NAT2 Acetylation Status Predicts Hepatotoxicity During Antituberculosis Therapy: Cumulative Risk Analysis of a Multiethnic Cohort
Abstract
1. Introduction
2. Results
2.1. Cohort Characteristics
2.2. Distribution of NAT2 Genotypes and ATDH
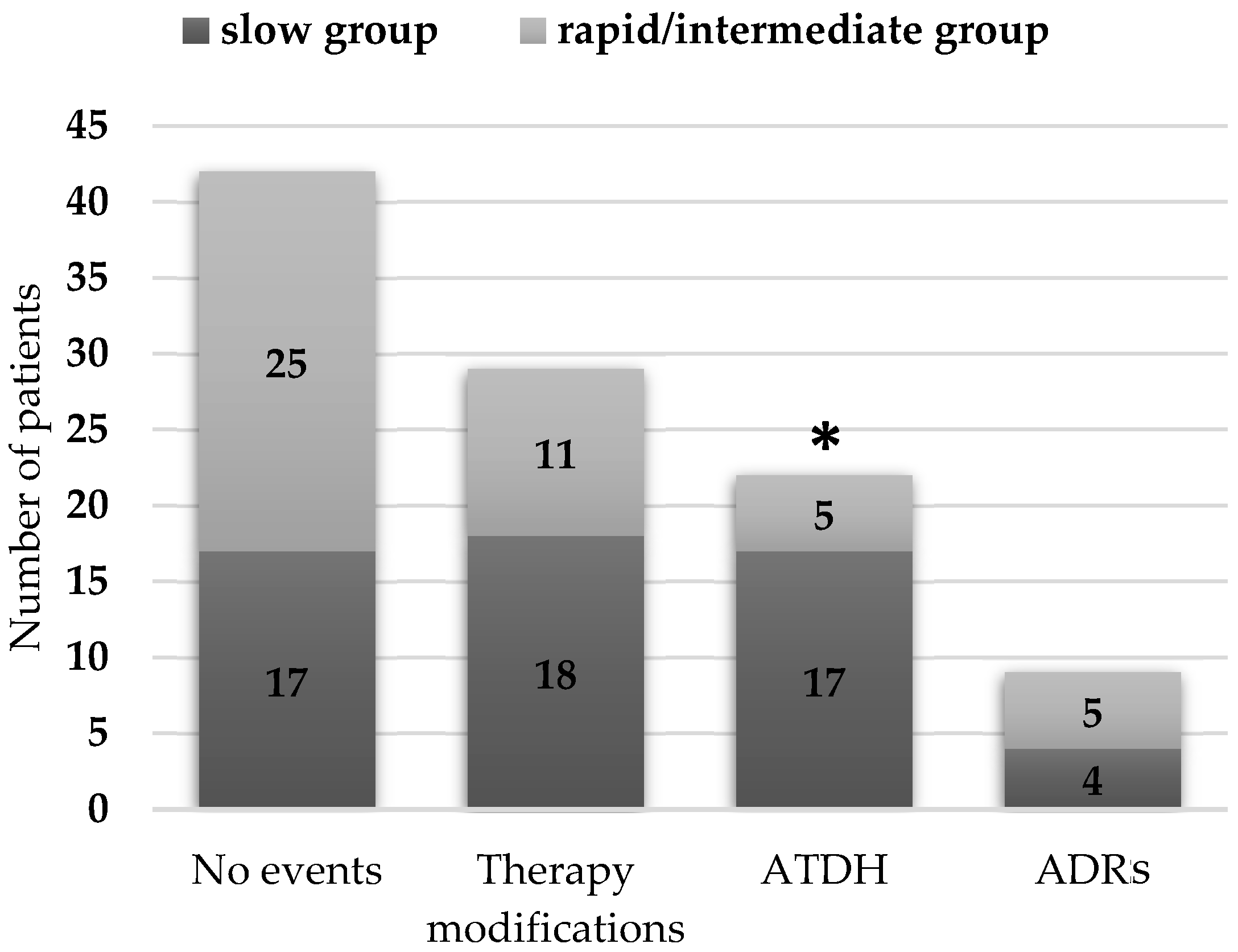
2.3. Acetylation Status and Treatment-Related Events
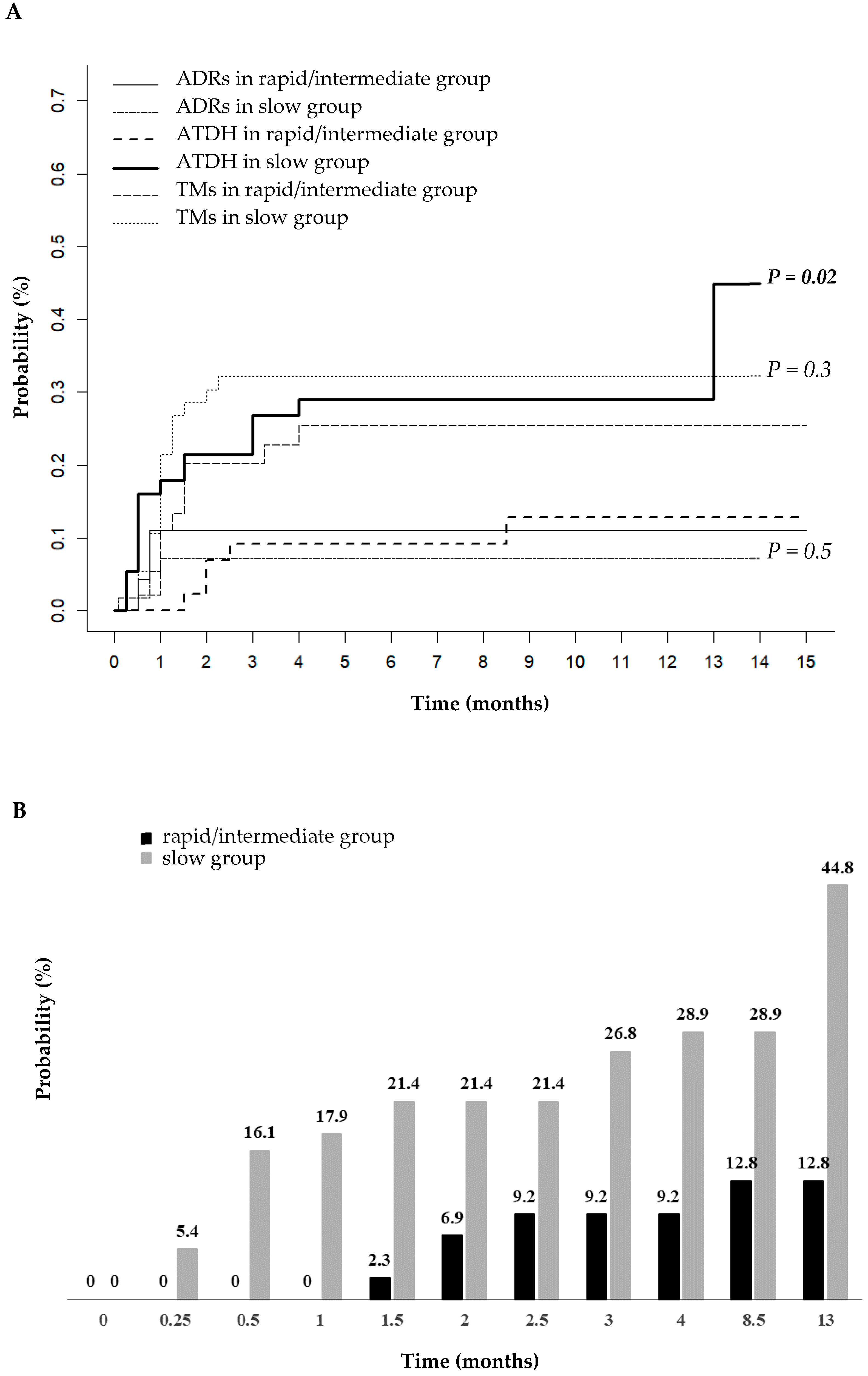
2.4. Cumulative Incidence of ATDH
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Acetylation Phenotype Prediction
4.3. Outcome of Interest: ATDH
4.4. Ethical Issues
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Adverse drug reaction |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| ATDH | Antituberculosis drug-induced hepatotoxicity |
| BIL | Bilirubin |
| BMI | Body mass index |
| CI | Confidence interval |
| DMEs | Drug-metabolizing enzymes |
| EMB | Ethambutol |
| INH | Isoniazid |
| IQR | Interquartile range |
| NAT2 | N-Acetyltransferase 2 |
| PZA | Pyrazinamide |
| RIF | Rifampin |
| SHR | Sub-distribution hazard ratio |
| TB | Tuberculosis |
| ULN | Upper limit of normal |
References
- Ramappa, V.; Aithal, G.P. Hepatotoxicity Related to Anti-tuberculosis Drugs: Mechanisms and Management. J. Clin. Exp. Hepatol. 2013, 3, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Tostmann, A.; Boeree, M.J.; Aarnoutse, R.E.; De Lange, W.C.; van der Ven, A.J.; Dekhuijzen, R. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol. 2008, 23, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shalimar; Bhatia, V.; Khanal, S.; Sreenivas, V.; Gupta, D.S.; Panda, S.K.; Acharya, S.K. Antituberculosis Therapy–Induced Acute Liver Failure: Magnitude, Profile, Prognosis, and Predictors of Outcome. Hepatology 2010, 51, 1665–1674. [Google Scholar] [CrossRef]
- WHO. WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Zha, B.S.; Nahid, P. Treatment of Drug-Susceptible Tuberculosis. Clin. Chest Med. 2019, 40, 763–774. [Google Scholar] [CrossRef]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a Prospective Study of Acute Liver Failure at 17 Tertiary Care Centers in the United States. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Dierkhising, R.; Kremers, W.K. Antituberculosis therapy drug-induced liver injury and acute liver failure†. Hepatology 2010, 52, 798–799. [Google Scholar] [CrossRef]
- Du, H.; Chen, X.; Fang, Y.; Yan, O.; Xu, H.; Li, L.; Li, W.; Huang, W. Slow N-acetyltransferase 2 genotype contributes to anti-tuberculosis drug-induced hepatotoxicity: A meta-analysis. Mol. Biol. Rep. 2013, 40, 3591–3596. [Google Scholar] [CrossRef]
- Ohno, M.; Yamaguchi, I.; Yamamoto, I.; Fukuda, T.; Yokota, S.; Maekura, R.; Ito, M.; Yamamoto, Y.; Ogura, T.; Maeda, K.; et al. Slow N-acetyltransferase 2 genotype affects the incidence of isoniazid and rifampicin-induced hepatotoxicity. Int. J. Tuberc. Lung Dis. 2000, 4, 256–261. [Google Scholar]
- Richardson, M.; Kirkham, J.; Dwan, K.; Sloan, D.J.; Davies, G.; Jorgensen, A.L. NAT2 variants and toxicity related to anti-tuberculosis agents: A systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 293–305. [Google Scholar] [CrossRef]
- Yang, S.; Hwang, S.J.; Park, J.Y.; Chung, E.K.; Lee, J.I. Association of genetic polymorphisms of CYP2E1, NAT2, GST and SLCO1B1 with the risk of anti-tuberculosis drug-induced liver injury: A systematic review and meta-analysis. BMJ Open 2019, 9, e027940. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Wilffert, B.; Tong, R.; van Soolingen, D.; Hof, S.v.D.; Alffenaar, J. The association between the NAT2 genetic polymorphisms and risk of DILI during anti-TB treatment: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2018, 84, 2747–2760. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Mandal, R.K.; Elasbali, A.M.; Dar, S.A.; Jawed, A.; Wahid, M.; Mahto, H.; Lohani, M.; Mishra, B.N.; Akhter, N.; et al. Pharmacogenetic association between NAT2 gene polymorphisms and isoniazid induced hepatotoxicity: Trial sequence meta-analysis as evidence. Biosci. Rep. 2019, 39, BSR20180845. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Sekhar Miraj, S.; Surulivelrajan, M.; Varma, M.; Sanju, C.S.; Rao, M. Influence of N -acetyltransferase 2 polymorphisms and clinical variables on liver function profile of tuberculosis patients. Expert Rev. Clin. Pharmacol. 2024, 17, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Liu, C.-H.; Hu, F.-C.; Chang, H.-C.; Liu, J.-L.; Chen, J.-M.; Yu, C.-J.; Lee, L.-N.; Kao, J.-H.; Yang, P.-C. Risk factors of hepatitis during Anti-tuberculous treatment and implications of hepatitis virus load. J. Infect. 2011, 62, 448–455. [Google Scholar] [CrossRef]
- Fountain, F.F.; Tolley, E.A.; Jacobs, A.R.; Self, T.H. Rifampin Hepatotoxicity Associated With Treatment of Latent Tuberculosis Infection. Am. J. Med. Sci. 2009, 337, 317–320. [Google Scholar] [CrossRef]
- Sarma, G.; Immanuel, C.; Kailasam, S.; Narayana, A.; Venkatesan, P. Rifampin-induced release of hydrazine from isoniazid. A possible cause of hepatitis during treatment of tuberculosis with regimens containing isoniazid and rifampin. Am. Rev. Respir. Dis. 1986, 133, 1072–1075. [Google Scholar] [CrossRef]
- McDonagh, E.M.; Boukouvala, S.; Aklillu, E.; Hein, D.W.; Altman, R.B.; Klein, T.E. PharmGKB summary. Pharmacogenetics Genom. 2014, 24, 409–425. [Google Scholar] [CrossRef]
- Fukunaga, K.; Kato, K.; Okusaka, T.; Saito, T.; Ikeda, M.; Yoshida, T.; Zembutsu, H.; Iwata, N.; Mushiroda, T. Functional Characterization of the Effects of N-acetyltransferase 2 Alleles on N-acetylation of Eight Drugs and Worldwide Distribution of Substrate-Specific Diversity. Front. Genet. 2021, 12, 652704. [Google Scholar] [CrossRef]
- Azuma, J.; Ohno, M.; Kubota, R.; Yokota, S.; Nagai, T.; Tsuyuguchi, K.; Okuda, Y.; Takashima, T.; Kamimura, S.; Fujio, Y.; et al. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: A randomized controlled trial for pharmacogenetics-based therapy. Eur. J. Clin. Pharmacol. 2013, 69, 1091–1101. [Google Scholar] [CrossRef]
- Suvichapanich, S.; Fukunaga, K.; Zahroh, H.; Mushiroda, T.; Mahasirimongkol, S.; Toyo-Oka, L.; Chaikledkaew, U.; Jittikoon, J.; Yuliwulandari, R.; Yanai, H.; et al. NAT2 ultra-slow acetylator and risk of anti-tuberculosis drug-induced liver injury. Pharmacogenetics Genom. 2018, 28, 167–176. [Google Scholar] [CrossRef]
- Chang, T.-E.; Su, W.-J.; Perng, C.-L.; Huang, Y.-H.; Hou, M.-C. The role of regular liver function monitoring in antituberculosis drug-induced liver injury. J. Chin. Med. Assoc. 2019, 82, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Saukkonen, J.J.; Cohn, D.L.; Jasmer, R.M.; Schenker, S.; Jereb, J.A.; Nolan, C.M.; Peloquin, C.A.; Gordin, F.M.; Nunes, D.; Strader, D.B.; et al. An Official ATS Statement: Hepatotoxicity of Antituberculosis Therapy. Am. J. Respir. Crit. Care Med. 2006, 174, 935–952. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, T.; Rebhan, K.; Lode, H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur. Respir. J. 1996, 9, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- Cheli, S.; Torre, A.; Schiuma, M.; Montrasio, C.; Civati, A.; Galimberti, M.; Battini, V.; Mariani, I.; Mosini, G.; Carnovale, C.; et al. NAT2 Slow Acetylator Phenotype as a Significant Risk Factor for Hepatotoxicity Caused by Antituberculosis Drugs: Results From a Multiethnic Nested Case-Control Study. Clin. Infect. Dis. 2024, ciae583, Erratum in Clin. Infect. Dis. 2025, 80, 697. [Google Scholar] [CrossRef]
- Fernández-Villar, A.; Sopeña, B.; Fernández-Villar, J.; Vázquez-Gallardo, R.; Ulloa, F.; Leiro, V.; Mosteiro, M.; Piñeiro, L. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int. J. Tuberc. Lung Dis. 2004, 8, 1499–1505. [Google Scholar]
- Noor, N.F.M.; Salleh, M.Z.; Zim, M.A.M.; Abu Bakar, Z.; Noorizhab, M.N.F.; Zakaria, N.I.; Lailanor, M.I.; Teh, L.K. NAT2 Polymorphism and Clinical Factors that Increase Antituberculosis Drug-Induced Hepatotoxicity. Pharmacogenomics 2022, 23, 531–541. [Google Scholar] [CrossRef]
- Cheng, F.; Jiang, X.-G.; Zheng, S.-L.; Wu, T.; Zhang, Q.; Ye, X.-C.; Liu, S.; Shi, J.-C. N-acetyltransferase 2 genetic polymorphisms and anti-tuberculosis-drug-induced liver injury: A correlation study. Front. Pharmacol. 2023, 14, 1171353. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Singh, R.; Patil, M.; Sheth, K.; Adarsh, C.K.; Balaraju, G. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J. Gastroenterol. Hepatol. 2012, 28, 161–167. [Google Scholar] [CrossRef]
- Abera, W.; Cheneke, W.; Abebe, G. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro Zone, South Ethiopia: A cohort study. Int. J. Mycobacteriol. 2016, 5, 14–20. [Google Scholar] [CrossRef]
- Shakya, R.; Rao, B.; Shrestha, B. Evaluation of Risk Factors for Antituberculosis Drugs-Induced Hepatotoxicity in Nepalese. Kathmandu Univ. J. Sci. Eng. Technol. 2006, 2. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, S.S.; Lee, J.M.; Cho, H.C.; Kim, W.S.; Kim, H.J.; Ha, C.Y.; Kim, H.J.; Kim, T.H.; Jung, W.T.; et al. Early monitoring for detection of antituberculous drug-induced hepatotoxicity. Korean J. Intern. Med. 2016, 31, 65–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, A.L.; Makmor-Bakry, M.; Islahudin, F.; Ting, C.Y.; Chan, S.K.; Tie, S.T. Adverse drug reactions of first-line antitubercular drugs: A retrospective study on characteristics, management, factors, and impacts. Asian Pac. J. Trop. Med. 2024, 17, 456–464. [Google Scholar] [CrossRef]
- Knowles, S.R.; Uetrecht, J.; Shear, N.H. Idiosyncratic drug reactions: The reactive metabolite syndromes. Lancet 2000, 356, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Ahadpour, M.; Eskandari, M.R.; Mashayekhi, V.; Tehrani, K.H.M.E.; Jafarian, I.; Naserzadeh, P.; Hosseini, M.-J. Mitochondrial oxidative stress and dysfunction induced by isoniazid: Study on isolated rat liver and brain mitochondria. Drug Chem. Toxicol. 2016, 39, 224–232. [Google Scholar] [CrossRef]
- Allison, R.; Guraka, A.; Shawa, I.T.; Tripathi, G.; Moritz, W.; Kermanizadeh, A. Drug induced liver injury—A 2023 update. J. Toxicol. Environ. Health Part B 2023, 26, 442–467. [Google Scholar] [CrossRef]
- Rusyn, I.; Arzuaga, X.; Cattley, R.C.; Corton, J.C.; Ferguson, S.S.; Godoy, P.; Guyton, K.Z.; Kaplowitz, N.; Khetani, S.R.; Roberts, R.A.; et al. Key Characteristics of Human Hepatotoxicants as a Basis for Identification and Characterization of the Causes of Liver Toxicity. Hepatology 2021, 74, 3486–3496. [Google Scholar] [CrossRef]
- Verma, R.; da Silva, K.E.; Rockwood, N.; Wasmann, R.E.; Yende, N.; Song, T.; Kim, E.; Denti, P.; Wilkinson, R.J.; Andrews, J.R. A Nanopore Sequencing-based Pharmacogenomic Panel to Personalize Tuberculosis Drug Dosing. Am. J. Respir. Crit. Care Med. 2024, 209, 1486–1496. [Google Scholar] [CrossRef]
- Verma, R.; Patil, S.; Zhang, N.; Moreira, F.M.F.; Vitorio, M.T.; Santos, A.d.S.; Wallace, E.; Gnanashanmugam, D.; Persing, D.H.; Savic, R.M.; et al. A Rapid Pharmacogenomic Assay to Detect NAT2 Polymorphisms and Guide Isoniazid Dosing for Tuberculosis Treatment. Am. J. Respir. Crit. Care Med. 2021, 204, 1317–1326. [Google Scholar] [CrossRef]
| Characteristics | Total n (%) | Slow Acetylator n (%) | Rapid/Intermediate Acetylator n (%) | p-Value * |
|---|---|---|---|---|
| Number of patients | 102 | 56 (54.9) | 46 (45.1) | |
| Age, yrs | ||||
| Median, (IQR) | 44 (34–55) | |||
| <60 | 82 (80.4) | 44 (78.6) | 38 (82.6) | |
| ≥60 | 20 (19.6) | 12 (21.4) | 8 (17.4) | 0.79 |
| Sex | ||||
| Males | 47 (46.1) | 28 (50) | 19 (41.3) | |
| Females | 55 (53.9) | 28 (50) | 27 (58.7) | 0.5 |
| BMI (kg/m2) | ||||
| Median (IQR) | 22.5 (19.6–26.9) | |||
| <18.5 | 15 (14.7) | 10 (17.9) | 5 (10.9) | |
| ≥18.5 | 87 (85.3) | 46 (82.1) | 41 (89.1) | 0.48 |
| Ethnicity | ||||
| European | 31 (30.4) | 18 (32.1) | 13 (28.3) | |
| African | 21 (20.6) | 13 (23.2) | 8 (17.4) | |
| Latin | 18 (17.6) | 9 (16.1) | 9 (19.6) | |
| Asian | 18 (17.6) | 8 (14.3) | 10 (21.7) | |
| Indian | 14 (13.8) | 8 (14.3) | 6 (13.0) | 0.82 |
| Tuberculosis localization in active tuberculosis (n = 88) | ||||
| Pulmonary | 36 (40.9) | 21 (43.8) | 15 (37.5) | |
| Non-pulmonary | 35 (39.8) | 18 (37.5) | 17 (42.5) | |
| Both | 17 (19.3) | 9 (18.7) | 8 (20.0) | 0.83 |
| Acetylator Status | n (%) | n (%) | n (%) | p-Value # |
|---|---|---|---|---|
| Overall | ATDH | No ATDH | 0.03 | |
| Slow | 56 (54.9) | 17 (77.3) | 39 (48.8) | |
| NAT2*5/*5 | 14 (13.7) | 5 (22.7) | 9 (11.3) | |
| NAT2*5/*6 | 15 (14.7) | 5 (22.7) | 10 (12.5) | |
| NAT2*5/*7 | 7 (6.9) | 2 (9.1) | 5 (6.3) | |
| NAT2*6/*6 | 9 (8.8) | 3 (13.6) | 6 (7.5) | |
| NAT2*6/*7 | 8 (7.8) | 2 (9.1) | 6 (7.5) | |
| NAT2*6/*14 | 1 (1.0) | 0 (0.0) | 1 (1.3) | |
| NAT2*7/*7 | 2 (2.0) | 0 (0.0) | 2 (2.5) | |
| Rapid/Intermediate | 46 (45.1) | 5 (22.7) | 41 (51.2) | |
| NAT2*1/*1 | 9 (8.8) | 0 (0.0) | 9 (11.3) | |
| NAT2*1/*5 | 15 (14.7) | 2 (9.1) | 13 (16.3) | |
| NAT2*1/*6 | 17 (16.7) | 1 (4.5) | 16 (20.0) | |
| NAT2*1/*7 | 5 (4.9) | 2 (9.1) | 3 (3.8) | |
| Total * | 102 (100) | 22 (100) | 75 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiuma, M.; Dinegro, S.; Battini, V.; Torre, A.; Covizzi, A.; Civati, A.; Galimberti, M.; Mariani, I.; Mosini, G.; Carnovale, C.; et al. NAT2 Acetylation Status Predicts Hepatotoxicity During Antituberculosis Therapy: Cumulative Risk Analysis of a Multiethnic Cohort. Int. J. Mol. Sci. 2025, 26, 3881. https://doi.org/10.3390/ijms26083881
Schiuma M, Dinegro S, Battini V, Torre A, Covizzi A, Civati A, Galimberti M, Mariani I, Mosini G, Carnovale C, et al. NAT2 Acetylation Status Predicts Hepatotoxicity During Antituberculosis Therapy: Cumulative Risk Analysis of a Multiethnic Cohort. International Journal of Molecular Sciences. 2025; 26(8):3881. https://doi.org/10.3390/ijms26083881
Chicago/Turabian StyleSchiuma, Marco, Sofia Dinegro, Vera Battini, Alessandro Torre, Alice Covizzi, Aurora Civati, Miriam Galimberti, Ilaria Mariani, Giulia Mosini, Carla Carnovale, and et al. 2025. "NAT2 Acetylation Status Predicts Hepatotoxicity During Antituberculosis Therapy: Cumulative Risk Analysis of a Multiethnic Cohort" International Journal of Molecular Sciences 26, no. 8: 3881. https://doi.org/10.3390/ijms26083881
APA StyleSchiuma, M., Dinegro, S., Battini, V., Torre, A., Covizzi, A., Civati, A., Galimberti, M., Mariani, I., Mosini, G., Carnovale, C., Riva, A., Gori, A., Antinori, S., Clementi, E., Radice, S., & Cheli, S. (2025). NAT2 Acetylation Status Predicts Hepatotoxicity During Antituberculosis Therapy: Cumulative Risk Analysis of a Multiethnic Cohort. International Journal of Molecular Sciences, 26(8), 3881. https://doi.org/10.3390/ijms26083881










