Osmoregulation and Physiological Response of Largemouth Bass (Micropterus salmoides) Juvenile to Different Salinity Stresses
Abstract
1. Introduction
2. Results
2.1. Determination of Median-Lethal Concentration
2.2. Histological Analysis of Gill and Intestine After 96 h Acute Salinity Exposure
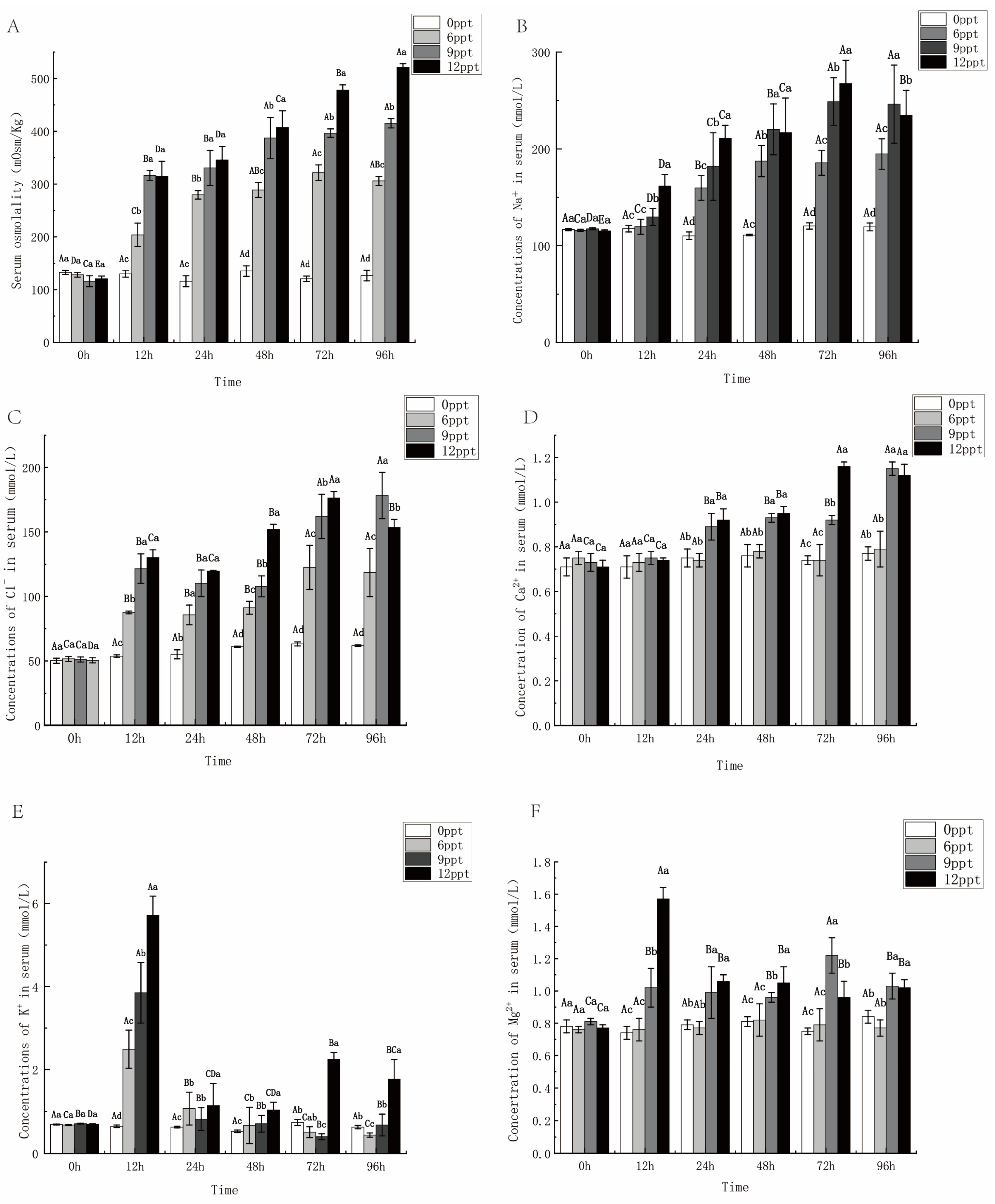
2.3. Effects of Different Salinity on Osmolality and Serum Ions
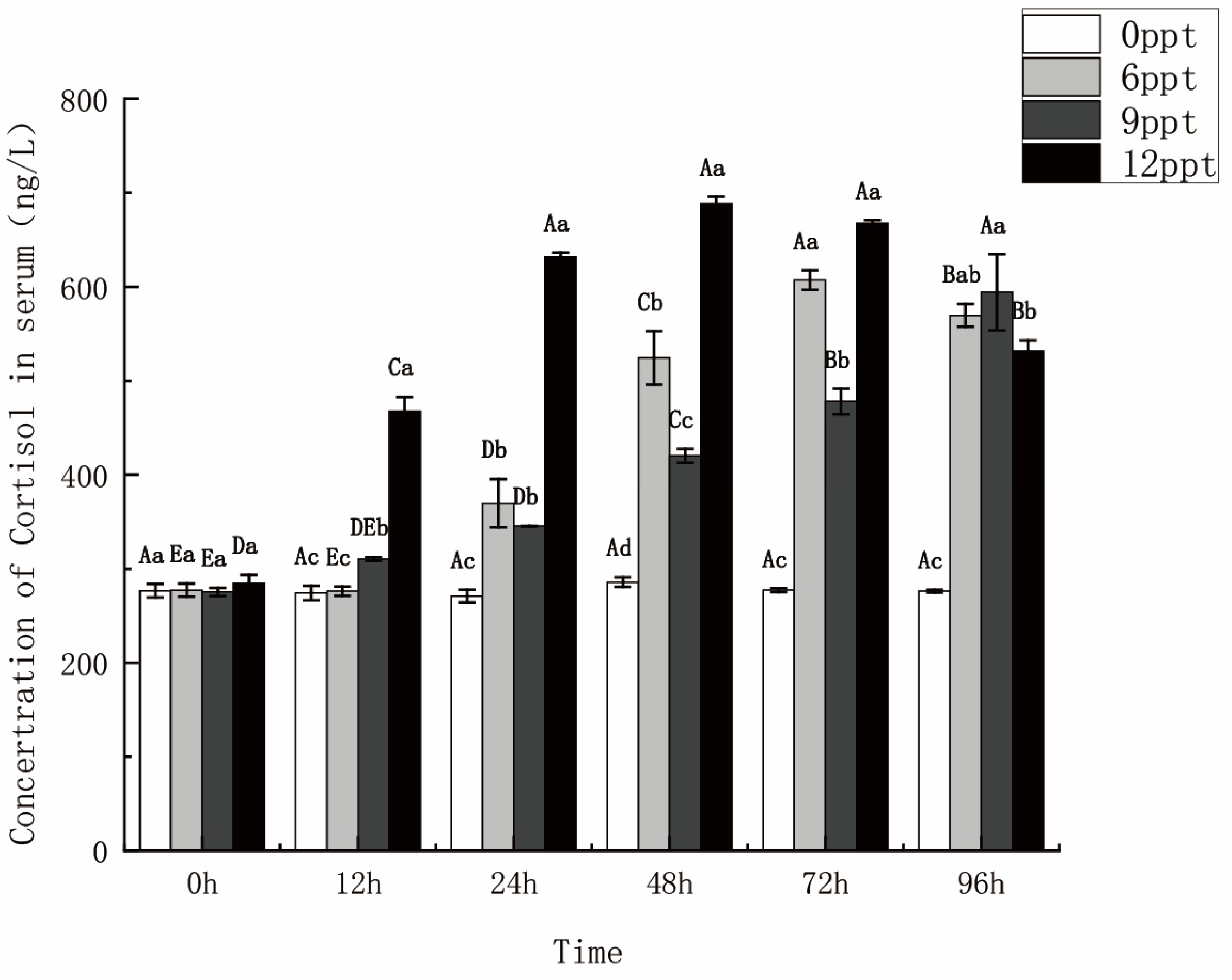
2.4. Serum Cortisol Concentration Increased with Salinity Increasing
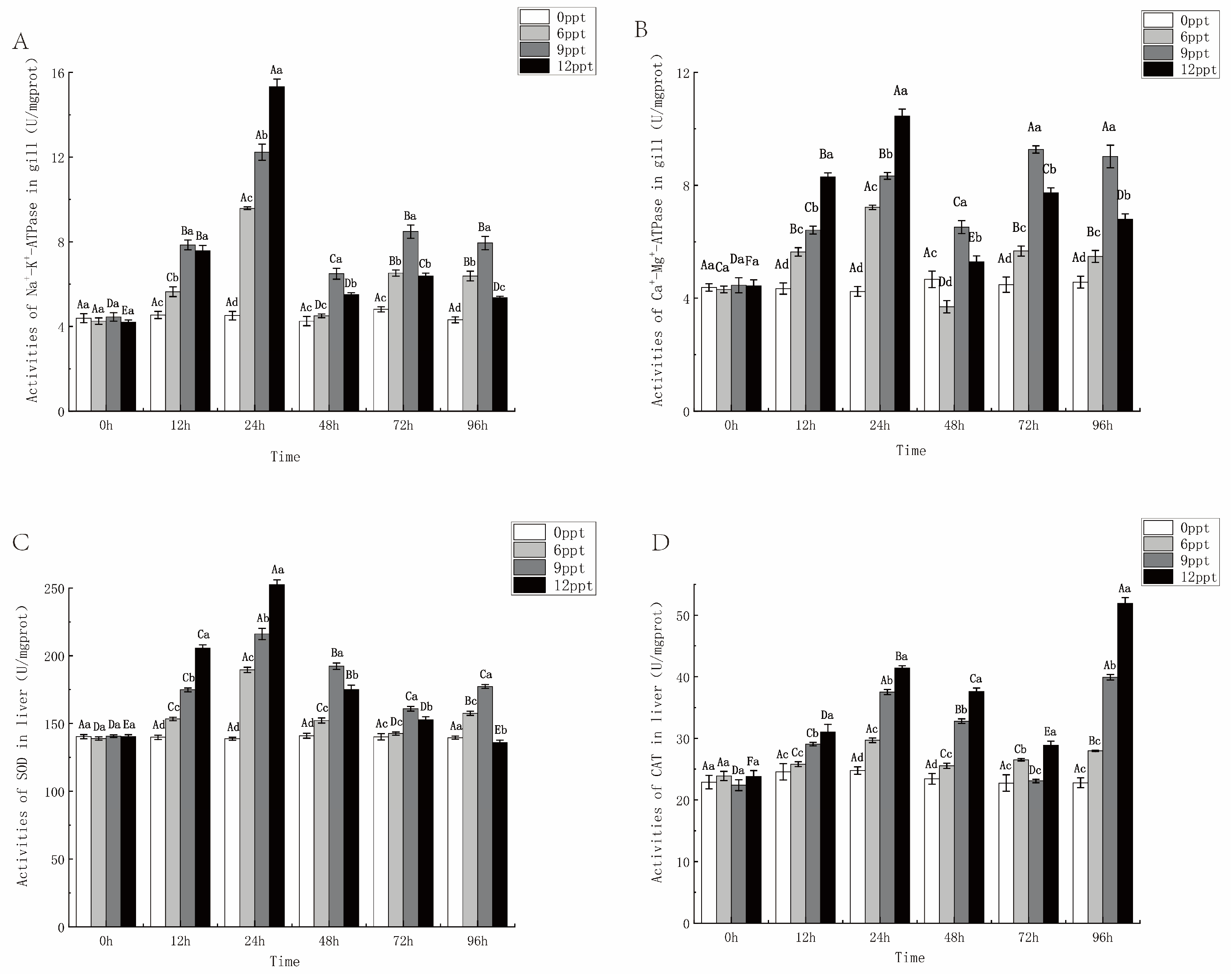
2.5. Enzyme Activity in Gill and Liver During 96 h Acute Salinity Exposure
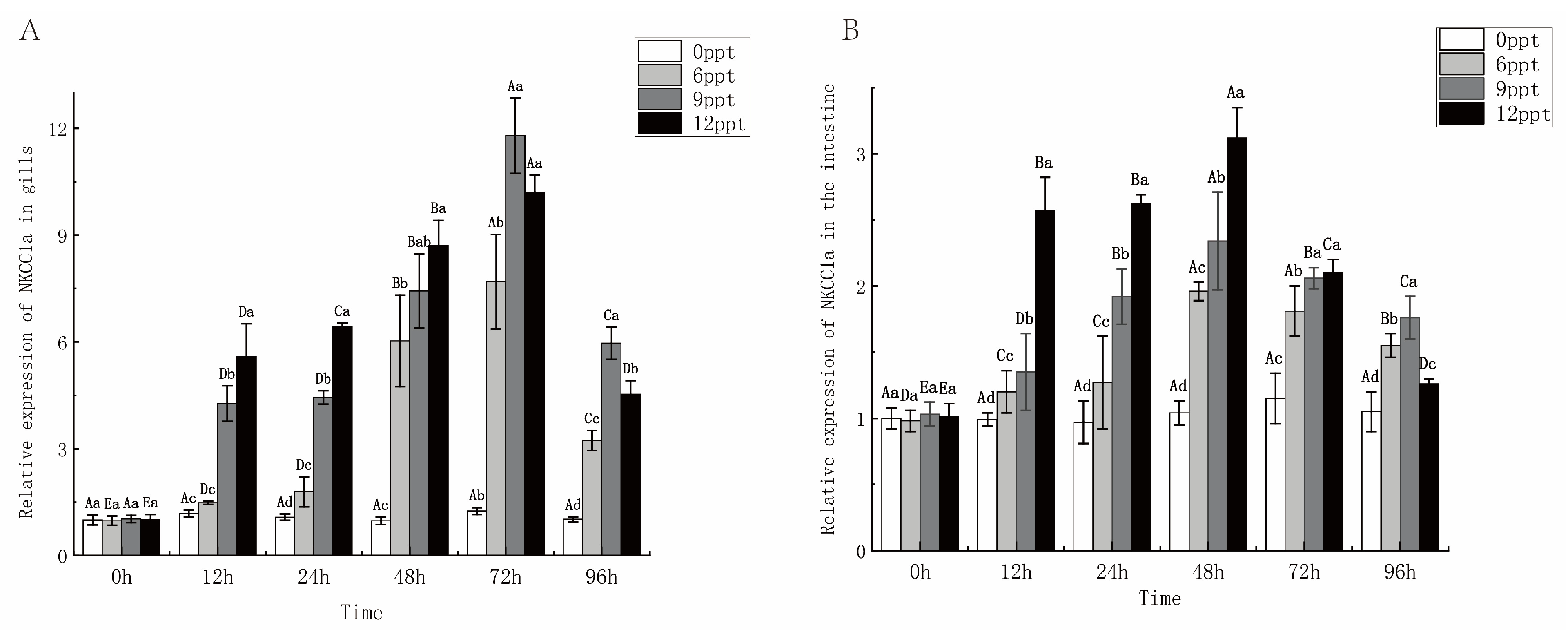
2.6. Effect of Different Salinity on NKCC1a Gene Expression
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Experimental Design and Sample Collection
4.3. Histological Analysis of Gill and Intestine
4.4. Analysis of Serum Indicators
4.5. Enzyme Activity Analysis
4.6. Total RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NKCC1a | Na-K-2Cl 1a |
| NKA | Na+/K+-ATPase |
| CMA | Ca2+-Mg2+-ATPase |
| SOD | superoxide dismutase |
| CAT | Catalase |
| COR | Cortisol |
References
- Kwasek, K.; Thorne-Lyman, A.L.; Phillips, M. Can human nutrition be improved through better fish feeding practices? A review paper. Crit. Rev. Food Sci. Nutr. 2020, 60, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Q.; Li, Y.; Yu, S.P.; Hu, B.Z.; Guo, H.; Jin, L.; Cong, D.L.; Meng, B.; Ding, J.N.; Liang, X.W. Saline-alkaline soil bacterial community structure and diversity analysis under different patterns of land-use in a lake wetland in songnen plain, China. Appl. Ecol. Environ. Res. 2021, 19, 1337–1352. [Google Scholar] [CrossRef]
- Velasco, J.; Gutiérrez-Cánovas, C.; Botella-Cruz, M.; Sánchez-Fernández, D.; Arribas, P.; Carbonell, J.A.; Millán, A.; Pallarés, S. Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 374, 1764. [Google Scholar] [CrossRef] [PubMed]
- Abiala, M.A.; Abdelrahman, M.; Burritt, D.J.; Tran, L.-S.P. Salt stress tolerance mechanisms and potential applications of legumes for sustainable reclamation of salt-degraded soils. Land Degrad. Dev. 2018, 29, 3812–3822. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Effects of saline-alkali stress on the tissue structure, antioxidation, immunocompetence and metabolomics of Eriocheir sinensis. Sci. Total Environ. 2023, 871, 162109. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, H.; Liu, Y.; Qi, X.; Sun, D.; Zhang, C.; Zhang, K.; Zhang, M.; Li, J.; Li, Y. Gill histological and transcriptomic analysis provides insights into the response of spotted sea bass (Lateolabrax maculatus) to alkalinity stress. Aquaculture 2023, 563, 738945. [Google Scholar] [CrossRef]
- Song, L.; Zhao, Y.; Song, Y.; Zhao, L.; Ma, C.; Zhao, J. Effects of saline-alkaline water on growth performance, nutritional processing, and immunity in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 544, 737036. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, J.-C.; Li, C.-C.; Morni, W.Z.; Suhaili, A.S.N.; Kuo, Y.-H.; Chang, Y.-H.; Chen, L.-L.; Tsui, W.-C.; Chen, Y.-Y.; et al. Modulation of the innate immune system in white shrimp Litopenaeus vannamei following long-term low salinity exposure. Fish. Shellfish. Immunol. 2012, 33, 324–331. [Google Scholar] [CrossRef]
- Liu, B.; Guo, H.-Y.; Zhu, K.-C.; Guo, L.; Liu, B.-S.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.-C. Growth, physiological, and molecular responses of golden pompano Trachinotus ovatus (Linnaeus, 1758) reared at different salinities. Fish. Physiol. Biochem. 2019, 45, 1879–1893. [Google Scholar] [CrossRef]
- Tian, L.; Tan, P.; Yang, L.; Zhu, W.; Xu, D. Effects of salinity on the growth, plasma ion concentrations, osmoregulation, non-specific immunity, and intestinal microbiota of the yellow drum (Nibea albiflora). Aquaculture 2020, 528, 735470. [Google Scholar] [CrossRef]
- Hegazi, M.A.M.; Attia, Z.I.; Hegazi, M.M. Salinity lessens the impact of high stocking densities and metabolic cost in white muscle and liver of Nile tilapia fingerlings. Aquac. Res. 2014, 45, 566–570. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, A.; Yuan, C.; Zhao, T.; Chang, H.; Zhang, J. Transcriptome analysis of liver lipid metabolism disorders of the turbot Scophthalmus maximus in response to low salinity stress. Aquaculture 2021, 534, 736273. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, M.; Zhang, J.-E.; Deng, Z. Effects of salinity on survival, growth and reproduction of the invasive aquatic snail Pomacea canaliculata (Gastropoda: Ampullariidae). Hydrobiologia 2020, 847, 3103–3114. [Google Scholar] [CrossRef]
- Huong, D.T.T.; Jasmani, S.; Jayasankar, V.; Wilder, M. Na/K-ATPase activity and osmo-ionic regulation in adult whiteleg shrimp Litopenaeus vannamei exposed to low salinities. Aquaculture 2010, 304, 88–94. [Google Scholar] [CrossRef]
- Xiong, Y.; Dong, S.; Huang, M.; Li, Y.; Wang, X.; Wang, F.; Ma, S.; Zhou, Y. Growth, osmoregulatory response, adenine nucleotide contents, and liver transcriptome analysis of steelhead trout (Oncorhynchus mykiss) under different salinity acclimation methods. Aquaculture 2020, 520, 734937. [Google Scholar] [CrossRef]
- Chang, R.J.A.; Celino-Brady, F.T.; Seale, A.P. Changes in cortisol and corticosteroid receptors during dynamic salinity challenges in Mozambique tilapia. Gen. Comp. Endocrinol. 2023, 342, 114340. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Gewaily, M.S.; Sewilam, H. The growth performance, antioxidative capacity, and histological features of intestines, gills, and livers of Nile tilapia reared in different water salinities and fed menthol essential oil. Aquaculture 2022, 554, 738122. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Sun, Y. Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus). Metabolites 2023, 13, 159. [Google Scholar] [CrossRef]
- Bai, J.; Lutz-Carrillo, D.J.; Quan, Y.; Liang, S. Taxonomic status and genetic diversity of cultured largemouth bass Micropterus salmoides in China. Aquaculture 2008, 278, 27–30. [Google Scholar] [CrossRef]
- Ran, F.Y.; Jin, W.J.; Huang, C. Research progress on the effects of salinity change on fish. J. Northwest Univ. (Nat. Sci. Ed.) 2020, 48, 10–18. [Google Scholar]
- Sun, S.; Gong, C.; Deng, C.; Yu, H.; Zheng, D.; Wang, L.; Sun, J.; Song, F.; Luo, J. Effects of salinity stress on the growth performance, health status, and intestinal microbiota of juvenile Micropterus salmoides. Aquaculture 2003, 576, 739888. [Google Scholar] [CrossRef]
- Lu, G.Z.; Yao, Z.Z.; Lai, Q.F. Growth performance, blood parameters, and texture characteristics of juvenile largemouth bass (Micropterus salmoides) exposed to highly saline-alkaline water. Prog. Fish. Sci. 2022, 43, 1–11. [Google Scholar]
- Ding, M.; Tao, Y.; Hua, J.; Dong, Y.; Lu, S.; Qiang, J.; He, J. Genome-Wide Association Study Reveals Growth-Related SNPs and Candidate Genes in Largemouth Bass (Micropterus salmoides) Adapted to Hypertonic Environments. Int. J. Mol. Sci. 2025, 26, 1834. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Liu, Y.; Du, J.; Lei, C.; Wang, C.; Li, S.; Song, H. Effects of short-term salt exposure on gill damage, serum components and gene expression patterns in juvenile Largemouth bass (Micropterus salmoides). Comp. Biochem. Physiol. Part. D Genom. Proteom. 2025, 53, 101365. [Google Scholar] [CrossRef]
- Árnason, T.; Magnadóttir, B.; Björnsson, B.; Steinarsson, A.; Björnsson, B.T. Effects of salinity and temperature on growth, plasma ions, cortisol and immune parameters of juvenile Atlantic cod (Gadus morhua). Aquaculture 2013, 380–383, 70–79. [Google Scholar] [CrossRef]
- Ghazi, S.; Diab, A.M.; Khalafalla, M.M.; Mohamed, R.A. Synergistic Effects of Selenium and Zinc Oxide Nanoparticles on Growth Performance, Hemato-biochemical Profile, Immune and Oxidative Stress Responses, and Intestinal Morphometry of Nile Tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2022, 200, 364–374. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Li, Y.; Gao, P.; Zhou, K.; Yao, Z.; Sun, Z.; Qin, H.; Lai, Q. Effects of saline and alkaline stresses on the survival, growth, and physiological responses in juvenile mandarin fish (Siniperca chuatsi). Aquaculture 2024, 591, 741143. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Z.; Song, Y.; Yang, J.; Lu, Y.; Lai, W.; Wu, Z.; Zhao, D.; Lin, H.; Zhang, Y.; et al. Effects of salinity on growth, physiology, biochemistry and gut microbiota of juvenile grass carp (Ctenopharyngodon idella). Aquat. Toxicol. 2023, 258, 106482. [Google Scholar] [CrossRef]
- Abozeid, A.M.; Abdel-Rahim, M.M.; Abouelenien, F.A.; Elkaradawy, A.; Mohamed, R.A. Quillaja saponaria and/or Yucca schidigera ameliorate water quality, growth performance, blood health, intestine and gills histomorphology of Nile tilapia, Oreochromis niloticus. Aquac. Res. 2021, 52, 6117–6131. [Google Scholar] [CrossRef]
- Gewaily, M.S.; Abdo, S.E.; Moustafa, E.M.; AbdEl-kader, M.F.; Abd El-Razek, I.M.; El-Sharnouby, M.; Alkafafy, M.; Raza, S.H.; El Basuini, M.F.; Van Doan, H.; et al. Dietary Synbiotics Can Help Relieve the Impacts of Deltamethrin Toxicity of Nile Tilapia Reared at Low Temperatures. Animals 2021, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yao, T.; Zhang, T.; Sun, M.; Ning, Z.; Chen, Y.; Mu, W. Effects of chronic saline-alkaline stress on gill, liver and intestinal histology, biochemical, and immune indexes in Amur minnow (Phoxinus lagowskii). Aquaculture 2024, 579, 740153. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Blood biochemical variables, antioxidative status, and histological features of intestinal, gill, and liver tissues of African catfish (Clarias gariepinus) exposed to high salinity and high-temperature stress. Environ. Sci. Pollut. Res. Int. 2022, 29, 56357–56369. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, Y.; Li, X.; Wu, F.; Song, C.; Liu, Y. The response and osmotic pressure regulation mechanism of Haliotis discus hannai (Mollusca, Gastropoda) to sudden salinity changes. Hydrobiologia 2017, 795, 181–198. [Google Scholar] [CrossRef]
- Lu, W.; Long, L.; Zhao, P.; Zhang, X.; Yan, C.; Dong, S.; Huang, Q. Perfluorinated compounds disrupted osmoregulation in Oryzias melastigma during acclimation to hypoosmotic environment. Ecotoxicol. Environ. Saf. 2021, 223, 112613. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Qi, X.; Zhang, K.; Liu, Y.; Fan, H.; Yu, P.; Tian, Y.; Li, Y. Na+-K+-ATPase and nka genes in spotted sea bass (Lateolabrax maculatus) and their involvement in salinity adaptation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 235, 69–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.-J.; Zhang, J.-Y.; Ndayambaje, E.; Lin, H.; Chen, J.; Hong, H. Chronic exposure to dichloroacetamide induces biochemical and histopathological changes in the gills of zebrafish. Environ. Toxicol. 2019, 34, 781–787. [Google Scholar] [CrossRef]
- Watanabe, S.; Itoh, K.; Kaneko, T. Prolactin and cortisol mediate the maintenance of hyperosmoregulatory ionocytes in gills of Mozambique tilapia: Exploring with an improved gill incubation system. Gen. Comp. Endocrinol. 2016, 232, 151–159. [Google Scholar] [CrossRef]
- Saravanan, M.; Ramesh, M.; Petkam, R.; Poopal, R.K. Influence of environmental salinity and cortisol pretreatment on gill Na+/K+ −ATPase activity and survival and growth rates in Cyprinus carpio. Aquac. Rep. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Yang, W.K.; Chung, C.H.; Cheng, H.C.; Tang, C.H.; Lee, T.H. Different expression patterns of renal Na+/K+-ATPase α-isoform-like proteins between tilapia and milkfish following salinity challenges. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 202, 23–30. [Google Scholar] [CrossRef]
- Bu, X.; Zhu, J.; Liu, S.; Wang, C.; Xiao, S.; Lu, M.; Li, E.; Wang, X.; Qin, J.G.; Chen, L. Growth, osmotic response and transcriptome response of the euryhaline teleost, Oreochromis mossambicus fed different myo-inositol levels under long-term salinity stress. Aquaculture 2021, 534, 736294. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2019, 215, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Xu, S.; Wang, D. Effects of acute low-salinity stress on osmoregulation, antioxidant capacity, and growth of the black sea bream (Acanthopagrus schlegelii). Fish. Physiol. Biochem. 2022, 48, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Zhao, Z.; Luo, L.; Zhang, R.; Guo, K.; Zhang, L.; Yang, Y. Effects of Alkalinity on the Antioxidant Capacity, Nonspecific Immune Response and Tissue Structure of Chinese Mitten Crab Eriocheir sinensis. Fishes 2022, 7, 206. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, C.; Qi, Q.; Wang, R.; Zhang, S.; Yan, R.; Li, B.; Li, S. Effects of salinity stress on anxiety behavior and antioxidant capability of guppy (Poecilia reticulata). Ecotoxicology 2023, 32, 598–605. [Google Scholar] [CrossRef]
- Hiroi, J.; Yasumasu, S.; McCormick, S.D.; Hwang, P.P.; Kaneko, T. Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J. Exp. Biol. 2008, 211, 2584–2599. [Google Scholar] [CrossRef]
- Hebert, S.C.; Mount, D.B.; Gamba, G. Molecular physiology of cation-coupled Cl- cotransport: The SLC12 family. Pflug. Arch. Eur. J. Physiol. 2004, 447, 580–593. [Google Scholar] [CrossRef]
- Cutler, C.P.; Cramb, G. Differential expression of absorptive cation-chloride-cotransporters in the intestinal and renal tissues of the European eel (Anguilla anguilla). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 63–73. [Google Scholar] [CrossRef]
- OECD. Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures; OECD Series on Testing and Assessment; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Hwang, P.-P.; Lee, T.-H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, T.; Zhao, Q.; Wang, S.; Chen, Y.; Ning, Z.; Sun, M.; Zhou, H.; Mu, W. Effects of dietary supplementation with astragalus (Astragalus membranaceus) root or leaf meal on the hematology, serum biochemical parameters, histomorphology, oxidative status, and resistance of Phoxinus lagowskii against bacterial infection. Aquaculture 2023, 565, 739135. [Google Scholar] [CrossRef]
- Takei, Y.; McCormick, S.D. 3-Hormonal Control of Fish Euryhalinity. Fish Physiol. 2013, 32, 69–123. [Google Scholar]
- Wang, J.; Yang, Y.; Wang, Z.; Xu, K.; Xiao, X.; Mu, W. Comparison of effects in sustained and diel-cycling hypoxia on hypoxia tolerance, histology, physiology and expression of clock genes in high latitude fish Phoxinus lagowskii. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 260, 111020. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

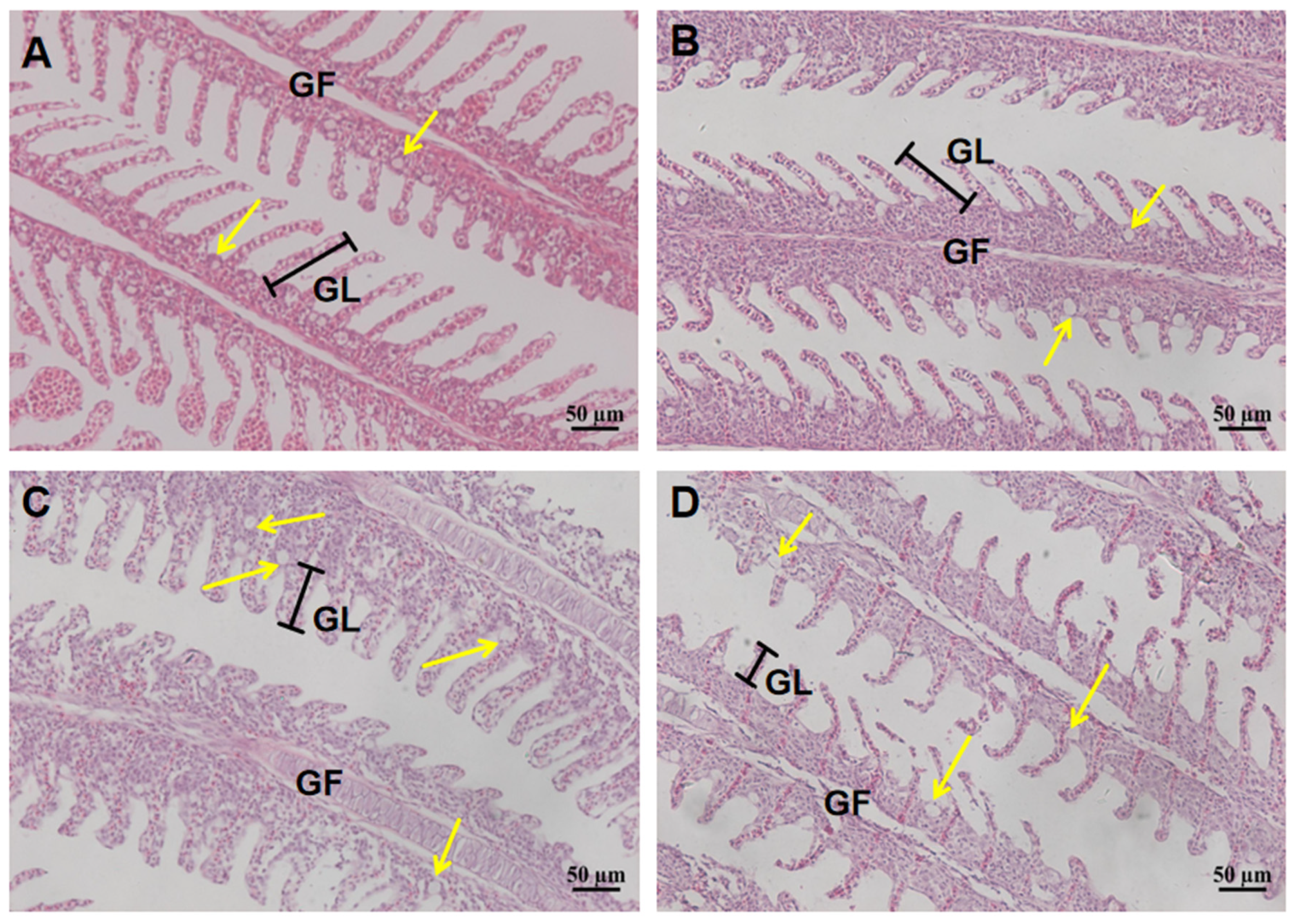

| Indexes | 0 ppt | 6 ppt | 9 ppt | 12 ppt |
|---|---|---|---|---|
| Chlorine cells diameter/µm | 5.3 ± 0.9 b | 5.6±0.7 b | 8.6 ± 1.3 a | 3.1 ± 1.1 c |
| Chlorine cells quantity | 8 ± 1.5 b | 8 ± 1 b | 11.5 ± 2.2 a | 5 ± 0.5 c |
| Gill filament length/µm | 31.7 ± 3.1 a | 29.3 ± 2.6 a | 23.5±2.4 b | 15.7 ± 1.1 c |
| Goblet cells quantity | 22.3 ± 1.5 b | 23.1 ± 1.8 b | 35.6 ± 3.1 a | 19.8 ± 3.7 c |
| Genes | GenBank No. | Primers (5′-3′) |
|---|---|---|
| NKCC1a | XM_038733157.1 | F: GATGATGGCAAAGCTCCAACT R: TGTGCCCTTCCCTTGTTTCTT |
| β-action | XM_020651307.1 | F: AAAGGGAAATCGTGCGTGAC R: AAGGAAGGCTGGAAGAGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tian, J.; Song, H.; Zhu, T.; Lei, C.; Du, J.; Li, S. Osmoregulation and Physiological Response of Largemouth Bass (Micropterus salmoides) Juvenile to Different Salinity Stresses. Int. J. Mol. Sci. 2025, 26, 3847. https://doi.org/10.3390/ijms26083847
Liu Y, Tian J, Song H, Zhu T, Lei C, Du J, Li S. Osmoregulation and Physiological Response of Largemouth Bass (Micropterus salmoides) Juvenile to Different Salinity Stresses. International Journal of Molecular Sciences. 2025; 26(8):3847. https://doi.org/10.3390/ijms26083847
Chicago/Turabian StyleLiu, Yang, Jing Tian, Hongmei Song, Tao Zhu, Caixia Lei, Jinxing Du, and Shengjie Li. 2025. "Osmoregulation and Physiological Response of Largemouth Bass (Micropterus salmoides) Juvenile to Different Salinity Stresses" International Journal of Molecular Sciences 26, no. 8: 3847. https://doi.org/10.3390/ijms26083847
APA StyleLiu, Y., Tian, J., Song, H., Zhu, T., Lei, C., Du, J., & Li, S. (2025). Osmoregulation and Physiological Response of Largemouth Bass (Micropterus salmoides) Juvenile to Different Salinity Stresses. International Journal of Molecular Sciences, 26(8), 3847. https://doi.org/10.3390/ijms26083847





