Systematic Inflammation and Oxidative Stress Elevation in Diabetic Retinopathy and Diabetic Patients with Macular Edema
Abstract
1. Introduction
2. Results
2.1. Main Biochemical Serum Parameters in the Studied Patients
- -
- Thickening approximately 500 μm from the center of the macula.
- -
- Hard exudates around the center of the macula, in case the underlying retina is also thickened.
- -
- Presence of an area of retinal thickening with a diameter of about 1500 µm or more, located at a distance of 1DD or less from the center of the macula.
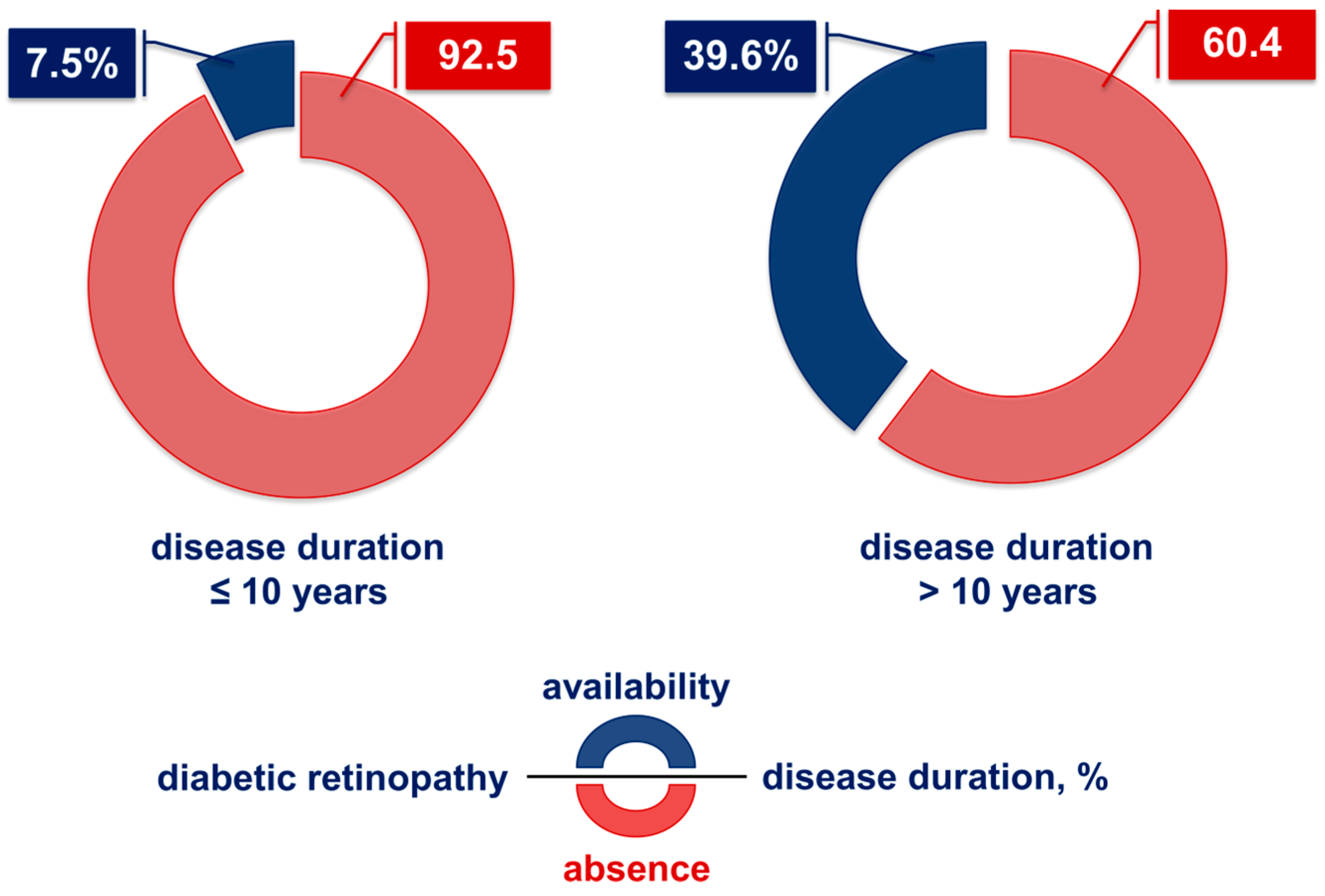
2.2. Influence of the Duration of Type 2 Diabetes on the Development of Diabetic Retinopathy
2.3. Influence of Disease Duration on the Manifestation of DME
2.4. Levels of ROS and AGEs
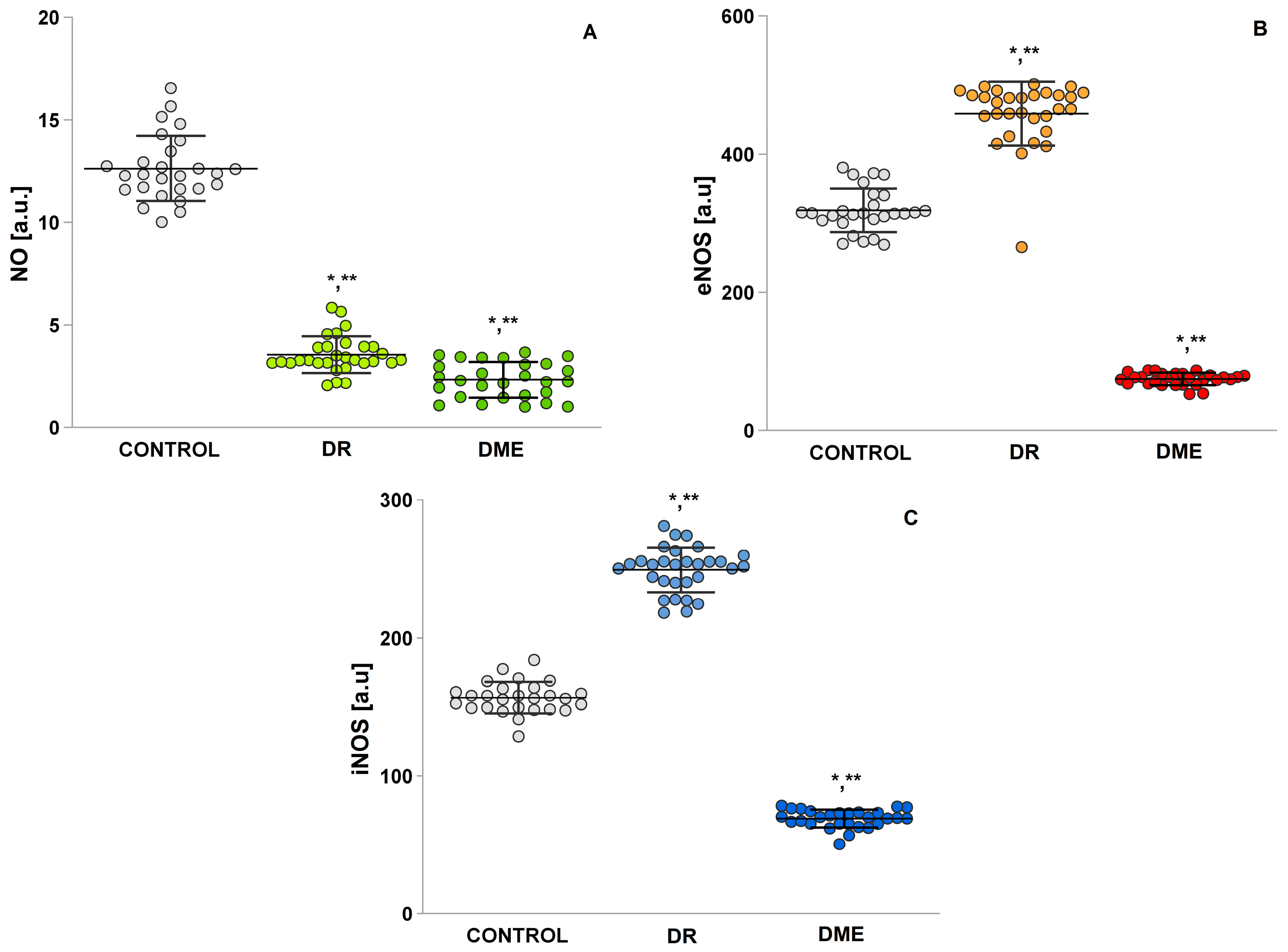
2.5. Serum Levels of NO, Endothelial Nitric Oxide Synthase (eNOS), and Inducible iNOS
2.6. Mean Serum Levels of the Pro-Oxidant Malondialdehyde (MDA) and Pro-Oxidant Molecules 4-Hydroxy-2-Nonenal (4-HNE)
2.7. DNA Oxidation Biomarker Study: 8-Hydroxy-2-Deoxyguanosine (8-OHdG) Level
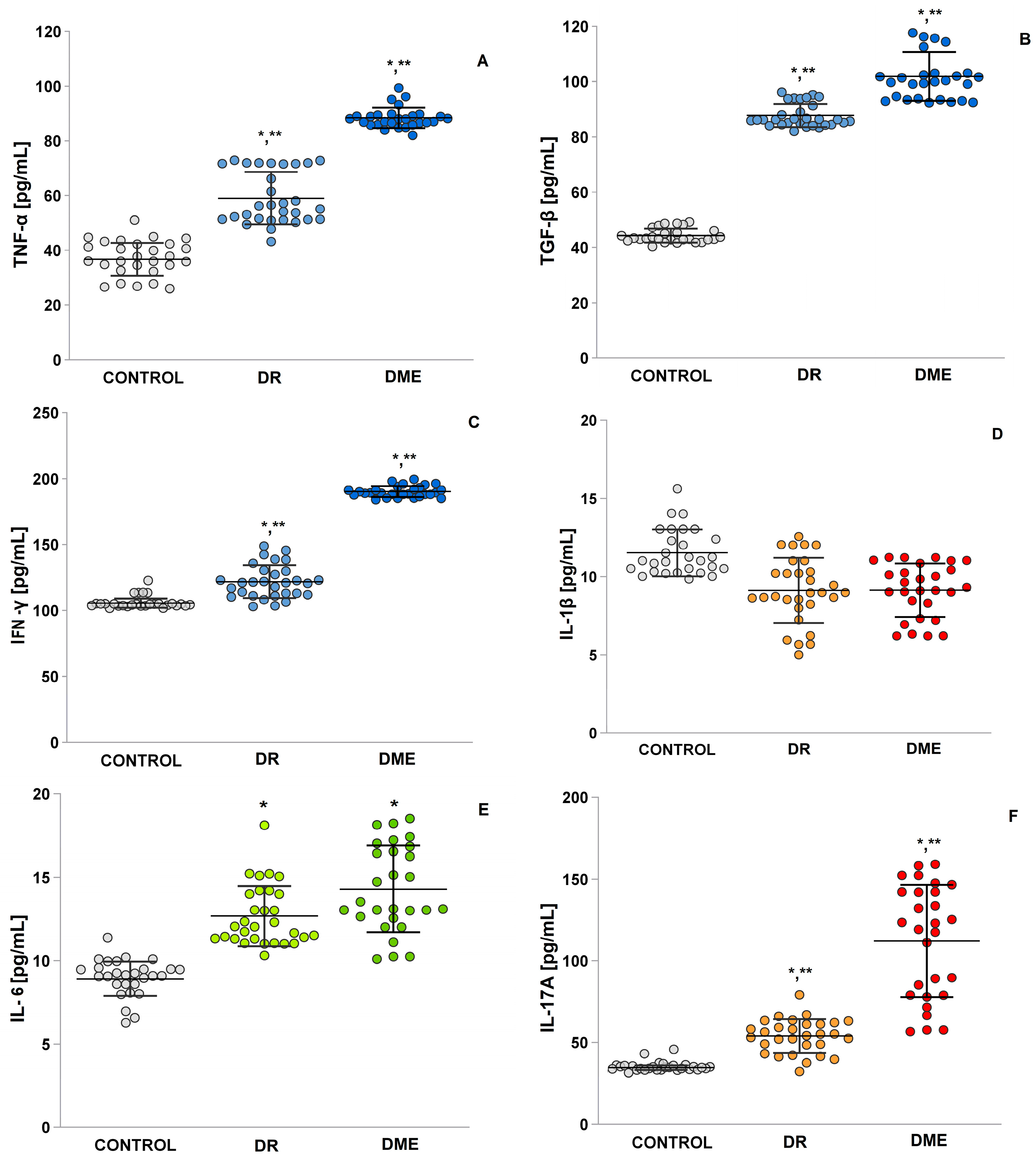
2.8. An Examination of the Levels of TNF-α, TGF-β, IFN-γ, IL-1β and IL-6, and IL-17A
3. Discussion
Limitations
- -
- Another limitation is the need to study several redox and inflammatory markers, since monitoring a limited number of them could not provide a complete picture of the redox state of the body. This requires the introduction of a multidisciplinary approach and personalized medical assessment.
- -
- At the same time, not all laboratories have the full range of specific equipment for the analysis, for example, an EPR spectrometer, which is the gold standard in assessing redox imbalance and levels of oxidants in the body. Instead, less sensitive and less specific spectrophotometric methods are used, which produce a high level of error. The assessment of redox imbalance and ROS and RNS levels by EPR introduces the need to create a single protocol for the analysis of serum samples from patients with DR-DME, which is currently not registered and unified.
- -
- There is individual variability between individual patients in terms of metabolism, body mass index (BMI); genetic and epigenetic factors; the activity of single-gene antioxidant systems (SOD, GPx, etc.); intracellular ROS metabolism (including mitochondrial damage); immune response (hyperinflammatory phenotype or immune dysfunction); presence of dysbiosis, etc. In certain patients, a non-specific correlation between individual markers can be observed, which explains why some patients with diabetes develop severe DR-DME compared to others despite similar therapeutic regimens.
- -
- Diet and intake of nutritional supplements with antioxidant properties, such as alpha-lipoic acid, vitamins, glutathione, or medications, for the management of DR-DME and chronic diseases that can influence or modify oxidative stress and inflammation to one degree or another are essential.
4. Materials and Methods
4.1. Ethics Statement
4.2. Ophthalmologic Examination
4.3. Electron Paramagnetic Resonance (EPR) Study
4.3.1. An Evaluation of the ROS Product Levels
4.3.2. An Evaluation of the •NO Radical Levels
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorour, O.A.; Levine, E.S.; Baumal, C.R.; Elnahry, A.G.; Braun, P.; Girgis, J.; Waheed, N.K. Persistent diabetic macular edema: Definition, incidence, biomarkers, and treatment methods. Surv. Ophthalmol. 2023, 68, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Čater, M.; Križančić Bombek, L. Protective Role of Mitochondrial Uncoupling Proteins against Age-Related Oxidative Stress in Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 1473. [Google Scholar] [CrossRef]
- Sanz-González, S.M.; García-Medina, J.J.; Zanón-Moreno, V.; López-Gálvez, M.I.; Galarreta-Mira, D.; Duarte, L.; Valero-Velló, M.; Ramírez, A.I.; Arévalo, J.F.; Pinazo-Durán, M.D.; et al. Clinical and Molecular-Genetic Insights into the Role of Oxidative Stress in Diabetic Retinopathy: Antioxidant Strategies and Future Avenues. Antioxidants 2020, 9, 1101. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Salobrar-García, E.; Pinazo-Durán, M.D.; Ramírez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen. Res. 2020, 15, 1408. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; de Julián-López, E.; Soler-Domínguez, C.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Ramírez, J.M.; Pinazo-Durán, M.D.; Salazar, J.J.; Ramírez, A.I. The Role of Autophagy in Eye Diseases. Life 2021, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Fragiotta, S.; Pinazo-Durán, M.D.; Scuderi, G. Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy. Nutrients 2022, 14, 792. [Google Scholar] [CrossRef]
- Li, J.; Zhao, T.; Sun, Y. Interleukin-17A in diabetic retinopathy: The crosstalk of inflammation and angiogenesis. Biochem. Pharmacol. 2024, 225, 116311. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.E.; Lee, C.A.; Zapadka, T.E.; Zhou, A.Y.; Barber, K.G.; Taylor, Z.R.R.; Howell, S.J.; Taylor, P.R. IL-17A Enhances Retinal Neovascularization. Int. J. Mol. Sci. 2023, 24, 1747. [Google Scholar] [CrossRef]
- Vofo, B.N.; Chowers, I. Suppressing Inflammation for the Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration: Dazdotuftide as a Potential New Multitarget Therapeutic Candidate. Biomedicines 2023, 11, 1562. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, H.; Bao, S.; Wang, N.; Gillies, M. CDiabetic macular edema: New concepts in patho-physiology and treatment. Cell Biosci. 2014, 4, 1. [Google Scholar] [CrossRef]
- Stewart, E.A.; Saker, S.; Amoaku, W.M. Dexamethasone reverses the effects of high glucose on human retinal endothelial cell permeability and proliferation in vitro. Exp. Eye Res. 2016, 151, 75. [Google Scholar] [CrossRef]
- Andrés-Blasco, I.; Gallego-Martínez, A.; Palos, S.K.; Lleó, A.; Casaroli, R.; Di Lauro, S.; Pinazo-Duran, M.D. Crosstalk between oxidative stress, inflammation, angiogenesis and apoptosis in diabetic macular edema. Acta Ophthalmol. 2024, 102. [Google Scholar] [CrossRef]
- Pessoa, B.B.D.S.T. The Role of Vitreous and Vitreoretinal Interface in the Management of Diabetic Macular Edema. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2022. [Google Scholar]
- Kowluru, R.A. Cross Talks between Oxidative Stress, Inflammation and Epigenetics in Diabetic Retinopathy. Cells 2023, 12, 300. [Google Scholar] [CrossRef]
- Karam-Palos, S.; Andrés-Blasco, I.; Campos-Borges, C.; Zanón-Moreno, V.; Gallego-Martínez, A.; Alegre-Ituarte, V.; García-Medina, J.J.; Pastor-Idoate, S.; Sellés-Navarro, I.; Vila-Arteaga, J.; et al. Oxidative Stress Mediates Epigenetic Modifications and the Expression of miRNAs and Genes Related to Apoptosis in Diabetic Retinopathy Patients. J. Clin. Med. 2024, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Milluzzo, A.; Maugeri, A.; Barchitta, M.; Sciacca, L.; Agodi, A. Epigenetic Mechanisms in Type 2 Diabetes Retinopathy: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10502. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Mukai, E.; Fujimoto, S.; Inagaki, N. Role of Reactive Oxygen Species in Glucose Metabolism Disorder in Diabetic Pancreatic β-Cells. Biomolecules 2022, 12, 1228. [Google Scholar] [CrossRef]
- Lin, W.; Shen, P.; Song, Y.; Huang, Y.; Tu, S. Reactive oxygen species in autoimmune cells: Function, differentiation, and metabolism. Front. Immunol. 2021, 12, 635021. [Google Scholar] [CrossRef]
- Rajlic, S.; Treede, H.; Münzel, T.; Daiber, A.; Duerr, G.D. Early Detection Is the Best Prevention—Characterization of Oxidative Stress in Diabetes Mellitus and Its Consequences on the Cardiovascular System. Cells 2023, 12, 583. [Google Scholar] [CrossRef]
- Suresh, V.; Reddy, A. Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impedement. J. Diabetes Metab. Disord. 2021, 20, 989. [Google Scholar] [CrossRef]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhadada, S.K. AGEs accumulation with vascular complications, glycemic control and metabolic syndrome: A narrative review. Bone 2023, 176, 116884. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Involvement of Cytokines in the Pathogenesis of Diabetic Macular Edema. Int. J. Mol. Sci. 2021, 22, 3427. [Google Scholar] [CrossRef]
- Tang, L.; Xu, G.; Zhang, J.F. Inflammation in diabetic retinopathy: Possible roles in pathogenesis and potential implications for therapy. Neural Regen. Res. 2023, 18, 976–982. [Google Scholar] [CrossRef]
- Nebbioso, M.; Franzone, F.; Lambiase, A.; Bonfiglio, V.; Limoli, P.G.; Artico, M.; Taurone, S.; Vingolo, E.M.; Greco, A.; Polimeni, A. Oxidative Stress Implication in Retinal Diseases—A Review. Antioxidants 2022, 11, 1790. [Google Scholar] [CrossRef]
- Park, Y.G.; Park, Y.S.; Kim, I.-B. Complement System and Potential Therapeutics in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 6851. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.D.; Kodati, B.; Stankowska, D.L.; Krishnamoorthy, R.R. Role of mitophagy in ocular neurodegeneration. Front. Neurosci. 2023, 17, 1299552. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Majsterek, I. Relationship between Biochemical Pathways and Non-Coding RNAs Involved in the Progression of Diabetic Retinopathy. J. Clin. Med. 2024, 13, 292. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Bokhary, K.; Aljaser, F.; Abudawood, M.; Tabassum, H.; Bakhsh, A.; Alhammad, S.; Alsubki, R. Role of oxidative stress and severity of diabetic retinopathy in type 1 and type 2 diabetes. Ophthalmic Res. 2021, 64, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Karan, B.M.; Little, K.; Augustine, J.; Stitt, A.W.; Curtis, T.M. Aldehyde Dehydrogenase and Aldo-Keto Reductase Enzymes: Basic Concepts and Emerging Roles in Diabetic Retinopathy. Antioxidants 2023, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Choudhury, A.D.; Agrawal, S.; Bisen, A.C.; Sanap, S.N.; Verma, S.K.; Bhatta, R.S. Recent insights into the etiopathogenesis of diabetic retinopathy and its management. J. Ocul. Pharmacol. Ther. 2024, 40, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Induction of 5-lipoxygenase by 4-hydroxynonenal via Nitric Oxide Generation in Vascular Smooth Muscle Cells. J. Korean Ophthalmic Opt. Soc. 2023, 28, 255. [Google Scholar] [CrossRef]
- Do, D.V.; Agarwal, A.; Nguyen, Q.D.; Haller, J.A. Retinal arterial Macroaneurysms. In Albert and Jakobiec’s Principles and Practice of Ophthalmology; Springer International Publishing: Cham, Switzerland, 2022; p. 3191. [Google Scholar] [CrossRef]
- Mohanty, K.; Dada, R.; Dada, T. Oxidative DNA damage and reduced expression of DNA repair genes: Role in primary open angle glaucoma (POAG). Ophthalmic Genet. 2017, 38, 446. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of oxidative stress in retinal disease and the early intervention strategies: A review. Oxidative Med. Cell. Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef]
- Tangvarasittichai, O.; Tangvarasittichai, S. Oxidative stress, ocular disease and diabetes retinopathy. Curr. Pharm. Des. 2018, 24, 4726. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- López-Contreras, A.K.; Martínez-Ruiz, M.G.; Olvera-Montaño, C.; Robles-Rivera, R.R.; Arévalo-Simental, D.E.; Castellanos-González, J.A.; Hernández-Chávez, A.; Huerta-Olvera, S.G.; Cardona-Muñoz, E.G.; Rodríguez-Carrizalez, A.D. Importance of the Use of Oxidative Stress Biomarkers and Inflammatory Profile in Aqueous and Vitreous Humor in Diabetic Retinopathy. Antioxidants 2020, 9, 891. [Google Scholar] [CrossRef]
- Chen, K.H.; Hsiang, E.L.; Hsu, M.Y.; Chou, Y.C.; Lin, T.C.; Chang, Y.L.; Hwang, D.K. Elevation of serum oxidative stress in patients with retina vein occlusions. Acta Ophthalmol. 2019, 97, 290. [Google Scholar] [CrossRef]
- Hussain, A.; Ashique, S.; Afzal, O.; Altamimi, M.A.; Malik, A.; Kumar, S.; Altamimi, A.S. A correlation between oxidative stress and diabetic retinopathy: An updated review. Exp. Eye Res. 2023, 236, 109650. [Google Scholar] [CrossRef] [PubMed]
- Youwakim, J.; Vallerand, D.; Girouard, H. Neurovascular Coupling in Hypertension Is Impaired by IL-17A through Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 3959. [Google Scholar] [CrossRef]
- Starace, V.; Battista, M.; Brambati, M.; Cavalleri, M.; Bertuzzi, F.; Amato, A.; Cicinelli, M.V. The role of inflammation and neurodegeneration in diabetic macular edema. Ther. Adv. Ophthalmol. 2021, 13. [Google Scholar] [CrossRef]
- Zhou, A.Y.; Taylor, B.E.; Barber, K.G.; Lee, C.A.; Taylor, Z.R.R.; Howell, S.J.; Taylor, P.R. Anti-IL17A Halts the Onset of Diabetic Retinopathy in Type I and II Diabetic Mice. Int. J. Mol. Sci. 2023, 24, 1347. [Google Scholar] [CrossRef] [PubMed]
- Zapadka, T.E.; Lindstrom, S.I.; Taylor, B.E.; Lee, C.A.; Tang, J.; Taylor, Z.R.R.; Howell, S.J.; Taylor, P.R. RORγt Inhibitor-SR1001 Halts Retinal Inflammation, Capillary Degeneration, and the Progression of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 3547. [Google Scholar] [CrossRef]
- Zapadka, T.E.; Lindstrom, S.I.; Batoki, J.C.; Lee, C.A.; Taylor, B.E.; Howell, S.J.; Taylor, P.R. Aryl Hydrocarbon Receptor Agonist VAF347 Impedes Retinal Pathogenesis in Diabetic Mice. Int. J. Mol. Sci. 2021, 22, 4335. [Google Scholar] [CrossRef]
- Howell, S.J.; Lee, C.A.; Batoki, J.C.; Zapadka, T.E.; Lindstrom, S.I.; Taylor, B.E.; Lee, C.A.; Tang, J.; Taylor, Z.R.R.; Howell, S.J.; et al. Retinal inflammation, oxidative stress, and vascular impairment is ablated in diabetic mice receiving XMD8-92 treatment. Front. Pharmacol. 2021, 12, 732630. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Carnt, N.; Pattamatta, U.; Samarawickrama, C.; White, A.; Calder, V. A review of the cytokine IL-17 in ocular surface and corneal disease. Curr. Eye Res. 2019, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haydinger, C.D.; Ferreira, L.B.; Williams, K.A.; Smith, J.R. Mechanisms of macular edema. Front. Med. 2023, 10, 1128811. [Google Scholar] [CrossRef]
- Wang, X.N.; Cai, X.; Li, T.T.; Long, D.; Wu, Q. Peripapillary vessel density and retinal nerve fiber layer thickness changes in early diabetes retinopathy. Int. J. Ophthalmol. 2022, 15, 1488–1495. [Google Scholar] [CrossRef]
- Minaker, S.A.; Mason, R.H.; Lahaie Luna, G.; Farahvash, A.; Garg, A.; Bhambra, N.; Bapat, P.; Muni, R.H. Changes in Aqueous and Vitreous Inflammatory Cytokine Levels in Diabetic Macular Oedema: A Systematic Review and Meta-analysis. Acta Ophthalmol. 2022, 100, 53. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.; Tran, T.; Sanfilippo, P.; Lim, L.L.; Sandhu, S.S.; Wickremasinghe, S. Elevated aqueous TNF-α levels are associated with more severe functional and anatomic findings in eyes with diabetic macular oedema. Clin. Exp. Ophthalmol. 2024, 52, 981–990. [Google Scholar] [CrossRef]
- Machalińska, A.; Kuligowska, A.; Ziontkowska-Wrzałek, A.; Stroynowska, B.; Pius-Sadowska, E.; Safranow, K.; Machaliński, J.; Mozolewska-Piotrowska, K.; Machaliński, B. The Severity of Diabetic Retinopathy Corresponds with Corneal Nerve Alterations and Ocular Discomfort of the Patient. Int. J. Mol. Sci. 2024, 25, 6072. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, G.; Puig, L. Pathogenic Role of IL-17 and Therapeutic Targeting of IL-17F in Psoriatic Arthritis and Spondyloarthropathies. Int. J. Mol. Sci. 2023, 24, 10305. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, H.; Huang, Z.; Liao, W.; Zou, T.; Shang, X.; Yu, H. M1 linear ubiquitination of LKB1 inhibits vascular endothelial cell injury in atherosclerosis through activation of AMPK. Hum. Cell 2023, 36, 1901–1914. [Google Scholar] [CrossRef]
- Richter, P.; Macovei, L.A.; Mihai, I.R.; Cardoneanu, A.; Burlui, M.A.; Rezus, E. Cytokines in Systemic Lupus Erythematosus—Focus on TNF-α and IL-17. Int. J. Mol. Sci. 2023, 24, 14413. [Google Scholar] [CrossRef] [PubMed]
- Gomułka, K.; Ruta, M. The Role of Inflammation and Therapeutic Concepts in Diabetic Retinopathy—A Short Review. Int. J. Mol. Sci. 2023, 24, 1024. [Google Scholar] [CrossRef]
- Gomaa, A.R.; Bedda, A.M.; ElGoweini, H.F.; Taleb, R.S.Z.; Saleh, A.M.A. Study of aqueous humour inflammatory mediators’ levels in a cohort of Egyptian patients with diabetic macular oedema. BMC Ophthalmol. 2023, 23, 456. [Google Scholar] [CrossRef]
- Bergandi, L.; Palladino, G.; Meduri, A.; De Luca, L.; Silvagno, F. Vitamin D and Sulforaphane Decrease Inflammatory Oxidative Stress and Restore the Markers of Epithelial Integrity in an In Vitro Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2024, 25, 6404. [Google Scholar] [CrossRef]
- Mohammad, H.M.F.; Eladl, M.A.; Abdelmaogood, A.K.K.; Elshaer, R.E.; Ghanam, W.; Elaskary, A.; Saleh, M.A.K.; Eltrawy, A.H.; Ali, S.K.; Moursi, S.M.M.; et al. Protective Effect of Topiramate against Diabetic Retinopathy and Computational Approach Recognizing the Role of NLRP3/IL-1β/TNF-αSignaling. Biomedicines 2023, 11, 3202. [Google Scholar] [CrossRef]
- ElSayed, M.H.; Elbayoumi, K.S.; Eladl, M.A.; Mohamed, A.A.; Hegazy, A.; El-Sherbeeny, N.A.; Zaitone, S.A. Memantine mitigates ROS/TXNIP/NLRP3 signaling and protects against mouse diabetic retinopathy: Histopathologic, ultrastructural and bioinformatic studies. Biomed. Pharmacother. 2023, 163, 114772. [Google Scholar] [CrossRef] [PubMed]
- Alshaman, R.; Kolieb, E.; El-Sayed, R.M.; Gouda, S.G.; Alattar, A.; Zaitone, S.A.; Abdelmaogood, A.K.K.; Elabbasy, L.M.; Eltrawy, A.H.; Sayd, F.Y.; et al. Computational and Experimental Approaches Exploring the Role of Hesperetin in Improving Autophagy in Rat Diabetic Retinopathy. Biomedicines 2024, 12, 552. [Google Scholar] [CrossRef]
- Hernandez, M.; Recalde, S.; González-Zamora, J.; Bilbao-Malavé, V.; Sáenz de Viteri, M.; Bezunartea, J.; Moreno-Orduña, M.; Belza, I.; Barrio-Barrio, J.; Fernandez-Robredo, P.; et al. Anti-Inflammatory and Anti-Oxidative Synergistic Effect of Vitamin D and Nutritional Complex on Retinal Pigment Epithelial and Endothelial Cell Lines against Age-Related Macular Degeneration. Nutrients 2021, 13, 1423. [Google Scholar] [CrossRef]
- Yoshida, S.; Sotozono, C.; Ikeda, T.; Kinoshita, S. Interleukin-6 (IL-6) production by cytokine-stimulated human Müller cells. Curr. Eye Res. 2001, 22, 341–347. [Google Scholar] [CrossRef]
- Li, D.S.; Liao, H.X.; Zhang, J.L.; Qin, B. Effect of aflibercept combined with triamcinolone acetonide on aqueous humor growth factor and inflammatory mediators in diabetic macular edema. Int. J. Ophthalmol. 2024, 17, 297–303. [Google Scholar] [CrossRef]
- Andrés-Blasco, I.; Gallego-Martínez, A.; Machado, X.; Cruz-Espinosa, J.; Di Lauro, S.; Casaroli-Marano, R.; Alegre-Ituarte, V.; Arévalo, F.; Pinazo-Durán, D. Oxidative stress, inflammatory, angiogenic, and apoptotic molecules in proliferative diabetic retinopathy and diabetic macular edema patients. Int. J. Mol. Sci. 2023, 24, 8227. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, M. Oxidative stress and inflammatory bowel disease in pediatrics. TJS 2023, 21, 375. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Fukuda, M. Classification and treatment of diabetic retinopathy. Diabetes Res. Clin. Pract. 1994, 24, 171. [Google Scholar] [CrossRef]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 115–121. [Google Scholar] [CrossRef]




| Variables | Controls (n = 94) | DMT2 with DR (n = 96) | DMT2 with DME (n = 38) | p |
|---|---|---|---|---|
| Age; mean ± SD | 63.35 ± 12.897 | 63.15 ± 10.23 | 62.13 ± 13.177 | <0.001 |
| Sex (M/F) | 15M/12F | 43M/53F | 19M/19F | 0.948 |
| Disease duration; mean ± SD | - | 12.70 ± 8.65 | 15.07 ± 1.11 | - |
| BMI (kg/m2); mean ± SD BMI > 30 kg/m2, n (%) | 29.23 ± 4.11 38 (40.4%) | 31.52 ± 5.89 54 (56.2%) | 32.34 ± 6.01 54 (59.01%) | <0.001 <0.001 |
| Blood sugar (mmol/L); mean ± SD | 4.97 ± 0.32 | 9.52 ± 5.18 | 8.77 ± 0.75 | <0.001 |
| HbA1c (%); mean ± SD | 5.06 ± 0.27 | 8.20 ± 2.06 | 8.21 ± 0.18 | <0.001 |
| Cholesterol (mmol/L) | 4.43 ± 0.76 | 5.12 ± 1.38 | 5.47 ± 0.6 | <0.001 |
| Triglycerides (mmol/L) | 1.52 ± 0.44 | 2.43 ± 1.27 | 2.513 ± 0.17 | <0.001 |
| HDL (mmol/L) | 1.01 ± 0.28 | 1.25 ± 0.38 | 1.95 ± 0.13 | <0.001 |
| LDL (mmol/L) | 2.32 ± 0.62 | 2.86 ± 1.10 | 2.32 ± 0.15 | <0.001 |
| SOD U/gHb | 121 ± 15.55 | 58.24 ± 15.31 | 47.26 ± 13.14 | <0.001 |
| CAT U/gHb | 48.59 ± 8.66 | 73.35 ± 8.64 | 70.87 ± 11.63 | <0.001 |
| GPx U/gHb | 289.87 ± 25.58 | 116.49 ± 18.11 | 76.53 ± 21.38 | <0.001 |
| Controls | DR | DME | |
|---|---|---|---|
| Central macular thickness CMT (μm) | 115.62 ± 0.21 | 226.92 ± 0.844 | 488.53 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova-Parlapanska, K.; Draganova, V.; Georgieva, E.; Goycheva, P.; Nikolova, G.; Karamalakova, Y. Systematic Inflammation and Oxidative Stress Elevation in Diabetic Retinopathy and Diabetic Patients with Macular Edema. Int. J. Mol. Sci. 2025, 26, 3810. https://doi.org/10.3390/ijms26083810
Petkova-Parlapanska K, Draganova V, Georgieva E, Goycheva P, Nikolova G, Karamalakova Y. Systematic Inflammation and Oxidative Stress Elevation in Diabetic Retinopathy and Diabetic Patients with Macular Edema. International Journal of Molecular Sciences. 2025; 26(8):3810. https://doi.org/10.3390/ijms26083810
Chicago/Turabian StylePetkova-Parlapanska, Kamelia, Valeria Draganova, Ekaterina Georgieva, Petya Goycheva, Galina Nikolova, and Yanka Karamalakova. 2025. "Systematic Inflammation and Oxidative Stress Elevation in Diabetic Retinopathy and Diabetic Patients with Macular Edema" International Journal of Molecular Sciences 26, no. 8: 3810. https://doi.org/10.3390/ijms26083810
APA StylePetkova-Parlapanska, K., Draganova, V., Georgieva, E., Goycheva, P., Nikolova, G., & Karamalakova, Y. (2025). Systematic Inflammation and Oxidative Stress Elevation in Diabetic Retinopathy and Diabetic Patients with Macular Edema. International Journal of Molecular Sciences, 26(8), 3810. https://doi.org/10.3390/ijms26083810









