and ultrastructural effects of alpha lipoic acid and cyanocobalamin administration in a rat model of sciatic nerve injury. J. Back Musculoskelet. Rehabil. 2017, 30, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, K.M.; Hackshaw, K.V. Nutritional Supplements for the Treatment of Neuropathic Pain. Biomedicines 2021, 9, 674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stein, J.; Geisel, J.; Obeid, R. Association between neuropathy and B-vitamins: A systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A.; Yang, J.; Zhang, G.B.; Feng, Y.H.; Wang, F.; Zhou, P.Y. Effect of mecobalamin treatment on the recovery of patients with posterior communicating artery aneurysm inducing oculomotor nerve palsy after operation. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2603–2607. [Google Scholar] [PubMed]
- Baltrusch, S. The Role of Neurotropic B Vitamins in Nerve Regeneration. Biomed. Res. Int. 2021, 2021, 9968228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, F.; Xu, K.; Liu, L.; Zhang, K.; Xia, L.; Zhang, M.; Teng, C.; Tong, H.; He, Y.; Xue, Y.; et al. Vitamin B12 Enhances Nerve Repair and Improves Functional Recovery After Traumatic Brain Injury by Inhibiting ER Stress-Induced Neuron Injury. Front. Pharmacol. 2019, 10, 406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, H.; Yang, T.; Li, Q.; Zhu, Z.; Wang, L.; Bai, G.; Li, D.; Li, Q.; Wang, W. Dexamethasone and vitamin B(12) synergistically promote peripheral nerve regeneration in rats by upregulating the expression of brain-derived neurotrophic factor. Arch. Med. Sci. 2012, 8, 924–930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gan, L.; Qian, M.; Shi, K.; Chen, G.; Gu, Y.; Du, W.; Zhu, G. Restorative effect and mechanism of mecobalamin on sciatic nerve crush injury in mice. Neural Regen. Res. 2014, 9, 1979–1984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jolivalt, C.G.; Mizisin, L.M.; Nelson, A.; Cunha, J.M.; Ramos, K.M.; Bonke, D.; Calcutt, N.A. B vitamins alleviate indices of neuropathic pain in diabetic rats. Eur. J. Pharmacol. 2009, 612, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Al-Saaeed, S.M.; Ali, H.A.; Ali, S.M.; Ali, S.A. Vitamins B therapy in regeneration of peripheral neuropathy associated with lipid profile. J. Phys. Conf. Ser. 2019, 1279, 012016. [Google Scholar]
- Manto, K.M.; Govindappa, P.K.; Martinazzi, B.; Han, A.; Hegarty, J.P.; Koroneos, Z.; Talukder, M.A.H.; Elfar, J.C. Erythropoietin-PLGA-PEG as a local treatment to promote functional recovery and neurovascular regeneration after peripheral nerve injury. J. Nanobiotechnol. 2022, 20, 461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, Z.S.; Zhang, H.; Bo, W.; Gao, W. Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats. AJNR Am. J. Neuroradiol. 2010, 31, 509–515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundem, L.; Chris Tseng, K.C.; Li, H.; Ketz, J.; Noble, M.; Elfar, J. Erythropoietin Enhanced Recovery After Traumatic Nerve Injury: Myelination and Localized Effects. J. Hand Surg. Am. 2016, 41, 999–1010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brzezinski, A. Melatonin in humans. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, A.Y.; Morina, A.A.; Bytyqi, C.I.; Mekaj, Y.H.; Duci, S.B. Application of topical pharmacological agents at the site of peripheral nerve injury and methods used for evaluating the success of the regenerative process. J. Orthop. Surg. Res. 2014, 9, 94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Kaya, Y.; Sarıkcıoğlu, L.; Aslan, M.; Kencebay, C.; Demir, N.; Derin, N.; Angelov, D.N.; Yıldırım, F.B. Comparison of the beneficial effect of melatonin on recovery after cut and crush sciatic nerve injury: A combined study using functional, electrophysiological, biochemical, and electron microscopic analyses. Childs Nerv. Syst. 2013, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Rateb, E.E.; Amin, S.N.; El-Tablawy, N.; Rashed, L.A.; El-Attar, S. Effect of melatonin supplemented at the light or dark period on recovery of sciatic nerve injury in rats. EXCLI J. 2017, 16, 138–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atik, B.; Erkutlu, I.; Tercan, M.; Buyukhatipoglu, H.; Bekerecioglu, M.; Pence, S. The effects of exogenous melatonin on peripheral nerve regeneration and collagen formation in rats. J. Surg. Res. 2011, 166, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Turgut, M.; Uyanikgil, Y.; Baka, M.; Tunç, A.T.; Yavaşoğlu, A.; Yurtseven, M.E.; Kaplan, S. Pinealectomy exaggerates and melatonin treatment suppresses neuroma formation of transected sciatic nerve in rats: Gross morphological, histological and stereological analysis. J. Pineal Res. 2005, 38, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, H.; Huang, H.; Bi, W.; Yan, R.; Tan, X.; Wen, W.; Wang, C.; Song, W.; Zhang, Y.; et al. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol. Med. Rep. 2018, 17, 4360–4368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikeda, K.; Yamauchi, D.; Osamura, N.; Hagiwara, N.; Tomita, K. Hyaluronic acid prevents peripheral nerve adhesion. Br. J. Plast. Surg. 2003, 56, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Smit, X.; van Neck, J.W.; Afoke, A.; Hovius, S.E. Reduction of neural adhesions by biodegradable autocrosslinked hyaluronic acid gel after injury of peripheral nerves: An experimental study. J. Neurosurg. 2004, 101, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Ozgenel, G.Y. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery 2003, 23, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, K.; Tanaka, H.F.; Ohkochi, H.; Miyasaka, M.; Yamanokuchi, H.; Yoshidad, K.; Yoshida, T. Hyaluronan tetrasaccharide promotes regeneration of peripheral nerve: In vivo analysis by film model method. Brain Res. 2011, 1385, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, J.A.; Warnke, P.H.; Pan, Y.C.; Shenaq, S. Increased axonal regeneration through a biodegradable amnionic tube nerve conduit: Effect of local delivery and incorporation of nerve growth factor/hyaluronic acid media. Ann. Plast. Surg. 2000, 44, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zor, F.; Deveci, M.; Kilic, A.; Ozdag, M.F.; Kurt, B.; Sengezer, M.; Sönmez, T.T. Effect of VEGF gene therapy and hyaluronic acid film sheath on peripheral nerve regeneration. Microsurgery 2014, 34, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, W.; Lee, J.S.; Youn, S.J.; Lee, H.; Baik, M.Y. Enhanced Antioxidant Capacity of Puffed Turmeric (Curcuma longa L.) by High Hydrostatic Pressure Extraction (HHPE) of Bioactive Compounds. Foods 2020, 9, 1690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suh, H.W.; Kang, S.; Kwon, K.S. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol. Cell Biochem. 2007, 298, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Omidi, A.; Karbalay-Doust, S. Curcumin protects the dorsal root ganglion and sciatic nerve after crush in rat. Pathol. Res. Pract. 2011, 207, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Chantemargue, B.; Richard, L.; Vignaud, L.; Favreau, F.; Faye, P.A.; Vignoles, P.; Sturtz, F.; Trouillas, P.; Vallat, J.M.; et al. Local low dose curcumin treatment improves functional recovery and remyelination in a rat model of sciatic nerve crush through inhibition of oxidative stress. Neuropharmacology 2018, 139, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, H.; Liu, J.; Chen, Y.; Wang, Q.; Xiang, L. Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. Neurosci. Lett. 2016, 610, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yao, Z.; Zhao, Y.; Zhang, T.; Wang, J.; Li, S.; Chen, Z. Electrical stimulation therapy for peripheral nerve injury. Front. Neurol. 2023, 14, 1081458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juckett, L.; Saffari, T.M.; Ormseth, B.; Senger, J.L.; Moore, A.M. The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury. Biomolecules 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, X.L.; Song, X.Z.; Li, Q.; Li, Y.R.; He, F.; Gu, X.S.; Ming, D. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen. Res. 2022, 17, 2185–2193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gisbert Roca, F.; Serrano Requena, S.; Monleón Pradas, M.; Martínez-Ramos, C. Electrical Stimulation Increases Axonal Growth from Dorsal Root Ganglia Co-Cultured with Schwann Cells in Highly Aligned PLA-PPy-Au Microfiber Substrates. Int. J. Mol. Sci. 2022, 23, 6362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Q.; Nasser, M.I.; He, J.; Deng, G.; Ouyang, Q.; Zhuang, D.; Deng, Y.; Hu, H.; Liu, N.; Li, Z.; et al. Engineered Schwann Cell-Based Therapies for Injury Peripheral Nerve Reconstruction. Front. Cell Neurosci. 2022, 16, 865266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics 2016, 13, 295–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, H.L.; Chiang, C.Y.; Lu, Z.H.; Cheng, F.C.; Chen, C.J.; Sheu, M.L.; Sheehan, J.; Pan, H.C. Late administration of high-frequency electrical stimulation increases nerve regeneration without aggravating neuropathic pain in a nerve crush injury. BMC Neurosci. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, P.; Li, C.; Yang, C.; Sun, M.; Hou, H.; Guan, Y.; Chen, J.; Liu, S.; Chen, K.; Ma, Y.; et al. A biodegradable and flexible neural interface for transdermal optoelectronic modulation and regeneration of peripheral nerves. Nat. Commun. 2024, 15, 4721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Witt, A.; Kristensen, R.S.; Fuglsang-Frederiksen, A.; Pedersen, T.H.; Finnerup, N.B.; Kasch, H.; Tankisi, H. Muscle velocity recovery cycles in neurogenic muscles. Clin. Neurophysiol. 2019, 130, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.E.S.; Jeyaratnam, S.; Lim, A.Y.T. Updates in peripheral nerve surgery of the upper extremity: Diagnosis and treatment options. Ann. Transl. Med. 2023, 11, 391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, R.H. Repair of the trigeminal nerve: A review. Aust. Dent. J. 2010, 55, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wolford, L.M.; Rodrigues, D.B. Autogenous grafts/allografts/conduits for bridging peripheral trigeminal nerve gaps. Atlas Oral Maxillofac Surg. Clin. N. Am. 2011, 19, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Matus, G.; Aravena, J.P.; Mariño, D.; Niklander, S.E. Decellularized allografts as an alternative for reconstruction of large inferior alveolar nerve defects: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2023, 28, e183–e190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, H.; Lin, Q.; Rui, X.; Huang, Y.; Wu, X.; Yang, W.; Yu, Z.; He, W. Research status of facial nerve repair. Regen. Ther. 2023, 24, 507–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, J.; Fisher, D. Facial nerve reconstruction using acellular nerve allograft. J. Craniofac. Surg. 2022, 33, e413–e414. [Google Scholar] [CrossRef]
- Aycart, M.A.; Perry, B.; Alhefzi, M.; Bueno, E.M.; Kueckelhaus, M.; Fischer, S.; Pomahac, B. Surgical Optimization of Motor Recovery in Face Transplantation. J. Craniofac. Surg. 2016, 27, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.E.; Ng, N.Y.; Riehle, M.O.; Kingham, P.J.; Dahlin, L.B.; Wiberg, M.; Hart, A.M. Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb. Cochrane Database Syst. Rev. 2022, 12, CD012574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rinker, B. Nerve Transfers in the Upper Extremity: A Practical User’s Guide. Ann. Plast. Surg. 2015, 74 (Suppl. S4), S222–S228. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.A.; Larocerie-Salgado, J.; Ross, D.C.; Miller, T.A.; Pripotnev, S. Assessment, patient selection, and rehabilitation of nerve transfers. Front. Rehabil. Sci. 2023, 4, 1267433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dubernard, J.M.; Petruzzo, P.; Lanzetta, M.; Parmentier, H.; Martin, X.; Dawahra, M.; Hakim, N.S.; Owen, E. Functional results of the first human double-hand transplantation. Ann. Surg. 2003, 238, 128–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suchyta, M.A.; Sabbagh, M.D.; Morsy, M.; Mardini, S.; Moran, S.L. Advances in peripheral nerve regeneration as it relates to vascularized composite allotransplantation. Vasc. Compos. Allotransplant. 2016, 3, 75–88. [Google Scholar] [CrossRef][Green Version]
- Elliott, R.M.; Tintle, S.M.; Levin, L.S. Upper extremity transplantation: Current concepts and challenges in an emerging field. Curr. Rev. Musculoskelet. Med. 2014, 7, 83–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheesborough, J.E.; Smith, L.H.; Kuiken, T.A.; Dumanian, G.A. Targeted muscle reinnervation and advanced prosthetic arms. Semin. Plast. Surg. 2015, 29, 62–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bates, T.J.; Fergason, J.R.; Pierrie, S.N. Technological Advances in Prosthesis Design and Rehabilitation Following Upper Extremity Limb Loss. Curr. Rev. Musculoskelet. Med. 2020, 13, 485–493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubiak, C.A.; Etra, J.W.; Brandacher, G.; Kemp, S.W.P.; Kung, T.A.; Lee, W.P.A.; Cederna, P.S. Prosthetic Rehabilitation and Vascularized Composite Allotransplantation following Upper Limb Loss. Plast. Reconstr. Plast. Reconstr. Surg. 2019, 143, 1688–1701. [Google Scholar] [CrossRef] [PubMed]
- Thatte, M.R.; Hiremath, A.; Goklani, M.S.; Patel, N.R.; Takwale, A.B. Peripheral Nerve Injury to the Lower Limb: Repair and Secondary Reconstruction. Indian J. Plast. Surg. 2019, 52, 93–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lezak, B.; Massel, D.H.; Varacallo, M.A. Peroneal Nerve Injury. [Updated 2024 February 25]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549859/ (accessed on 20 February 2025).
- Glazebrook, M.A.; Paletz, J.L. Treatment of posttraumatic injuries to the nerves in the foot and ankle. Foot Ankle Clin. 2006, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dellon, A.L. Reconstruction of a painful post-traumatic medial plantar neuroma with a bioabsorbable nerve conduit: A case report. J. Foot Ankle Surg. 2001, 40, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Purnell, C.A.; Cheesborough, J.E.; Kelikian, A.S.; Dumanian, G.A. Treatment of Foot and Ankle Neuroma Pain With Processed Nerve Allografts. Foot Ankle Int. 2016, 37, 1098–1105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Ray, W.Z.; Chenard, K.E.; Tung, T.; Mackinnon, S.E. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand 2009, 4, 239–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, A.M.; Aral, A.M.; Zamora, R.; Gharpure, N.; El-Dehaibi, F.; Zor, F.; Kulahci, Y.; Karagoz, H.; Barclay, D.A.; Yin, J.; et al. Peripheral nerve repair is associated with augmented cross-tissue inflammation following vascularized composite allotransplantation. Front. Immunol. 2023, 14, 1151824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Premera Blue Cross. Medical Policy 7.01.584—Nerve Repair for Peripheral Nerve Injuries Using Synthetic Conduits or Allografts. Updated 23 December 2024. Effective 1 January 2025. Available online: https://www.premera.com/wa/provider/reference/hmo-medical-policies-search/ (accessed on 6 April 2025).
- Safa, B.; Buncke, G. Autograft Substitutes: Conduits and Processed Nerve Allografts. Hand Clin. 2016, 32, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Axogen: Avance Nerve Graft. Available online: https://axogeninc.eu/avance-nerve-graft/ (accessed on 7 April 2025).
- Axogen:Axoguard Nerve Connector. Available online: https://axogeninc.eu/axoguard-nerve-connector/ (accessed on 7 April 2025).
- Axogen: Axoguard Nerve Protector. Available online: https://axogeninc.eu/axoguard-nerve-protector/ (accessed on 7 April 2025).
- Integra LifeSciences: NeuraGen® Nerve Guide. Available online: https://products.integralife.com/neuragen-nerve-guide/product/nerve-tendon-neuragen-nerve-guide (accessed on 7 April 2025).
- Integra LifeSciences: NeuraGen® 3D Nerve Guide Matrix. Available online: https://products.integralife.com/neuragen-3d-nerve-guide-matrix/product/nerve-tendon-neuragen-3d-nerve-guide-matrix#CaseStudies (accessed on 7 April 2025).
- Integra LifeSciences: NeuraWrap® Nerve Protector. Available online: https://products.integralife.com/neurawrap-nerve-protector/product/nerve-tendon-neurawrap-nerve-protector (accessed on 7 April 2025).
- Stryker: Neuroflex. Available online: https://www.stryker.com/us/en/trauma-and-extremities/products/neuroflex.html (accessed on 7 April 2025).
- Stryker: NeuroMatrix. Available online: https://www.stryker.com/us/en/trauma-and-extremities/products/neuromatrix.html (accessed on 7 April 2025).
- Stryker: NeuroMend. Available online: https://www.stryker.com/us/en/trauma-and-extremities/products/neuromend.html (accessed on 7 April 2025).
- Regenity-Nerve Repair: Neurolac® Nerve Guide. Available online: https://regenity.com/solution/nerve-repair/ (accessed on 8 April 2025).
- Karabekmez, F.E.; Duymaz, A.; Moran, S.L. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand 2009, 4, 245–249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, M.S.; Rinker, B.D.; Weber, R.V.; Chao, J.D.; Ingari, J.V.; Brooks, D.; Buncke, G.M. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J. Hand Surg. Am. 2012, 37, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Safa, B.; Jain, S.; Desai, M.J.; Greenberg, J.A.; Niacaris, T.R.; Nydick, J.A.; Leversedge, F.J.; Megee, D.M.; Zoldos, J.; Rinker, B.D.; et al. Peripheral nerve repair throughout the body with processed nerve allografts: Results from a large multicenter study. Microsurgery 2020, 40, 527–537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, X.; Dong, Y.; Alhaskawi, A.; Zhou, H.; Ezzi, S.H.A.; Kota, V.G.; Abdulla, M.H.A.H.; Abdalbary, S.A.; Lu, H.; Wang, C. Techniques and graft materials for repairing peripheral nerve defects. Front. Neurol. 2024, 14, 1307883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frostadottir, D.; Perez, R.; Dahlin, L.B. Socioeconomic factors and outcome after repair and reconstruction of digital and major nerve trunk injuries in the upper limb. Sci. Rep. 2024, 14, 7242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Burks, S.S.; Anderson, K.D.; Dididze, M.; Khan, A.; Dietrich, W.D. The Use of Autologous Schwann Cells to Supplement Sciatic Nerve Repair with a Large Gap: First in Human Experience. Cell Transpl. 2016, 25, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Braga-Silva, J.; Gehlen, D.; Padoin, A.V.; Machado, D.C.; Garicochea, B.; Costa da Costa, J. Can local supply of bone marrow mononuclear cells improve the outcome from late tubular repair of human median and ulnar nerves? J. Hand Surg. Eur. Vol. 2008, 33, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Grimoldi, N.; Colleoni, F.; Tiberio, F.; Vetrano, I.G.; Cappellari, A.; Costa, A.; Belicchi, M.; Razini, P.; Giordano, R.; Spagnoli, D.; et al. Stem cell salvage of injured peripheral nerve. Cell Transpl. 2015, 24, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.N.; Olson, J.L.; Morhart, M.J.; Chan, K.M. Electrical stimulation enhances sensory recovery: A randomized controlled trial. Ann. Neurol. 2015, 77, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiu, X.; Wang, P.; Han, Y.; Chang, W.; Zhao, J. Intraoperative electrical stimulation promotes the short-term recovery of patients with cubital tunnel syndrome after surgery. J. Orthop. Surg. Res. 2023, 18, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, H.; Johnson, K.; Beale, S.; Miller, C. Rehabilitation of nerve injuries. In Peripheral Nerve Tissue Engineering and Regeneration; Phillips, J., Hercher, D., Hausner, T., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]

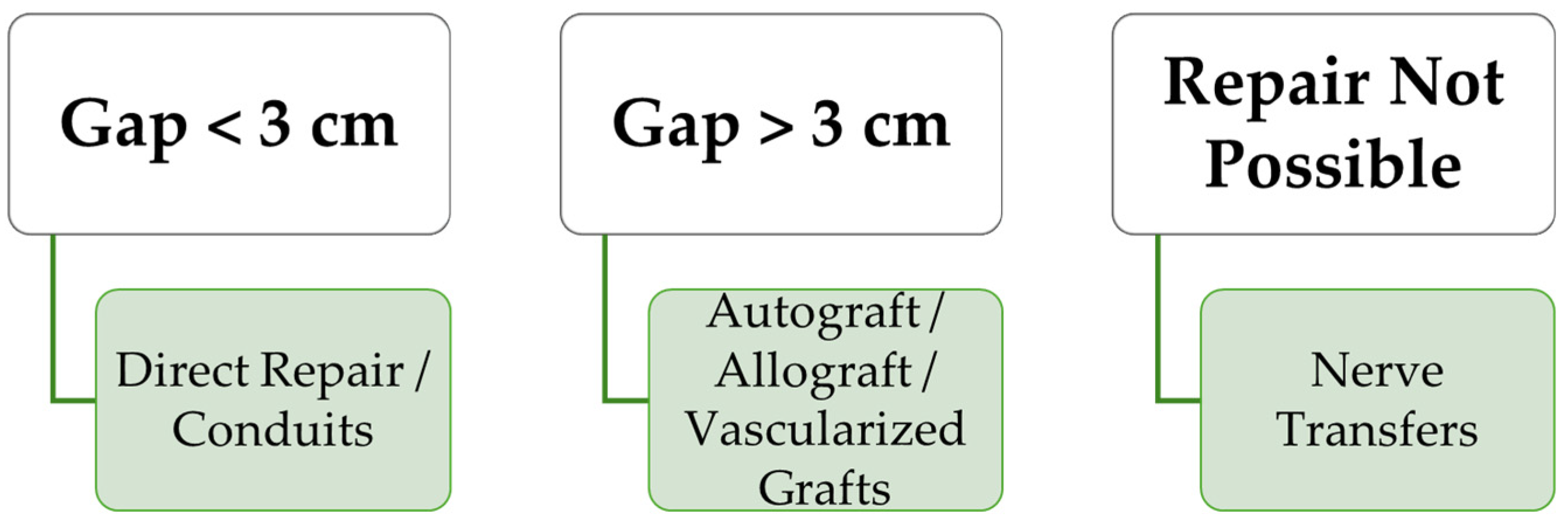

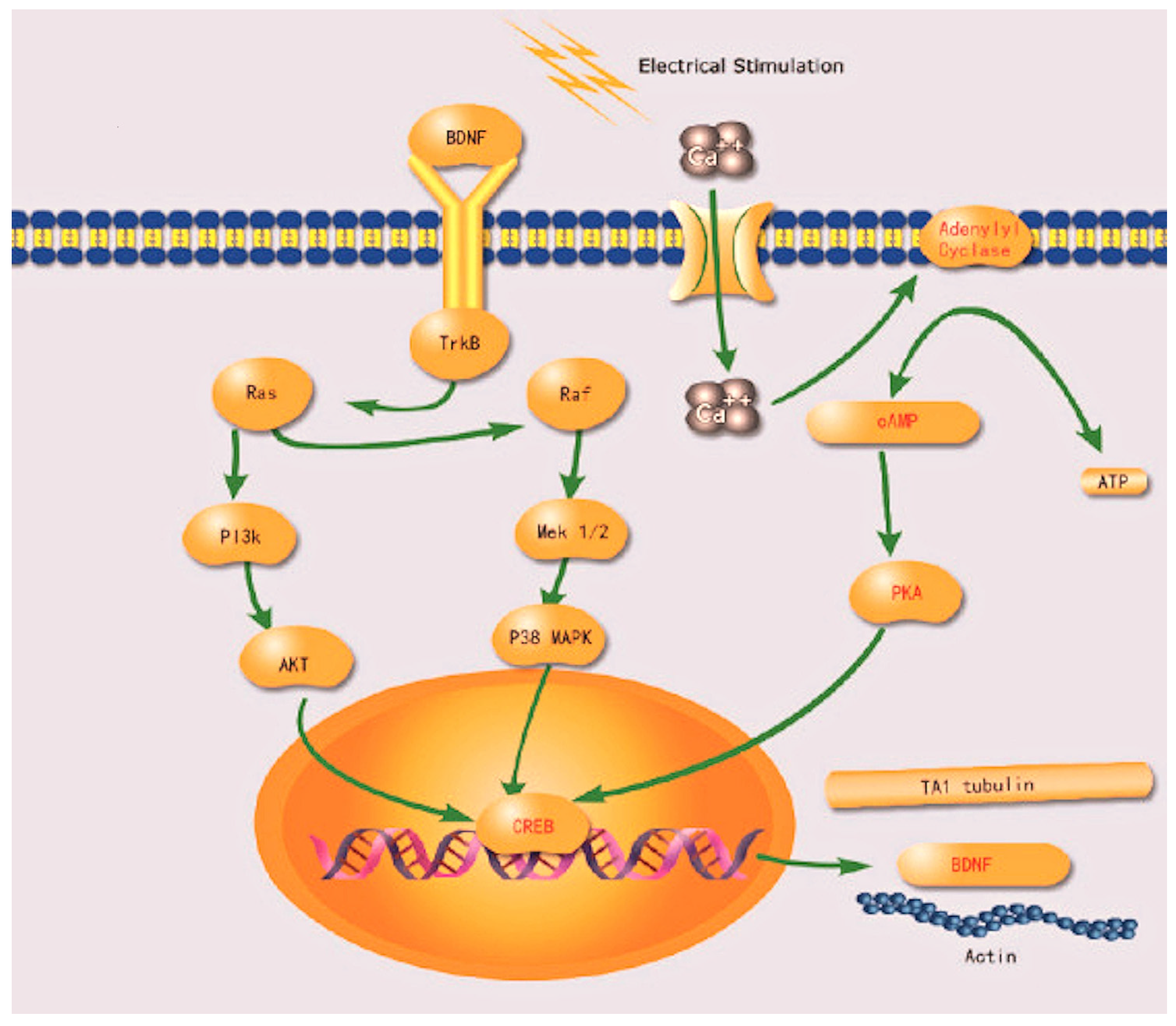
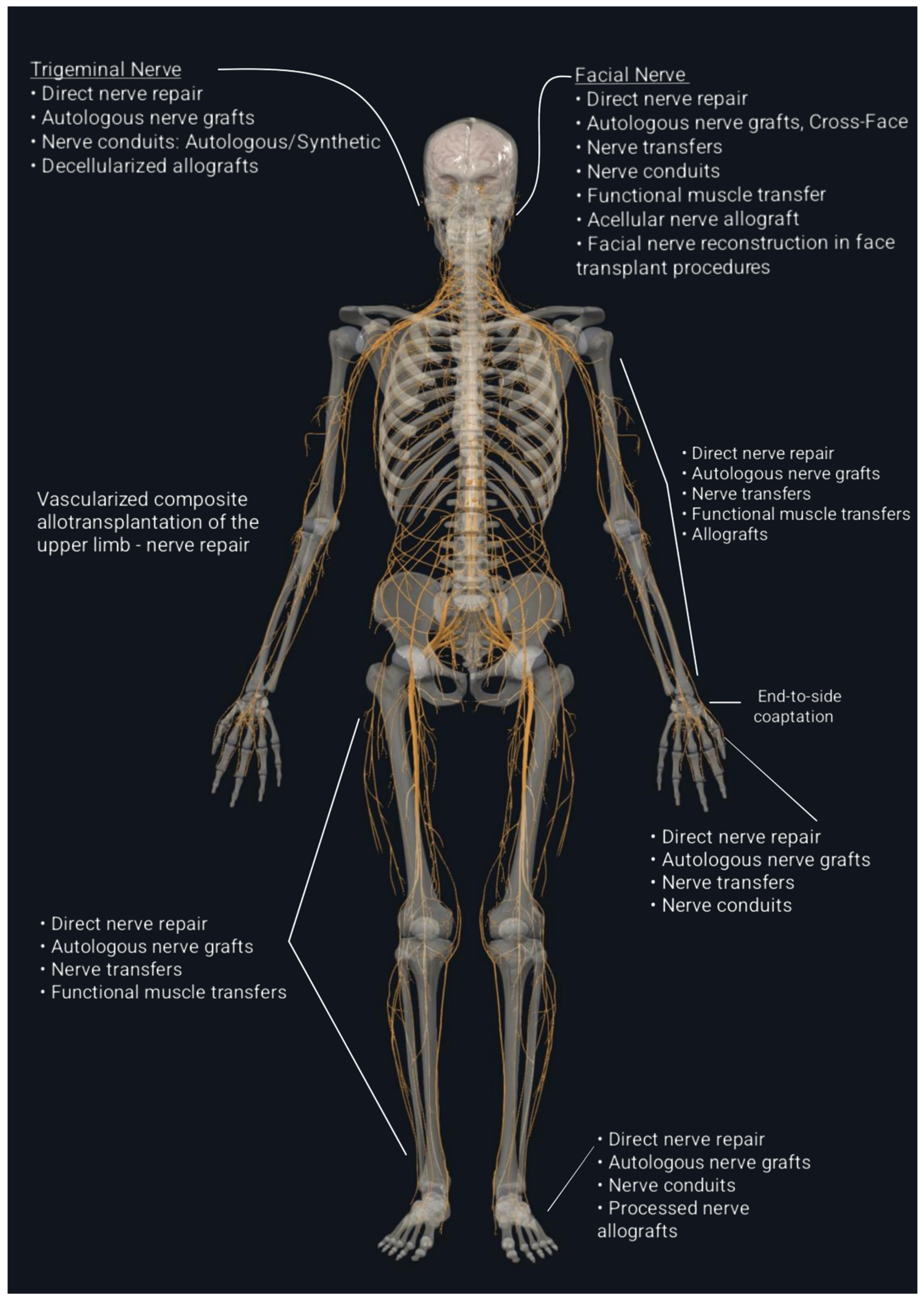
| Classification System | Type/Grade | Description | Pathophysiology | Prognosis |
|---|---|---|---|---|
| Seddon Classification | Neurapraxia | Temporary conduction block | Localized demyelination without axonal disruption. | Complete recovery expected within days to weeks. |
| Axonotmesis | Lesion in continuity with, axonal injury with intact connective tissue | Axonal damage with preservation of endoneurium, perineurium, and epineurium. Wallerian degeneration occurs distal to the injury. | Recovery possible without surgical intervention; axonal regrowth occurs at approximately 1 mm per day. | |
| Neurotmesis | Complete nerve severance | Total disruption of the nerve fiber, including axon and connective tissue. | If the nerve is completely transected, no recovery is possible without surgical repair. | |
| Sunderland Classification | Grade I | Equivalent to neurapraxia | Conduction block without structural damage. | Full recovery expected within weeks. |
| Grade II | Axonal disruption with intact endoneurium | Axonal damage with intact endoneurium; Wallerian degeneration occurs distal to the lesion. | Recovery occurs as axons regenerate along intact endoneurial sheaths. | |
| Grade III | Disruption of axon and endoneurium | Damage to axon and endoneurium, with perineurium intact; intrafascicular fibrosis may develop. | Partial recovery; misdirection of axons possible. | |
| Grade IV | Disruption of axon, endoneurium, perineurium | Preservation of only the epineurium; significant scarring impedes regeneration. | Recovery unlikely without surgical intervention. | |
| Grade V | Complete transection | Total severance of the nerve trunk, including all connective tissue structures. | Surgical intervention is essential; prognosis depends on timely and appropriate repair. | |
| Grade VI | Mixed injury (Mackinnon & Dellon) | Combination of injury severities within the same nerve. | Recovery varies; tailored surgical approaches may be necessary. |
| Nerve Conduit Type | Biomaterial | Key Properties | Clinical Applications | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Collagen Nerve Conduits | Type I collagen (from bovine or porcine sources) | Biodegradable and biocompatible Permeable Moderate mechanical strength | Repair of sensory and mixed nerves Nerve gaps up to 3 cm Commonly used in digital nerve injuries in the hand | Supports cell adhesion and migration Minimal immune response FDA-approved products available | Risk of disease transmission (animal-derived) Limited use in large nerve gaps |
| Polyglycolic Acid (PGA) Conduits | Synthetic polymer (example- Neurotube®) | Biodegradable High permeability Good flexibility | Repair of peripheral nerve gaps up to 3 cm Suitable for small-caliber nerves | Well-studied material Predictable degradation rate FDA-approved | Rapid degradation may lead to loss of support Degradation products can cause inflammation |
| Polycaprolactone (PCL) Conduits | Synthetic polymer | Biodegradable with slow degradation rate Good mechanical strength Low swelling | Repair of longer nerve gaps (up to 3 cm) Potential use in larger nerves due to mechanical strength | Long-term support for axonal growth Customizable properties through copolymerization | Slow degradation may impede tissue remodelling Limited clinical data compared to other materials |
| Chitosan Nerve Conduits | Natural polysaccharide derived from chitin | Biocompatible and biodegradable Antimicrobial properties Permeable | Experimental use in peripheral nerve injuries Potential for both sensory and motor nerve repair | Promotes nerve regeneration Low immunogenicity | Not widely available clinically Variability in material properties |
| Silicone Nerve Conduits | Non-biodegradable synthetic polymer | Biostable and non-degradable Flexible Impermeable (unless fenestrated) | Used in situations where long-term guidance is needed Historically used as a conduit before biodegradable options were available | Reusable and can be removed after regeneration Good mechanical protection | Requires second surgery for removal Risk of compression neuropathy due to non-degradability |
| Polyurethane Nerve Conduits | Synthetic polymer | Biodegradable Elastic and flexible Tunable permeability | Repair of small to medium nerve gaps Potential use in both peripheral and central nervous system injuries (experimental) | Mechanical properties similar to native nerve tissue Supports cell infiltration | Degradation products may cause inflammation Limited clinical use |
| Gelatin-Based Conduits | Natural protein derived from collagen | Biodegradable Good biocompatibility Permeable | Experimental applications in peripheral nerve repair Short nerve gaps | Supports cell adhesion and proliferation Easily modified with growth factors | Mechanical weakness Rapid degradation may not provide sufficient support |
| Hybrid Conduits (e.g., PCL/Collagen blends) | Combination of synthetic and natural materials | Tailored degradation rates Improved mechanical strength Enhanced biocompatibility | Repair of complex nerve injuries Potential for longer nerve gaps due to improved properties | Combines advantages of both materials Customizable to specific injury requirements | Complexity in manufacturing Regulatory approval challenges |
| Pathway | Effect |
|---|---|
| PI3K/Akt | Promotes cell survival, axonal elongation, and remyelination. |
| MAPK/ERK | Regulates neuronal differentiation and Schwann cell proliferation. |
| JNK/c-Jun | Controls neuronal plasticity and inflammatory responses. |
| RhoA/ROCK | Inhibits axonal outgrowth; its suppression enhances nerve regeneration. |
| JAK/STAT | Facilitates Schwann cell differentiation and myelin formation. |
| Nrg1-ErbB | Facilitates Schwann cell differentiation and myelin formation. |
| Nerve Repair Technique | Advantages | Disadvantages |
|---|---|---|
| Autogenous Nerve Grafting |
|
|
| Nerve Transfers |
|
|
| End-to-Side Coaptation |
|
|
| Nerve Allografts |
|
|
| Nerve Conduits |
|
|
| Product Name | Manufacturer | Type | Clinical Indications | Description |
|---|---|---|---|---|
| Avance® Nerve Graft | Axogen® | Processed allograft | Used for bridging peripheral nerve defects. | A three-dimensional scaffold designed to bridge nerve gaps, composed of a decellularized and cleansed extracellular matrix that supports remodeling and nerve regeneration. It is offered in various lengths and diameters to accommodate different gap sizes and anatomical requirements [299]. |
| Axoguard® Nerve Connector | Axogen® | Porcine ECM conduit | Coaptation of severed peripheral nerves short gaps. | A porcine submucosa extracellular matrix proposed for the approximation and tensionless repair of severed peripheral nerves with less than a 5 mm gap, allowing for natural healing where cells incorporate into the ECM to form tissue similar to the nerve’s epineural connective tissue [300]. |
| Axoguard ® Nerve Protector | Axogen® | Porcine ECM wrap | Protect injured peripheral nerves without gaps, preventing scar tissue formation. | A porcine submucosa ECM surgical implant proposed for the separation and protection of injured nerves without gaps, preventing soft tissue adherence while allowing cell incorporation to remodel a separating tissue layer. It is suitable for nerves up to 40 mm [301]. |
| NeuraGen® Nerve Guide | Integra LifeSciences | Collagen conduit | Suitable for bridging small peripheral nerve gaps. | A resorbable, semi-permeable bovine, type I collagen-based tubular implant for the repair of peripheral nerve defects (of 3 cm or less), maintaining an interface between the nerve and surrounding tissue and serving as a conduit for axonal growth [302]. |
| NeuraGen® 3D Nerve Guide Matrix | Integra LifeSciences | Collagen conduit filled with collagen-glycosaminoglycan (C6S) matrix | Optimize environment for mid-gap nerve regeneration. | 3D micro-architecture designed to mimic the natural structure of peripheral nerve tissue. The outer collagen conduit acts as a semi-permeable membrane, allowing the diffusion of small nutrient molecules into the guide while retaining larger molecules, such as nerve growth factors, within the conduit, promoting organized axonal regeneration. Addresses the nerve gaps of a maximum length of 3 cm, with diameters ranging from 1.5 mm to 7 mm [303]. |
| NeuraWrap® Nerve Protector | Integra LifeSciences | Collagen wrap | Encase the injured nerves, protecting against external compression and minimizing neuroma risk. | A resorbable bovine, type I collagen implant serving as an interface between the nerve and surrounding tissue through its porous outer layer and a semi-permeable internal membrane that allows for diffusion of small molecules while retaining nerve growth factors. Can wrap nerves from 3 mm to 10 mm in diameter with a maximum length of 4 cm [304]. |
| Neuroflex® Connector | Stryker | Collagen conduit | Severed nerves across joints, treatment of symptomatic or painful neuromas. | A resorbable type I collagen-based tubular matrix with corrugated side walls, it is highly flexible and kink-resistant—bends up to 60° without occlusion, maintains conduit shape during joint motion, and reduces risk of compression or collapse in dynamic areas. It is available in various diameters with a length of 2.5 cm [305]. |
| NeuroMatrix ® Connector | Stryker | Collagen conduit | Severed nerve injuries in straight anatomical path. | A resorbable, type I collagen-based conduit designed to provide structural support and tensionless repair across straight gaps. The only available length is 2.5 cm [306]. |
| NeuroMend® Collagen Wrap | Stryker | Collagen wrap | Protect crushed or compressed nerves from mechanical irritation. | A resorbable, semi-permeable collagen wrap that provides a protective sheath around injured peripheral nerves, helping to reduce suturing and supporting regeneration. It is suitable for nerves ranging from 2.0 mm to 12.0 mm in diameter, being available in 2 lengths (2.5 cm and 5.0 cm) [307]. |
| Neurolac® and Neurolac® Thin wall(TW) | Polyganics | Synthetic conduit made from poly(DL-lactide-ε-caprolactone) (PLCL) | Short nerve gaps repair, prevent neuroma formation/ | These bioabsorbable conduits are designed for the reconstruction of peripheral nerve gaps up to 20 mm, commonly in the hand and wrist. The TW version has a thinner wall structure to improve flexibility and allow easier handling or needle penetration when needed [308]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu-Bularda, A.; Vancea, C.-V.; Hodea, F.-V.; Cretu, A.; Bordeanu-Diaconescu, E.-M.; Dumitru, C.-S.; Ratoiu, V.-A.; Teodoreanu, R.-N.; Lascar, I.; Hariga, C.-S. Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3895. https://doi.org/10.3390/ijms26083895
Grosu-Bularda A, Vancea C-V, Hodea F-V, Cretu A, Bordeanu-Diaconescu E-M, Dumitru C-S, Ratoiu V-A, Teodoreanu R-N, Lascar I, Hariga C-S. Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(8):3895. https://doi.org/10.3390/ijms26083895
Chicago/Turabian StyleGrosu-Bularda, Andreea, Cristian-Vladimir Vancea, Florin-Vlad Hodea, Andrei Cretu, Eliza-Maria Bordeanu-Diaconescu, Catalina-Stefania Dumitru, Vladut-Alin Ratoiu, Razvan-Nicolae Teodoreanu, Ioan Lascar, and Cristian-Sorin Hariga. 2025. "Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies—A Narrative Review" International Journal of Molecular Sciences 26, no. 8: 3895. https://doi.org/10.3390/ijms26083895
APA StyleGrosu-Bularda, A., Vancea, C.-V., Hodea, F.-V., Cretu, A., Bordeanu-Diaconescu, E.-M., Dumitru, C.-S., Ratoiu, V.-A., Teodoreanu, R.-N., Lascar, I., & Hariga, C.-S. (2025). Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies—A Narrative Review. International Journal of Molecular Sciences, 26(8), 3895. https://doi.org/10.3390/ijms26083895





