Imide Polymers with Bipolar-Type Redox-Active Centers for High-Performance Aqueous Zinc Ion Battery Cathodes and Electrochromic Materials
Abstract
1. Introduction
2. Results and Discussion
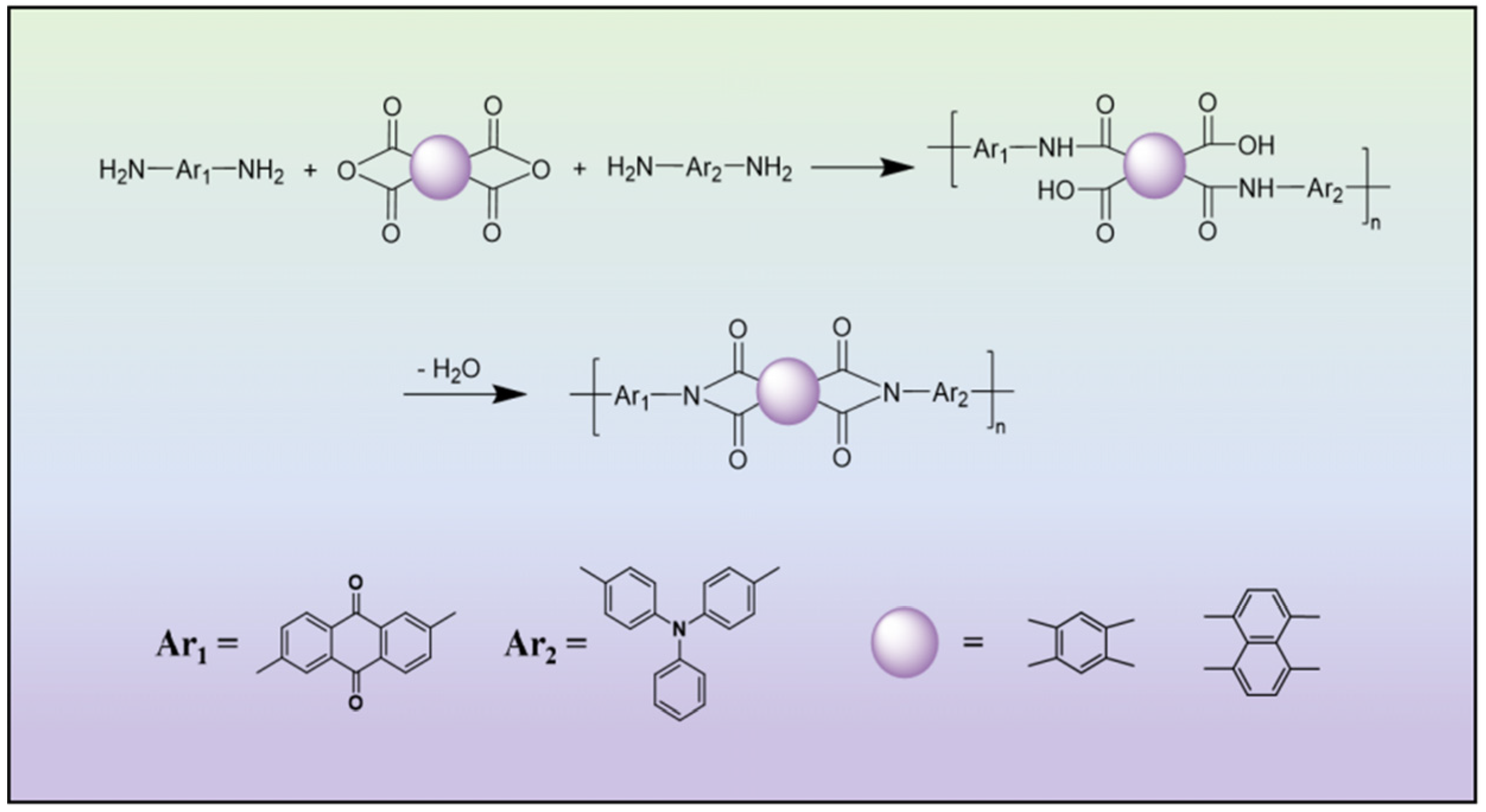
2.1. Physicochemical Characterizations
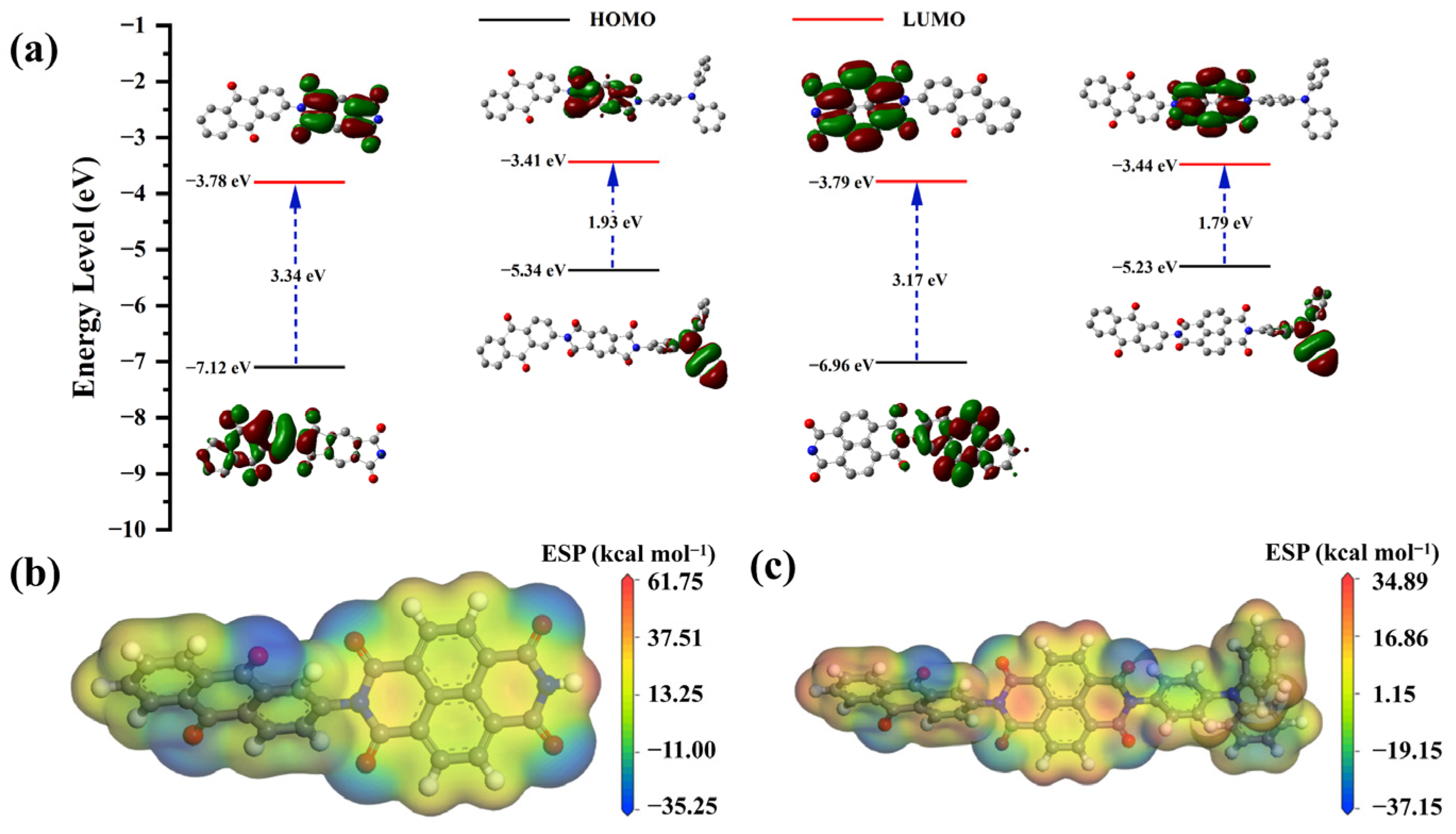
2.2. Quantum Chemistry Calculation
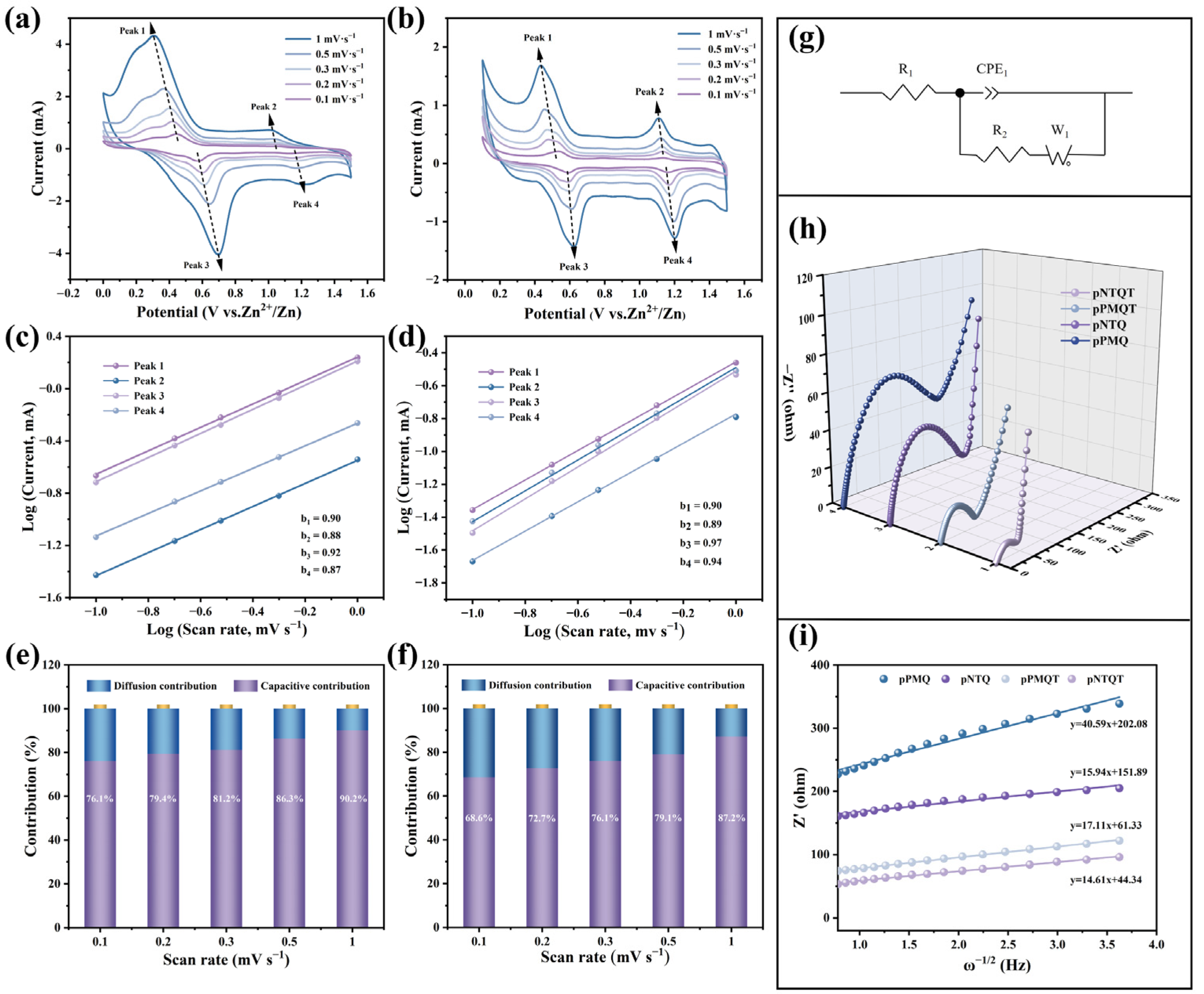
2.3. Electrochemical Analyses
2.4. Analysis of Redox Kinetics
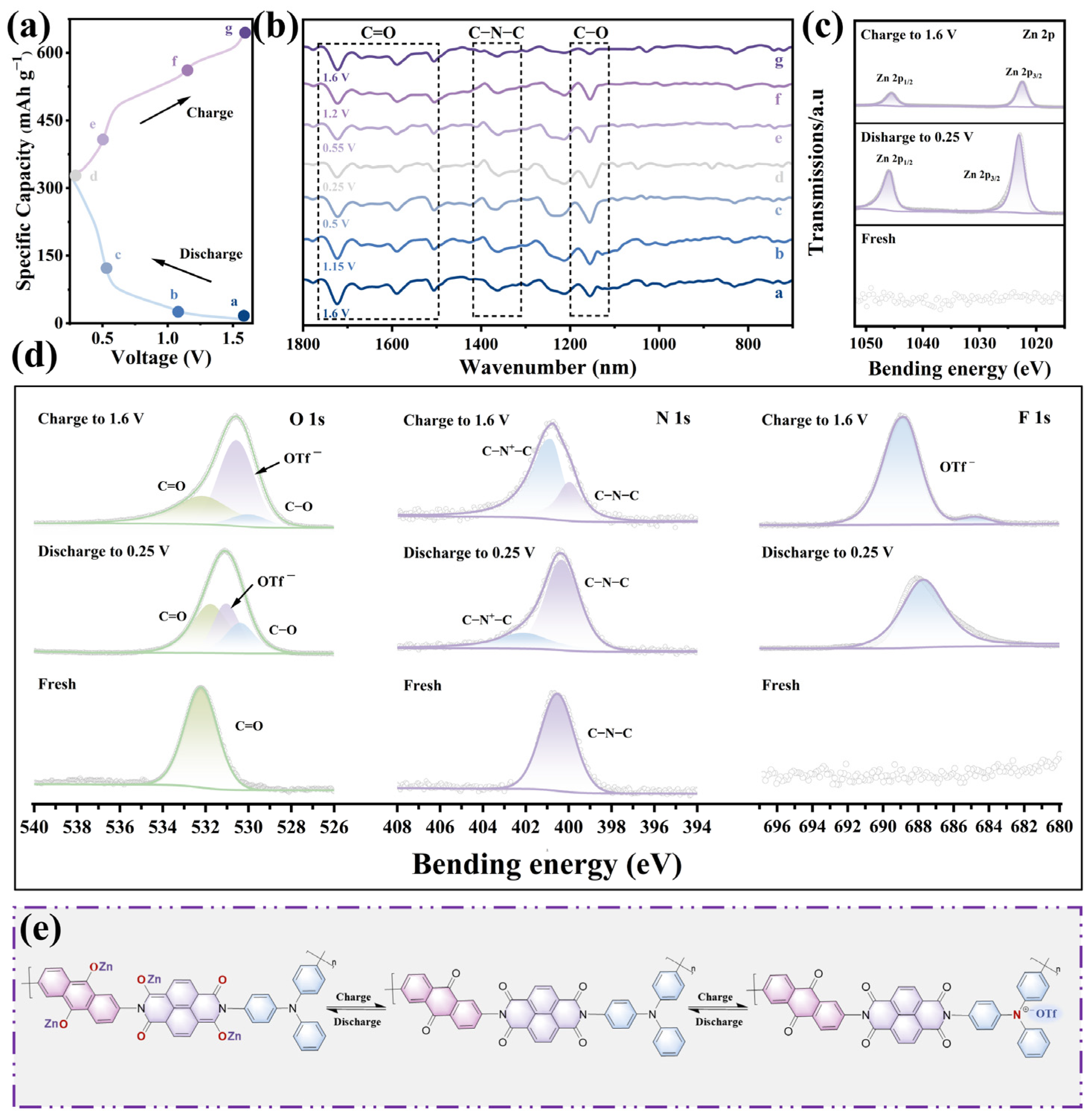
2.5. Electrochemical Reaction Mechanism
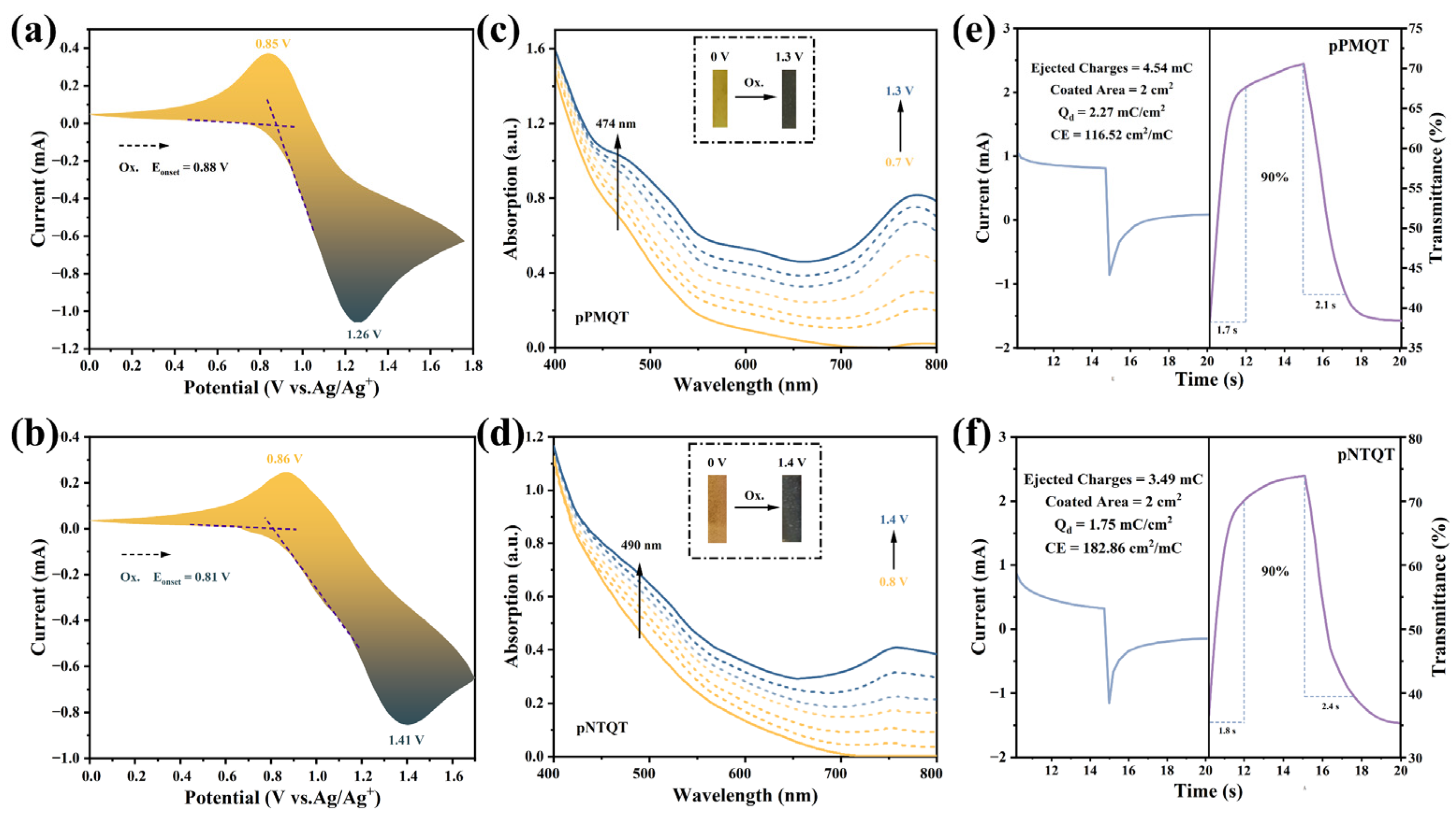
2.6. Electrochromic Properties of pPMQT and pNTQT
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of pPMQ and pNTQ
3.2.2. Synthesis of pPMQT and pNTQT
3.2.3. Electrochemical Measurements
3.2.4. Polymer Films
3.3. Characterizations
3.4. Calculation Methods
3.4.1. Theoretical Specific Capacity
3.4.2. Calculation of Electron Transfer Number
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gou, Q.Z.; Luo, H.R.; Zheng, Y.J.; Zhang, Q.; Li, C.; Wang, J.C.; Odunmbaku, O.; Zheng, J.; Xue, J.M.; Sun, K.; et al. Construction of Bio-inspired Film with Engineered Hydrophobicity to Boost Interfacial Reaction Kinetics of Aqueous Zinc-Ion Batteries. Small 2022, 18, 2201732. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, D.; Liu, Z.; Shang, X.; Liu, Q.; He, D. Dual functional MoS2/graphene interlayer as an efficient polysulfide barrier for advanced lithium-sulfur batteries. Electrochim. Acta 2017, 256, 28–36. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, S.Z.; Yao, Y.; Dong, P.; Song, H.J. Transition Metal Compounds Family for Li-S Batteries: The DFT-Guide for Suppressing Polysulfides Shuttle. Adv. Funct. Mater. 2023, 33, 2300825. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y. Functional Nanostructuring for Efficient Energy Conversion and Storage. Adv. Energy Mater. 2016, 6, 1600461. [Google Scholar] [CrossRef]
- Liu, J.L.; Guo, C.X.; Vasileff, A.; Qiao, S.Z. Nanostructured 2D Materials: Prospective Catalysts for Electrochemical CO2 Reduction. Small Methods 2017, 1, 1600006. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.D.; Zhao, J.; McDowell, M.T.; Lee, H.W.; Zhao, W.T.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanitechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P. Solar Technologies go Hybrid. Adv. Energy Mater. 2017, 7, 1701977. [Google Scholar] [CrossRef]
- Suddard-Bangsund, J.; Traverse, C.J.; Young, M.; Patrick, T.J.; Zhao, Y.M.; Lunt, R.R. Organic Salts as a Route to Energy Level Control in Low Bandgap, High Open-Circuit Voltage Organic and Transparent Solar Cells that Approach the Excitonic Voltage Limit. Adv. Energy Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Wang, D.H.; Chen, A.; Li, X.L.; Huang, Z.D.; Liang, G.J.; Wang, Y.; Zhi, C.Y. Tellurium: A High-Performance Cathode for Magnesium Ion Batteries Based on a Conversion Mechanism. ACS Nano 2022, 16, 5349–5357. [Google Scholar] [CrossRef]
- Kumar, M.; Nagaiah, T.C. High energy density aqueous rechargeable sodium-ion/sulfur batteries in “water in salt” electrolyte. Energy Storage Mater. 2022, 49, 390–400. [Google Scholar] [CrossRef]
- Li, Z.N.; Sami, I.; Yang, J.U.; Li, J.T.; Kumar, R.V.; Chhowalla, M. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium-sulfur batteries. Nat. Energy 2023, 8, 84–93. [Google Scholar] [CrossRef]
- Liu, J.P.; Gong, N.; Peng, W.C.; Li, Y.; Zhang, F.B.; Fan, X.B. Vertically aligned 1 T phase MoS2 nanosheet array for high-performance rechargeable aqueous Zn-ion batteries. Chem. Eng. J. 2022, 428, 130981. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, W.Z.; Sun, C.F. A potassium-tellurium battery. Energy Storage Mater. 2020, 28, 10–16. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Lin, Q.F.; Li, Y.L.; Han, W.; Wang, L.L. Effects of Multiple Ion Reactions Based on a CoSe2/MXene Cathode in Aluminum-Ion Batteries. Adv. Mater. 2023, 35, 2211527. [Google Scholar] [CrossRef]
- Dai, C.L.; Hu, L.Y.; Chen, H.; Jin, X.T.; Han, Y.Y.; Wang, Y.; Li, X.Y.; Zhang, X.Q.; Song, L.; Xu, M.W.; et al. Enabling fast-charging selenium-based aqueous batteries via conversion reaction with copper ions. Nat. Commun. 2022, 13, 1863. [Google Scholar] [CrossRef]
- Li, C.L.; Li, M.; Xu, H.T.; Zhao, F.; Gong, S.Q.; Wang, H.H.; Qi, J.J.; Wang, Z.Y.; Fan, X.B.; Peng, W.C.; et al. Constructing hollow nanotube-like amorphous vanadium oxide and carbon hybrid via in-situ electrochemical induction for high-performance aqueous zinc-ion batteries. J. Colloid Interf. Sci. 2022, 623, 277–284. [Google Scholar] [CrossRef]
- Xu, H.; Yang, W.; Liu, H.; Li, M.; Gong, S.; Zhao, F.; Li, C.; Qi, J.; Wang, H.; Peng, W.; et al. Boosting kinetics of tellurium redox reaction for high-performance aqueous zinc-tellurium batteries. Chem. Eng. J. 2023, 465, 142896. [Google Scholar] [CrossRef]
- Xue, Y.T.; Shen, X.P.; Zhou, H.; Cao, J.Y.; Pu, J.R.; Ji, Z.Y.; Kong, L.R.; Yuan, A.H. Vanadium hexacyanoferrate nanoparticles connected by cross-linked carbon nanotubes conductive networks for aqueous zinc-ion batteries. Chem. Eng. J. 2022, 448, 137657. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, S.Q.; Xu, H.T.; Li, M.; Li, L.A.; Qi, J.J.; Wang, H.H.; Wang, Z.Y.; Hu, Y.Q.; Fan, X.B.; et al. In situ constructing amorphous V2O5@Ti3C2Tx heterostructure for high-performance aqueous zinc-ion batteries. J. Power Sources 2022, 544, 231883. [Google Scholar] [CrossRef]
- Du, G.; Pang, H. Recent advancements in Prussian blue analogues: Preparation and application in batteries. Energy Storage Material. 2021, 36, 387–408. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, N.; Xin, T.; Yuan, X.; Li, Y.; Zhang, Q.; Liu, J. A high-voltage and high-capacity aqueous rechargeable Zn-organic battery using ion-selective membranes. J. Power Sources 2023, 564, 232865. [Google Scholar] [CrossRef]
- Yong, B.; Ma, D.T.; Wang, Y.Y.; Mi, H.W.; He, C.X.; Zhang, P.X. Understanding the Design Principles of Advanced Aqueous Zinc-Ion Battery Cathodes: From Transport Kinetics to Structural Engineering, and Future Perspectives. Adv. Energy Mater. 2020, 10, 2002354. [Google Scholar] [CrossRef]
- Huang, W.W.; Zhu, Z.Q.; Wang, L.J.; Wang, S.W.; Li, H.; Tao, Z.L.; Shi, J.F.; Guan, L.H.; Chen, J. Quasi-Solid-State Rechargeable Lithium-Ion Batteries with a Calix[4]quinone Cathode and Gel Polymer Electrolyte. Angew. Chem. Int. Ed. 2013, 52, 9162–9166. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Mao, L.J.; Wei, Z.X. Recent Progress in Polymeric Carbonyl-Based Electrode Materials for Lithium and Sodium Ion Batteries. Macromol. Rapid Commun. 2019, 40, 1800565. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.; Julien, C.; Paolella, A.; Armand, M.; Zaghib, K. Recent Progress on Organic Electrodes Materials for Rechargeable Batteries and Supercapacitors. Materials 2019, 12, 1770. [Google Scholar] [CrossRef]
- Oubaha, H.; Gohy, J.F.; Melinte, S. Carbonyl-Based π-Conjugated Materials: From Synthesis to Applications in Lithium-Ion Batteries. ChemPlusChem 2019, 84, 1179–1214. [Google Scholar] [CrossRef]
- Schon, T.B.; An, S.Y.; Tilley, A.J.; Seferos, D.S. Unusual Capacity Increases with Cycling for Ladder-Type Microporous Polymers. ACS Appl. Mater. Interfaces 2019, 11, 1739–1747. [Google Scholar] [CrossRef]
- Wang, H.G.; Yuan, S.; Ma, D.L.; Huang, X.L.; Meng, F.L.; Zhang, X.B. Tailored Aromatic Carbonyl Derivative Polyimides for High-Power and Long-Cycle Sodium-Organic Batteries. Adv. Energy Mater. 2014, 4, 1301651–1301657. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, H.; Beldjoudi, Y.; Kwon, T.W.; Kim, D.J.; Stoddart, J.F. Redox-Active Phenanthrenequinone Triangles in Aqueous Rechargeable Zinc Batteries. J. Am. Chem. Soc. 2020, 142, 2541–2548. [Google Scholar] [CrossRef]
- Friebe, C.; Schubert, U.S. High-Power-Density Organic Radical Batteries. Top. Curr. Chem. 2017, 375, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Glatz, H.; Lizundia, E.; Pacifico, F.; Kundu, D. An Organic Cathode Based Dual-Ion Aqueous Zinc Battery Enabled by a Cellulose Membrane. ACS Appl. Energy Mater. 2019, 2, 1288. [Google Scholar] [CrossRef]
- Koshika, K.; Sano, N.; Oyaizu, K.; Nishide, H. An Aqueous, Electrolyte-Type, Rechargeable Device Utilizing a Hydrophilic Radical Polymer-Cathode. Macromol. Chem. Phys. 2009, 210, 1989–1995. [Google Scholar] [CrossRef]
- Tie, Z.W.; Niu, Z.Q. Design Strategies for High-Performance Aqueous Zn/Organic Batteries. Angew. Chem. Int. Ed. 2020, 59, 21293–21303. [Google Scholar] [CrossRef]
- Chen, Z.X.; Mei, S.W.; Li, W.J.; Xu, N.; Dong, Y.J.; Jin, Y.X.; Ouyang, M.; Zhang, C. Study of multi-electron redox mechanism via electrochromic behavior in hexaazatrinaphthylene-based polymer as the cathode of lithium-organic batteries. J. Mater. Chem. A 2021, 9, 27010–27018. [Google Scholar] [CrossRef]
- Ciobanu, M.; Klein, J.; Middendorf, M.; Beladi Mousavi, S.M.; Carl, F.; Haase, M.; Walder, L. High contrast hybrid electrochromic film based on cross-linked phosphonated triarylamine on mesoporous antimony doped tin oxide. Sol. Energy Mater. Sol. Cell 2019, 203, 110186. [Google Scholar] [CrossRef]
- Ma, X.C.; Niu, H.J.; Wen, H.L.; Wang, S.H.; Lian, Y.F.; Jiang, X.K.; Wang, C.; Bai, X.D.; Wang, W. Synthesis, electrochromic, halochromic and electro-optical properties of polyazomethines with a carbazole core and triarylamine units serving as functional groups. J. Mater. Chem. C 2015, 3, 3482–3493. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhong, L.F.; Xie, J.H.; Yang, F.; Liu, X.Q.; Lu, X.H. A COF-Like N-Rich Conjugated Microporous Polytriphenylamine Cathode with Pseudocapacitive Anion Storage Behavior for High-Energy Aqueous Zinc Dual-Ion Batteries. Adv. Mater. 2021, 33, 2101857. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Lansåker, P.C.; Mlyuka, N.R.; Niklasson, G.A.; Avendaño, E. Progress in chromogenics: New results for electrochromic and thermochromic materials and devices. Sol. Energy Mater. Sol. Cell 2009, 93, 2032–2039. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Hsu, P.-N.; Chen, W.-H.; Lin, S.-L. High Glass Transitions of New Polyamides, Polyimides, and Poly(amide−imide)s Containing a Triphenylamine Group: Synthesis and Characterization. Macromolecules 2002, 35, 4669–4676. [Google Scholar] [CrossRef]
- Wang, J.L.; Lv, H.; Huang, L.L.; Li, J.H.; Xie, H.J.; Wang, G.; Gu, T.T. Anhydride-Based Compound with Tunable Redox Properties as Advanced Organic Cathodes for High-Performance Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 49447–49457. [Google Scholar] [CrossRef] [PubMed]
- Ba, Z.; Wang, Z.; Luo, M.; Li, H.-b.; Li, Y.; Huang, T.; Dong, J.; Zhang, Q.; Zhao, X. Benzoquinone-Based Polyimide Derivatives as High-Capacity and Stable Organic Cathodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.L.; Zhu, C.; Song, M.; Liang, P.; Zhang, X.; Tiep, N.H.; Zhao, H.F.; Wang, J.; Wang, R.M.; Zhang, H.; et al. A High-Rate and Stable Quasi-Solid-State Zinc-Ion Battery with Novel 2D Layered Zinc Orthovanadate Array. Adv. Mater. 2018, 30, 1803181. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yan, M.Y.; Zhang, G.B.; Sun, R.M.; Chen, L.N.; An, Q.Y.; Mai, L.Q. Layered VS2 Nanosheet-Based Aqueous Zn Ion Battery Cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Zhao, W.T.; Li, H.; Xu, Q. Cross-Conjugated Polycatechol Organic Cathode for Aqueous Zinc-Ion Storage. ChemSusChem 2020, 13, 188–195. [Google Scholar] [CrossRef]
- Ning, J.Y.; Zhang, X.P.; Xie, D.J.; He, Q.; Hu, J.; Tang, J.J.; Li, R.; Meng, H.; Yao, K.X. Unveiling Phenoxazine’s Unique Reversible Two-Electron Transfer Process and Stable Redox Intermediates for High-Performance Aqueous Zinc-ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202319796. [Google Scholar] [CrossRef]
- Wang, W.X.; Kale, V.S.; Cao, Z.; Lei, Y.J.; Kandambeth, S.; Zou, G.D.; Zhu, Y.P.; Abouhamad, E.; Shekhah, O.; Cavallo, L.; et al. Molecular Engineering of Covalent Organic Framework Cathodes for Enhanced Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2103617. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.X.; Wu, F.; Chen, R.J.; Li, L. High-Performance Aqueous Zinc Batteries Based on Organic/Organic Cathodes Integrating Multiredox Centers. Adv. Mater. 2021, 33, 2106469. [Google Scholar] [CrossRef]
- Wang, S.M.; Guo, Q.F.; Liu, H.R.; Zhang, L.H.; Zhang, C.F.; Zhou, T.F.; Ma, Q.W.; Li, H.B.; Wang, R.; Zheng, Y. Design of a bipolar organic small-molecule cathode with mesoporous nanospheres structure for long lifespan and high-rate Li-storage performance. Chem. Sci. 2024, 15, 1051–1060. [Google Scholar] [CrossRef]
- Kapaev, R.R.; Scherbakov, A.G.; Shestakov, A.F.; Stevenson, K.J.; Troshin, P.A. m-Phenylenediamine as a Building Block for Polyimide Battery Cathode Materials. ACS Appl. Energy Mater. 2021, 4, 4465–4472. [Google Scholar] [CrossRef]
- Labasan, K.B.; Lin, H.-J.; Baskoro, F.; Togonon, J.J.H.; Wong, H.Q.; Chang, C.-W.; Arco, S.D.; Yen, H.-J. Dicyanotriphenylamine-Based Polyimides as High-Performance Electrodes for Next Generation Organic Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 17467–17477. [Google Scholar] [CrossRef]
- Wang, H.-g.; Li, Q.; Wu, Q.; Si, Z.; Lv, X.; Liang, X.; Wang, H.; Sun, L.; Shi, W.; Song, S. Conjugated Microporous Polymers with Bipolar and Double Redox-Active Centers for High-Performance Dual-Ion, Organic Symmetric Battery. Adv. Energy Mater. 2021, 11, 2100381. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, S.; Zhang, W.; Song, W. Dual functional electrochromic/electrofluorochromic conjugated D-A polymer based on thieno[3,4-c]pyrrole-4,6-dione. Dye. Pigment. 2023, 219, 111566. [Google Scholar] [CrossRef]
- Zhao, J.X.; Zhou, M.J.; Zhang, Y.C.; Berda, E.B.; Chao, D.M. Water-Soluble Hyperbranched Polyamidoamine Bearing Viologen Groups toward Electrochromic/Electrofluorochromic Dual-Mode Aqueous Phase Devices. Macromol. Mater. Eng. 2022, 307, 2100977. [Google Scholar] [CrossRef]
- Keller, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Copper(I) and silver(I) complexes of 9,9-dimethyl-4,5-bis(di-tert-butylphosphino) xanthene: Photophysical properties and structural rigidity under pressure. Photochemical. Photobiol. Sci. 2018, 17, 375–385. [Google Scholar] [CrossRef]
- Li, Z.H.; Tan, J.; Gao, C.Y.; Wang, Y.; Wang, Y.G.; Ye, M.X.; Shen, J.F. Building better aqueous Zn-organic batteries. Energ Environ. Sci. 2023, 16, 2398–2431. [Google Scholar] [CrossRef]
- Shitahun, A.; Atlabachew, M.; Aragaw, B.A.; Benor, A.; Metto, M.; Abebe, A. Synthesis, characterization, and application of a novel electrochemical sensor based on poly [Mn(Chr)3]Cl2/PGE for the determination of ciprofloxacin in pharmaceuticals and urine samples. Int. J. Electrochem. Sci. 2025, 20, 100937. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, Y.; Mei, B.; Liu, J.; Niu, H.; Hou, Y. Imide Polymers with Bipolar-Type Redox-Active Centers for High-Performance Aqueous Zinc Ion Battery Cathodes and Electrochromic Materials. Int. J. Mol. Sci. 2025, 26, 3838. https://doi.org/10.3390/ijms26083838
Liu Z, Li Y, Mei B, Liu J, Niu H, Hou Y. Imide Polymers with Bipolar-Type Redox-Active Centers for High-Performance Aqueous Zinc Ion Battery Cathodes and Electrochromic Materials. International Journal of Molecular Sciences. 2025; 26(8):3838. https://doi.org/10.3390/ijms26083838
Chicago/Turabian StyleLiu, Zixuan, Yan Li, Binhua Mei, Jiaxue Liu, Haijun Niu, and Yanjun Hou. 2025. "Imide Polymers with Bipolar-Type Redox-Active Centers for High-Performance Aqueous Zinc Ion Battery Cathodes and Electrochromic Materials" International Journal of Molecular Sciences 26, no. 8: 3838. https://doi.org/10.3390/ijms26083838
APA StyleLiu, Z., Li, Y., Mei, B., Liu, J., Niu, H., & Hou, Y. (2025). Imide Polymers with Bipolar-Type Redox-Active Centers for High-Performance Aqueous Zinc Ion Battery Cathodes and Electrochromic Materials. International Journal of Molecular Sciences, 26(8), 3838. https://doi.org/10.3390/ijms26083838






