Tandemly Repeated G-Quadruplex Structures in the Pseudorabies Virus Genome: Implications for Epiberberine-Based Antiviral Therapy
Abstract
1. Introduction
2. Results
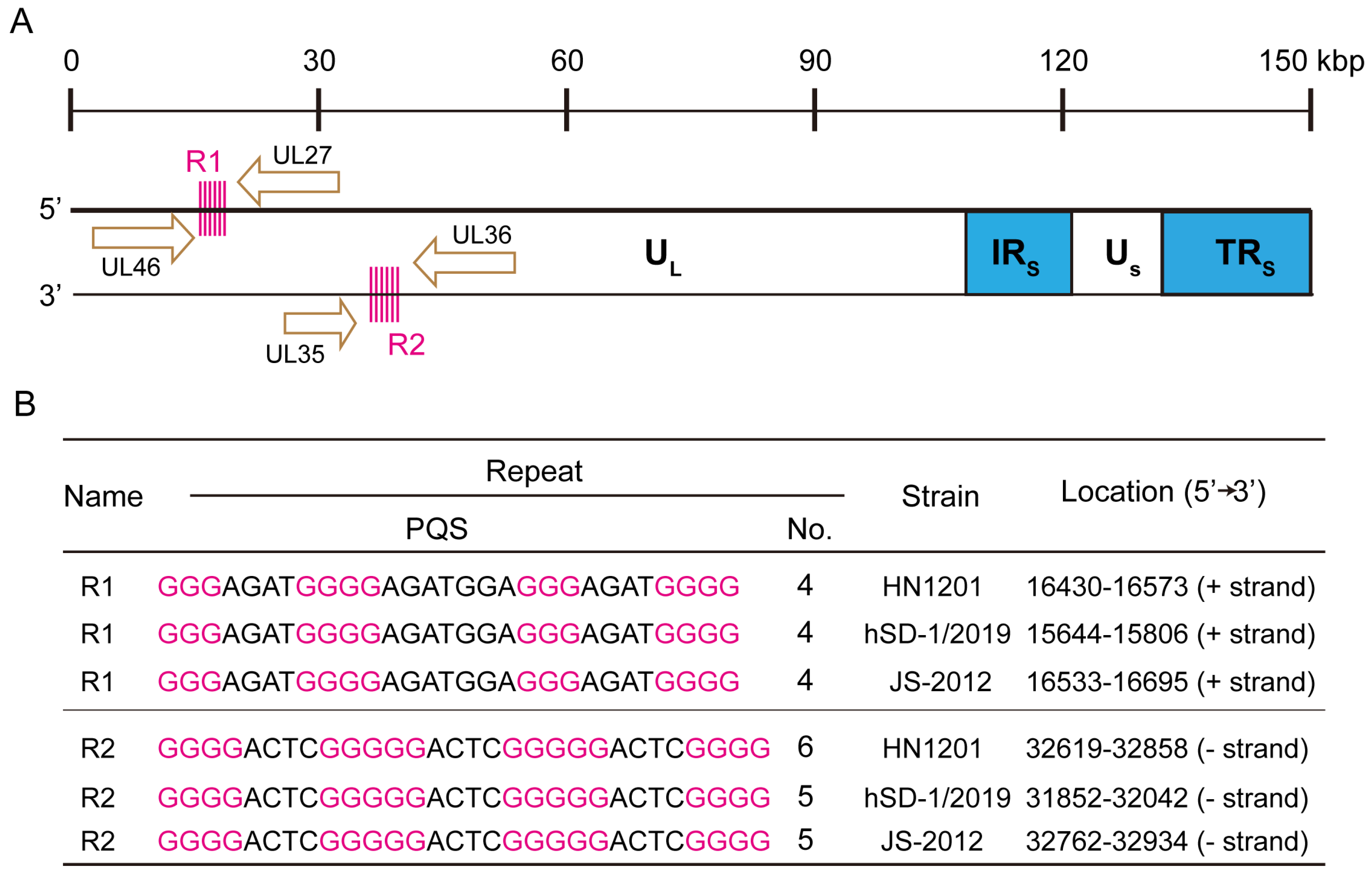
2.1. Identification of Tandemly Arranged G-Quadruplex Sequences in the PRV Genome
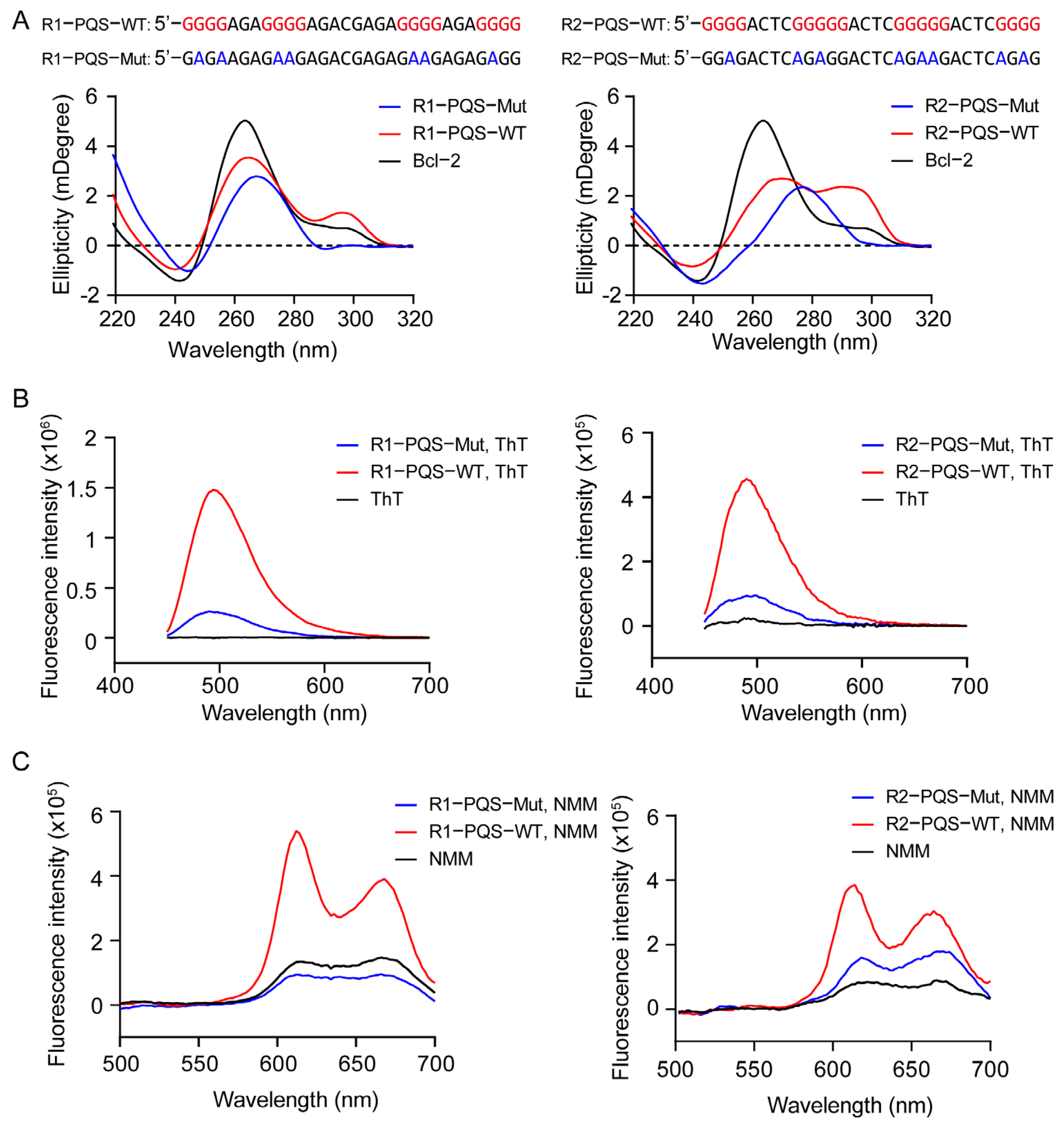
2.2. R1-PQS and R2-PQS Repeats Fold into Hybrid G-Quadruplex Structures
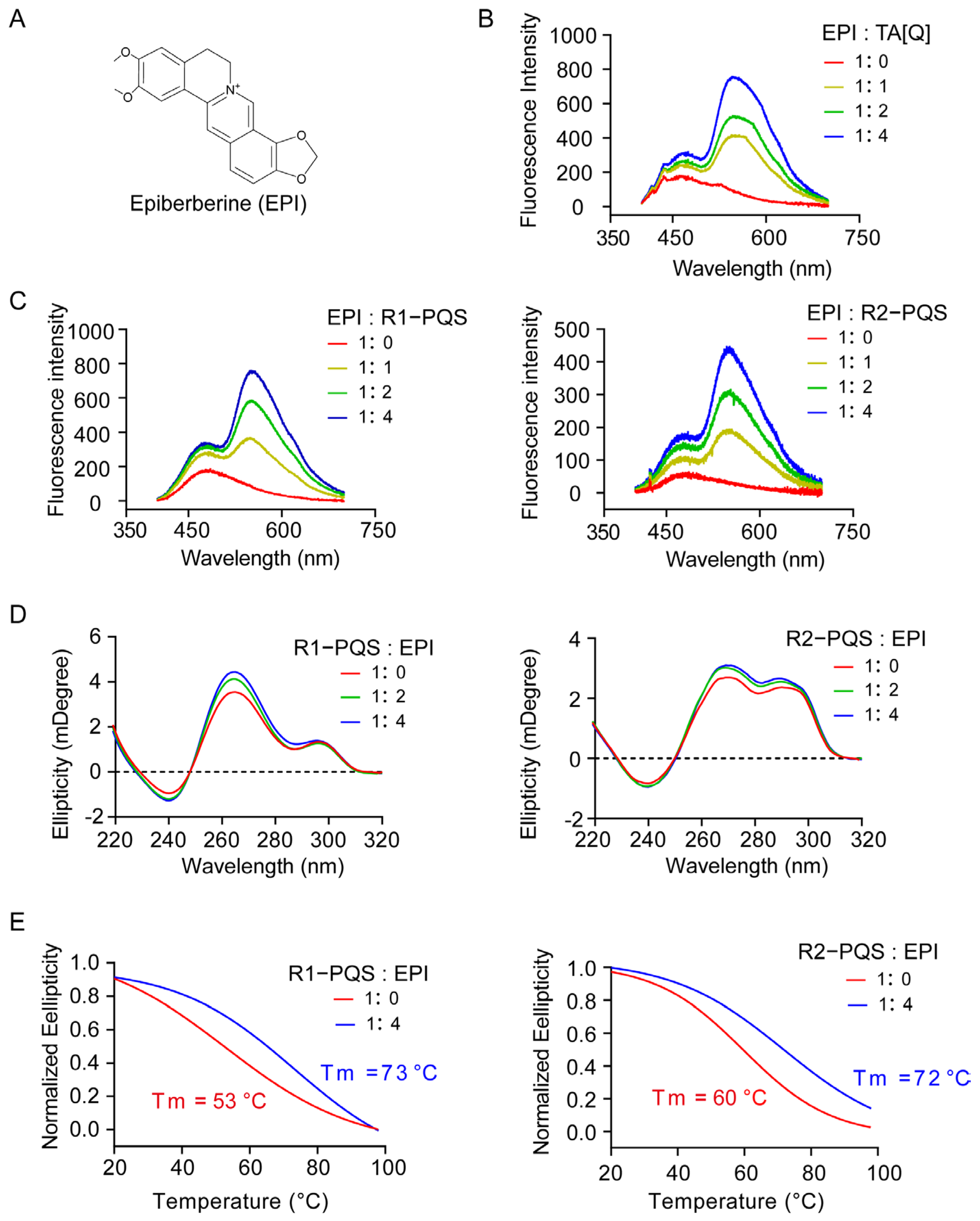
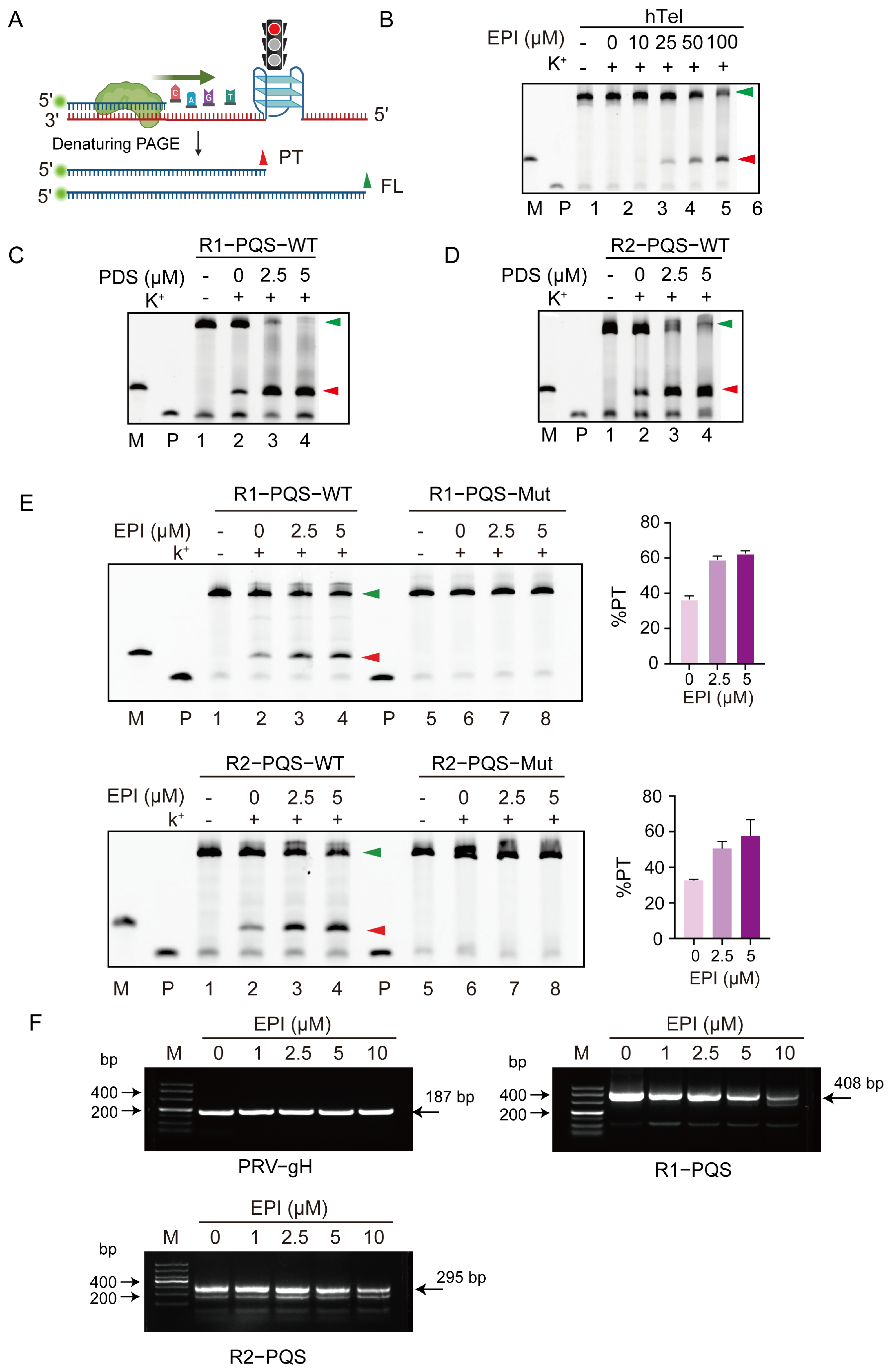
2.3. EPI Binds to and Stabilizes G4 Structures Formed in R1-PQS and R2-PQS
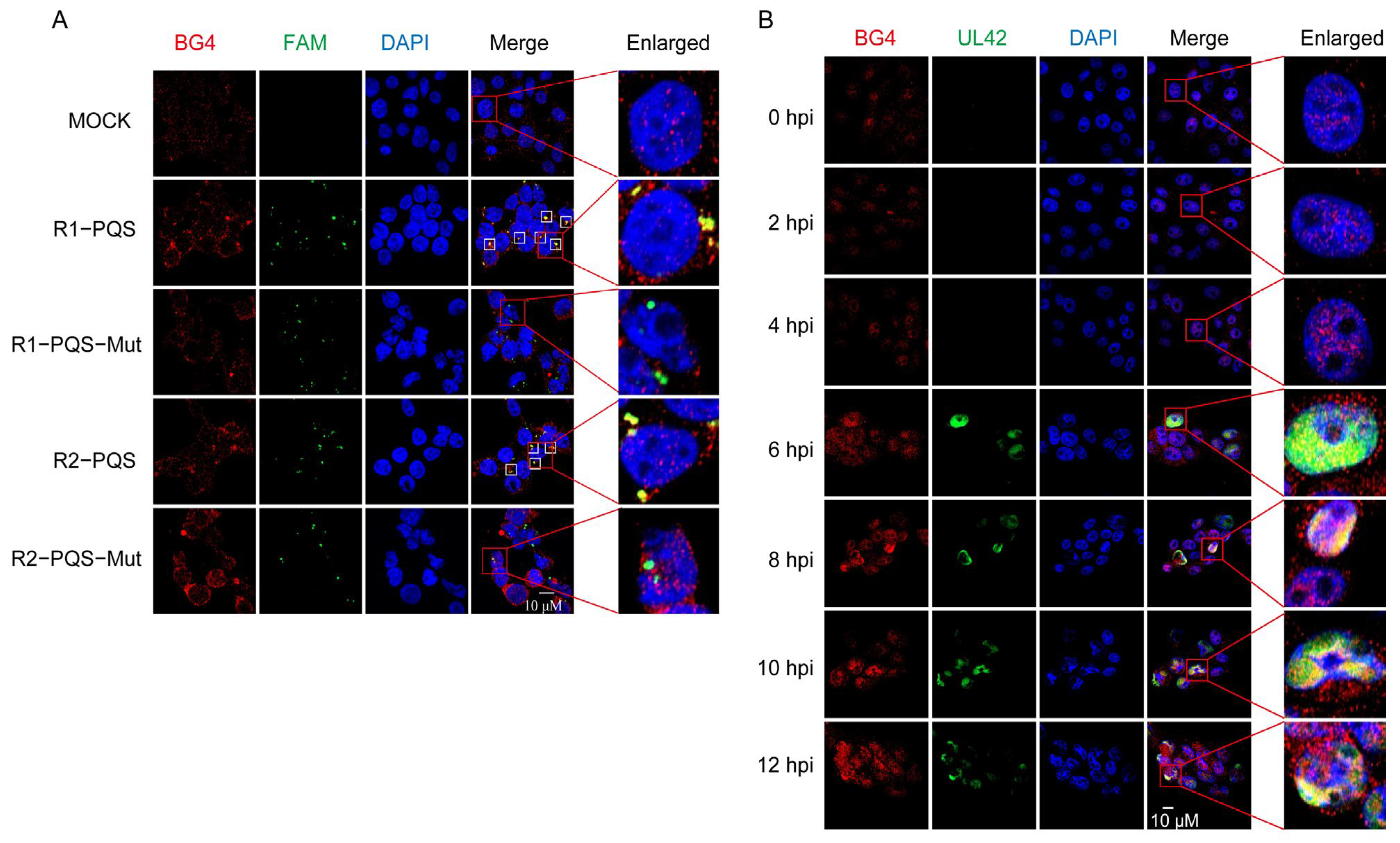
2.4. R1-PQS and R2-PQS Form G4 Structures in Cells
2.5. EPI Impairs DNA Synthesis by Targeting G4 Structures in R1-PQS and R2-PQS
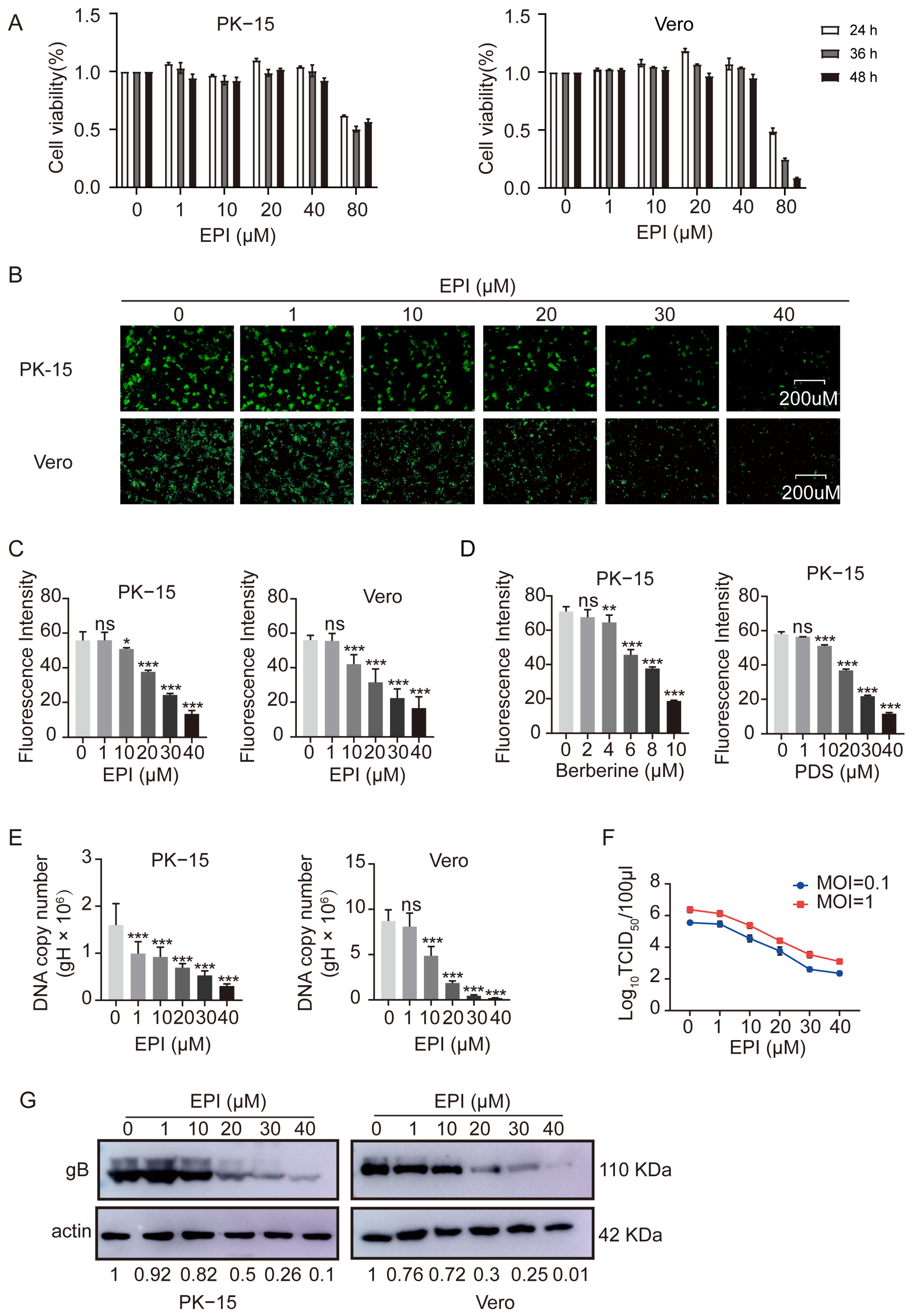
2.6. Inhibition of PRV Proliferation by EPI in Infected Cells
2.7. EPI Selectively Inhibits the Replication Phase of PRV in Infected Cells
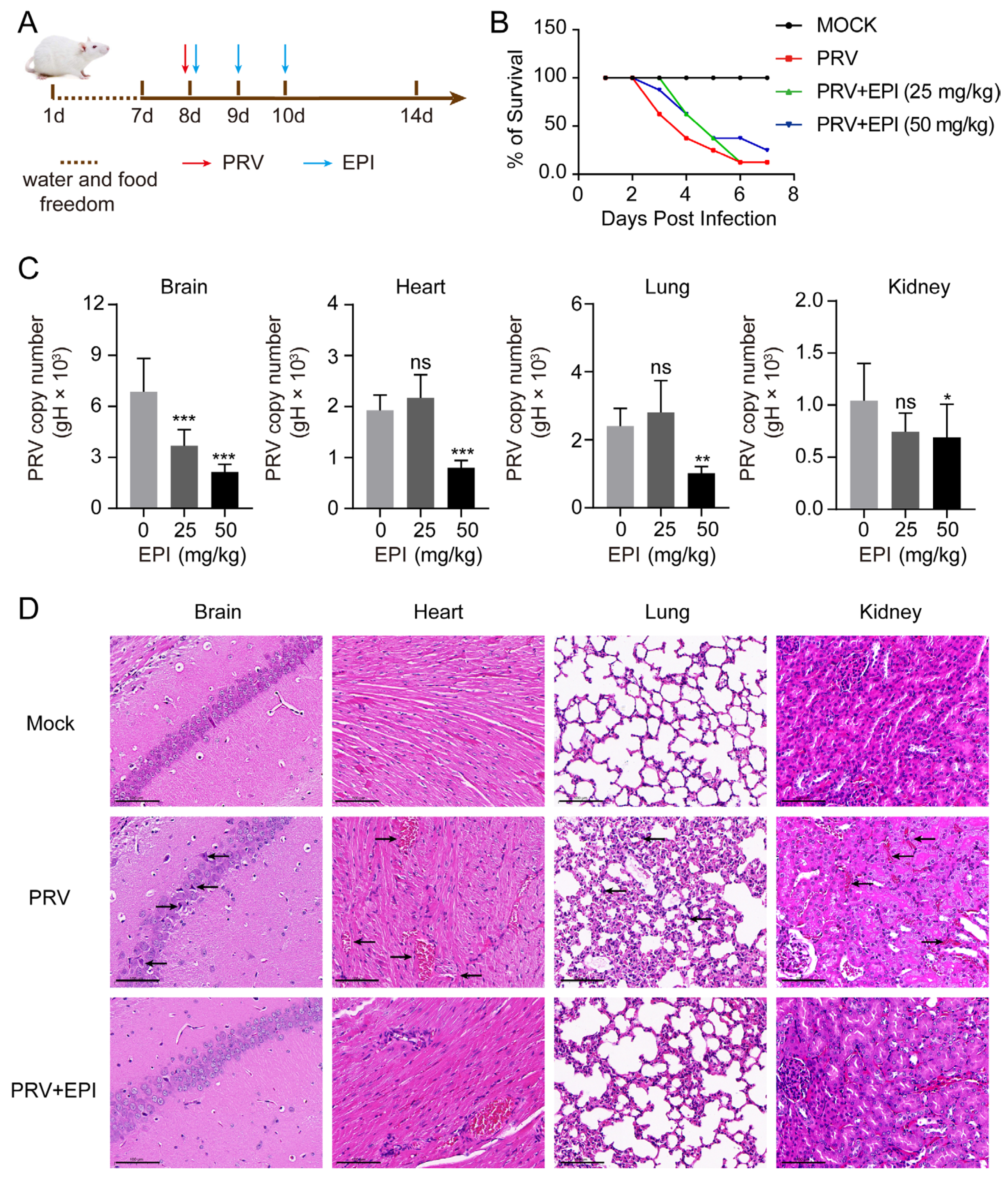
2.8. EPI Reduces Viral Load in PRV-Challenged Mice
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Materials and Oligonucleotides
4.3. Cell Viability Analysis
4.4. Flow Cytometry Assay
4.5. Viral Titration
4.6. Western Blot Assay
4.7. DNA Extraction and Quantitative Polymerase Chain Reaction
4.8. Circular Dichroism Spectroscopy
4.9. Fluorescence Turn-On Assay
4.10. Taq Polymerase Stop Assay
4.11. Immunofluorescence Analysis
4.12. Experiments in Mice
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef]
- Zok, T.; Kraszewska, N.; Miskiewicz, J.; Pielacinska, P.; Zurkowski, M.; Szachniuk, M. ONQUADRO: A database of experimentally determined quadruplex structures. Nucleic Acids Res. 2022, 50, D253–D258. [Google Scholar] [CrossRef]
- Robinson, J.; Raguseo, F.; Nuccio, S.P.; Liano, D.; Di Antonio, M. DNA G-quadruplex structures: More than simple roadblocks to transcription? Nucleic Acids Res. 2021, 49, 8419–8431. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Lipps, H.J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.P.; Terracciano, M.; Oliviero, G.; Roviello, G.N.; Borbone, N. Exploring the Relationship between G-Quadruplex Nucleic Acids and Plants: From Plant G-Quadruplex Function to Phytochemical G4 Ligands with Pharmaceutic Potential. Pharmaceutics 2022, 14, 2377. [Google Scholar] [CrossRef]
- Yadav, P.; Kim, N.; Kumari, M.; Verma, S.; Sharma, T.K.; Yadav, V.; Kumar, A. G-Quadruplex Structures in Bacteria: Biological Relevance and Potential as an Antimicrobial Target. J. Bacteriol. 2021, 203, e0057720. [Google Scholar] [CrossRef]
- Henderson, A.; Wu, Y.; Huang, Y.C.; Chavez, E.A.; Platt, J.; Johnson, F.B.; Brosh, R.M., Jr.; Sen, D.; Lansdorp, P.M. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014, 42, 860–869. [Google Scholar] [CrossRef]
- Ji, X.; Sun, H.; Zhou, H.; Xiang, J.; Tang, Y.; Zhao, C. Research progress of RNA quadruplex. Nucleic Acid Ther. 2011, 21, 185–200. [Google Scholar] [CrossRef]
- Amrane, S.; Kerkour, A.; Bedrat, A.; Vialet, B.; Andreola, M.L.; Mergny, J.L. Topology of a DNA G-quadruplex structure formed in the HIV-1 promoter: A potential target for anti-HIV drug development. J. Am. Chem. Soc. 2014, 136, 5249–5252. [Google Scholar] [CrossRef]
- Murat, P.; Zhong, J.; Lekieffre, L.; Cowieson, N.P.; Clancy, J.L.; Preiss, T.; Balasubramanian, S.; Khanna, R.; Tellam, J. G-quadruplexes regulate Epstein-Barr virus-encoded nuclear antigen 1 mRNA translation. Nat. Chem. Biol. 2014, 10, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Tluckova, K.; Marusic, M.; Tothova, P.; Bauer, L.; Sket, P.; Plavec, J.; Viglasky, V. Human papillomavirus G-quadruplexes. Biochemistry 2013, 52, 7207–7216. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Kim, Y.E.; Bansal, V.; Ghosh, A.; Hur, J.; Subramani, V.K.; Pradhan, S.; Lee, M.K.; Kim, K.K.; Ahn, J.H. Genome-wide analysis of regulatory G-quadruplexes affecting gene expression in human cytomegalovirus. PLoS Pathog. 2018, 14, e1007334. [Google Scholar] [CrossRef]
- Artusi, S.; Nadai, M.; Perrone, R.; Biasolo, M.A.; Palu, G.; Flamand, L.; Calistri, A.; Richter, S.N. The Herpes Simplex Virus-1 genome contains multiple clusters of repeated G-quadruplex: Implications for the antiviral activity of a G-quadruplex ligand. Antivir. Res. 2015, 118, 123–131. [Google Scholar] [CrossRef]
- Majee, P.; Kumar Mishra, S.; Pandya, N.; Shankar, U.; Pasadi, S.; Muniyappa, K.; Nayak, D.; Kumar, A. Identification and characterization of two conserved G-quadruplex forming motifs in the Nipah virus genome and their interaction with G-quadruplex specific ligands. Sci. Rep. 2020, 10, 1477. [Google Scholar] [CrossRef]
- Bian, W.X.; Xie, Y.; Wang, X.N.; Xu, G.H.; Fu, B.S.; Li, S.; Long, G.; Zhou, X.; Zhang, X.L. Binding of cellular nucleolin with the viral core RNA G-quadruplex structure suppresses HCV replication. Nucleic Acids Res. 2019, 47, 56–68. [Google Scholar] [CrossRef]
- Majee, P.; Pattnaik, A.; Sahoo, B.R.; Shankar, U.; Pattnaik, A.K.; Kumar, A.; Nayak, D. Inhibition of Zika virus replication by G-quadruplex-binding ligands. Mol. Ther. Nucleic Acids 2021, 23, 691–701. [Google Scholar] [CrossRef]
- Giraud, G.; Rodà, M.; Huchon, P.; Michelet, M.; Maadadi, S.; Jutzi, D.; Montserret, R.; Ruepp, M.D.; Parent, R.; Combet, C.; et al. G-quadruplexes control hepatitis B virus replication by promoting cccDNA transcription and phase separation in hepatocytes. Nucleic Acids Res. 2024, 52, 2290–2305. [Google Scholar] [CrossRef]
- Biswas, B.; Kandpal, M.; Vivekanandan, P. A G-quadruplex motif in an envelope gene promoter regulates transcription and virion secretion in HBV genotype B. Nucleic Acids Res. 2017, 45, 11268–11280. [Google Scholar] [CrossRef]
- Molnár, O.R.; Végh, A.; Somkuti, J.; Smeller, L. Characterization of a G-quadruplex from hepatitis B virus and its stabilization by binding TMPyP4, BRACO19 and PhenDC3. Sci. Rep. 2021, 11, 23243. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, L. G-quadruplexes in the monkeypox virus are potential antiviral targets. J. Med. Virol. 2023, 95, e28299. [Google Scholar] [CrossRef] [PubMed]
- Madireddy, A.; Purushothaman, P.; Loosbroock, C.P.; Robertson, E.S.; Schildkraut, C.L.; Verma, S.C. G-quadruplex-interacting compounds alter latent DNA replication and episomal persistence of KSHV. Nucleic Acids Res. 2016, 44, 3675–3694. [Google Scholar] [CrossRef] [PubMed]
- Kusov, Y.; Tan, J.; Alvarez, E.; Enjuanes, L.; Hilgenfeld, R. A G-quadruplex-binding macrodomain within the “SARS-unique domain is essential for the activity of the SARS-coronavirus replication-transcription complex. Virology 2015, 484, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, X.; Zhu, Y.; Li, Y.; Wang, Z.; Li, P.; Pan, J.; Wang, J.; Chu, B.; Yang, G.; et al. Identification of a conserved G-quadruplex within the E165R of African swine fever virus (ASFV) as a potential antiviral target. J. Biol. Chem. 2024, 300, 107453. [Google Scholar] [CrossRef]
- Muturi, E.; Meng, F.; Liu, H.; Jiang, M.; Wei, H.; Yang, H. Comprehensive Analysis of G-Quadruplexes in African Swine Fever Virus Genome Reveals Potential Antiviral Targets by G-Quadruplex Stabilizers. Front. Microbiol. 2021, 12, 798431. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Szabat, M.; Zielińska, K.; Kierzek, R. Identification and Structural Aspects of G-Quadruplex-Forming Sequences from the Influenza A Virus Genome. Int. J. Mol. Sci. 2021, 22, 6031. [Google Scholar] [CrossRef]
- Brázda, V.; Porubiaková, O.; Cantara, A.; Bohálová, N.; Coufal, J.; Bartas, M.; Fojta, M.; Mergny, J.L. G-quadruplexes in H1N1 influenza genomes. BMC Genom. 2021, 22, 77. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, L. Characterization of G-Quadruplexes in Enterovirus A71 Genome and Their Interaction with G-Quadruplex Ligands. Microbiol. Spectr. 2022, 10, e0046022. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, G.; Niu, J.; Wang, Z.; Wang, C.; Ren, J.; Qu, X. Targeting RNA G-Quadruplex in SARS-CoV-2: A Promising Therapeutic Target for COVID-19? Angew. Chem. (Int. Ed. Engl.) 2021, 60, 432–438. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, L. G-Quadruplexes Are Present in Human Coronaviruses Including SARS-CoV-2. Front. Microbiol. 2020, 11, 567317. [Google Scholar] [CrossRef]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. MMBR 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Longobardi, C.; D’Ambrosi, F.; Amoroso, M.G.; D’Alessio, N.; Damiano, S.; Ciarcia, R.; Iovane, V.; Iovane, G.; Pagnini, U.; et al. Aujeszky’s Disease in South-Italian Wild Boars (Sus scrofa): A Serological Survey. Animals 2021, 11, 3298. [Google Scholar] [CrossRef] [PubMed]
- Sehl, J.; Teifke, J.P. Comparative Pathology of Pseudorabies in Different Naturally and Experimentally Infected Species-A Review. Pathogens 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Bai, C.; Sun, J.; Chang, S.; Zhang, X. Emergence of virulent pseudorabies virus infection in northern China. J. Vet. Sci. 2013, 14, 363–365. [Google Scholar] [CrossRef] [PubMed]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef]
- Ai, J.W.; Weng, S.S.; Cheng, Q.; Cui, P.; Li, Y.J.; Wu, H.L.; Zhu, Y.M.; Xu, B.; Zhang, W.H. Human Endophthalmitis Caused by Pseudorabies Virus Infection, China, 2017. Emerg. Infect. Dis. 2018, 24, 1087–1090. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Xie, C.; Ding, S.; Yang, H.; Guo, S.; Li, J.; Qin, L.; Ban, F.; Wang, D.; et al. A Novel Human Acute Encephalitis Caused by Pseudorabies Virus Variant Strain. Clin. Infect. Dis. 2021, 73, e3690–e3700. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, W.; Zhang, C. G-Quadruplexes Formation at the Upstream Region of Replication Origin (OriL) of the Pseudorabies Virus: Implications for Antiviral Targets. Viruses 2021, 13, 2219. [Google Scholar] [CrossRef]
- Kong, J.-N.; Zhang, C.; Zhu, Y.-C.; Zhong, K.; Wang, J.; Chu, B.-B.; Yang, G.-Y. Identification and characterization of G-quadruplex formation within the EP0 promoter of pseudorabies virus. Sci. Rep. 2018, 8, 14029. [Google Scholar] [CrossRef]
- Deng, H.; Gong, B.; Yang, Z.; Li, Z.; Zhou, H.; Zhang, Y.; Niu, X.; Liu, S.; Wei, D. Intensive Distribution of G(2)-Quaduplexes in the Pseudorabies Virus Genome and Their Sensitivity to Cations and G-Quadruplex Ligands. Molecules 2019, 24, 774. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.C.; Ravichandran, S.; Park, D.; Lee, G.M.; Kim, Y.E.; Choi, Y.; Song, M.J.; Kim, K.K.; Ahn, J.H. G-quadruplexes formed by Varicella-Zoster virus reiteration sequences suppress expression of glycoprotein C and regulate viral cell-to-cell spread. PLoS Pathog. 2023, 19, e1011095. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, X.; Tang, Q.; Huang, T.; Tang, X.; Jiao, B.; Wang, R.; Zhu, X.; Ye, X.; Ma, H.; et al. Epiberberine inhibits Helicobacter pylori and reduces host apoptosis and inflammatory damage by down-regulating urease expression. J. Ethnopharmacol. 2024, 318, 117046. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Han, Z.; Li, J.; Du, Y. c-MYC and HIF1α promoter G-quadruplexes dependent metabolic regulation mechanism of berberine in colon cancer. J. Gastrointest. Oncol. 2022, 13, 1152–1168. [Google Scholar] [CrossRef]
- Wang, K.B.; Liu, Y.; Li, J.; Xiao, C.; Wang, Y.; Gu, W.; Li, Y.; Xia, Y.Z.; Yan, T.; Yang, M.H.; et al. Structural insight into the bulge-containing KRAS oncogene promoter G-quadruplex bound to berberine and coptisine. Nat. Commun. 2022, 13, 6016. [Google Scholar] [CrossRef]
- Oliva, R.; Mukherjee, S.; Manisegaran, M.; Campanile, M.; Del Vecchio, P.; Petraccone, L.; Winter, R. Binding Properties of RNA Quadruplex of SARS-CoV-2 to Berberine Compared to Telomeric DNA Quadruplex. Int. J. Mol. Sci. 2022, 23, 5690. [Google Scholar] [CrossRef]
- Deng, N.; Xia, J.; Wickstrom, L.; Lin, C.; Wang, K.; He, P.; Yin, Y.; Yang, D. Ligand Selectivity in the Recognition of Protoberberine Alkaloids by Hybrid-2 Human Telomeric G-Quadruplex: Binding Free Energy Calculation, Fluorescence Binding, and NMR Experiments. Molecules 2019, 24, 1574. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Shao, Y.; Lin, C.; Jia, H.; Chen, G.; Yang, D.; Wang, Y. Selective lighting up of epiberberine alkaloid fluorescence by fluorophore-switching aptamer and stoichiometric targeting of human telomeric DNA G-quadruplex multimer. Anal. Chem. 2015, 87, 730–737. [Google Scholar] [CrossRef]
- Klupp, B.G.; Hengartner, C.J.; Mettenleiter, T.C.; Enquist, L.W. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 2004, 78, 424–440. [Google Scholar] [CrossRef]
- Kikin, O.; D’Antonio, L.; Bagga, P.S. QGRS Mapper: A web-based server for predicting G-quadruplexes in nucleotide sequences. Nucleic Acids Res. 2006, 34, W676–W682. [Google Scholar] [CrossRef]
- Kejnovska, I.; Renciuk, D.; Palacky, J.; Vorlickova, M. CD Study of the G-Quadruplex Conformation. Methods Mol. Biol. 2019, 2035, 25–44. [Google Scholar] [PubMed]
- Dai, J.; Dexheimer, T.S.; Chen, D.; Carver, M.; Ambrus, A.; Jones, R.A.; Yang, D. An intramolecular G-quadruplex structure with mixed parallel/antiparallel G-strands formed in the human BCL-2 promoter region in solution. J. Am. Chem. Soc. 2006, 128, 1096–1098. [Google Scholar] [CrossRef] [PubMed]
- Yett, A.; Lin, L.Y.; Beseiso, D.; Miao, J.; Yatsunyk, L.A. N-methyl mesoporphyrin IX as a highly selective light-up probe for G-quadruplex DNA. J. Porphyr. Phthalocya. 2019, 23, 1195–1215. [Google Scholar] [CrossRef]
- Sabharwal, N.C.; Savikhin, V.; Turek-Herman, J.R.; Nicoludis, J.M.; Szalai, V.A.; Yatsunyk, L.A. N-methylmesoporphyrin IX fluorescence as a reporter of strand orientation in guanine quadruplexes. FEBS J. 2014, 281, 1726–1737. [Google Scholar] [CrossRef]
- Mohanty, J.; Barooah, N.; Dhamodharan, V.; Harikrishna, S.; Pradeepkumar, P.I.; Bhasikuttan, A.C. Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA. J. Am. Chem. Soc. 2013, 135, 367–376. [Google Scholar] [CrossRef]
- Yi, J.; Ye, X.; Wang, D.; He, K.; Yang, Y.; Liu, X.; Li, X. Safety evaluation of main alkaloids from Rhizoma Coptidis. J. Ethnopharmacol. 2013, 145, 303–310. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef]
- Du, W.; Wang, Y.; Huang, L.; Wei, Y.; Chen, D.; Sun, J.; Wu, H.; Feng, L.; Liu, C. Characterization of monoclonal antibodies that recognize the amino- and carboxy-terminal epitopes of the pseudorabies virus UL42 protein. Appl. Microbiol. Biotechnol. 2016, 100, 181–192. [Google Scholar] [CrossRef]
- Lin, C.; Wu, G.; Wang, K.; Onel, B.; Sakai, S.; Shao, Y.; Yang, D. Molecular Recognition of the Hybrid-2 Human Telomeric G-Quadruplex by Epiberberine: Insights into Conversion of Telomeric G-Quadruplex Structures. Angew. Chem. (Int. Ed. Engl.) 2018, 57, 10888–10893. [Google Scholar] [CrossRef]
- Ruggiero, E.; Richter, S.N. G-quadruplexes and G-quadruplex ligands: Targets and tools in antiviral therapy. Nucleic Acids Res. 2018, 46, 3270–3283. [Google Scholar] [CrossRef]
- Wong, G.; Lu, J.; Zhang, W.; Gao, G.F. Pseudorabies virus: A neglected zoonotic pathogen in humans? Emerg. Microbes Infect. 2019, 8, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Jiang, H.; Deng, H.; Dong, C.; Shen, W.; Chen, H.; Gao, C.; Xiao, S.; Liu, Z.F.; et al. G(2)-quadruplex in the 3’UTR of IE180 regulates Pseudorabies virus replication by enhancing gene expression. RNA Biol. 2020, 17, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Casas-Delucchi, C.S.; Daza-Martin, M.; Williams, S.L.; Coster, G. The mechanism of replication stalling and recovery within repetitive DNA. Nat. Commun. 2022, 13, 3953. [Google Scholar] [CrossRef] [PubMed]
- Prorok, P.; Artufel, M.; Aze, A.; Coulombe, P.; Peiffer, I.; Lacroix, L.; Guedin, A.; Mergny, J.L.; Damaschke, J.; Schepers, A.; et al. Involvement of G-quadruplex regions in mammalian replication origin activity. Nat. Commun. 2019, 10, 3274. [Google Scholar] [CrossRef]
- Prioleau, M.N. G-Quadruplexes and DNA Replication Origins. Adv. Exp. Med. Biol. 2017, 1042, 273–286. [Google Scholar]
- Lopes, J.; Piazza, A.; Bermejo, R.; Kriegsman, B.; Colosio, A.; Teulade-Fichou, M.P.; Foiani, M.; Nicolas, A. G-quadruplex-induced instability during leading-strand replication. EMBO J. 2011, 30, 4033–4046. [Google Scholar] [CrossRef]
- Renciuk, D.; Rynes, J.; Kejnovska, I.; Foldynova-Trantirkova, S.; Andang, M.; Trantirek, L.; Vorlickova, M. G-quadruplex formation in the Oct4 promoter positively regulates Oct4 expression. Biochim. Biophys. Acta 2017, 1860, 175–183. [Google Scholar] [CrossRef]
- Cogoi, S.; Paramasivam, M.; Membrino, A.; Yokoyama, K.K.; Xodo, L.E. The KRAS promoter responds to Myc-associated zinc finger and poly(ADP-ribose) polymerase 1 proteins, which recognize a critical quadruplex-forming GA-element. J. Biol. Chem. 2010, 285, 22003–22016. [Google Scholar] [CrossRef]
- Siddiqui-Jain, A.; Grand, C.L.; Bearss, D.J.; Hurley, L.H. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. USA 2002, 99, 11593–11598. [Google Scholar] [CrossRef]
- Teng, F.Y.; Jiang, Z.Z.; Guo, M.; Tan, X.Z.; Chen, F.; Xi, X.G.; Xu, Y. G-quadruplex DNA: A novel target for drug design. Cell. Mol. Life Sci. CMLS 2021, 78, 6557–6583. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Chang, X.; Qiao, Y.; Zhao, X.; Zhao, J.; Zhu, H.; Han, Y.; Zhang, C. Tandemly Repeated G-Quadruplex Structures in the Pseudorabies Virus Genome: Implications for Epiberberine-Based Antiviral Therapy. Int. J. Mol. Sci. 2025, 26, 3764. https://doi.org/10.3390/ijms26083764
Fan S, Chang X, Qiao Y, Zhao X, Zhao J, Zhu H, Han Y, Zhang C. Tandemly Repeated G-Quadruplex Structures in the Pseudorabies Virus Genome: Implications for Epiberberine-Based Antiviral Therapy. International Journal of Molecular Sciences. 2025; 26(8):3764. https://doi.org/10.3390/ijms26083764
Chicago/Turabian StyleFan, Songjie, Xiaotian Chang, Yan Qiao, Xiaoxiao Zhao, Jiafu Zhao, Heshui Zhu, Yingqian Han, and Chao Zhang. 2025. "Tandemly Repeated G-Quadruplex Structures in the Pseudorabies Virus Genome: Implications for Epiberberine-Based Antiviral Therapy" International Journal of Molecular Sciences 26, no. 8: 3764. https://doi.org/10.3390/ijms26083764
APA StyleFan, S., Chang, X., Qiao, Y., Zhao, X., Zhao, J., Zhu, H., Han, Y., & Zhang, C. (2025). Tandemly Repeated G-Quadruplex Structures in the Pseudorabies Virus Genome: Implications for Epiberberine-Based Antiviral Therapy. International Journal of Molecular Sciences, 26(8), 3764. https://doi.org/10.3390/ijms26083764





