Immunohistochemical Profiling of IDO1 and IL4I1 in Head and Neck Squamous Cell Carcinoma: Interplay for Metabolic Reprogramming?
Abstract
1. Introduction
2. Results
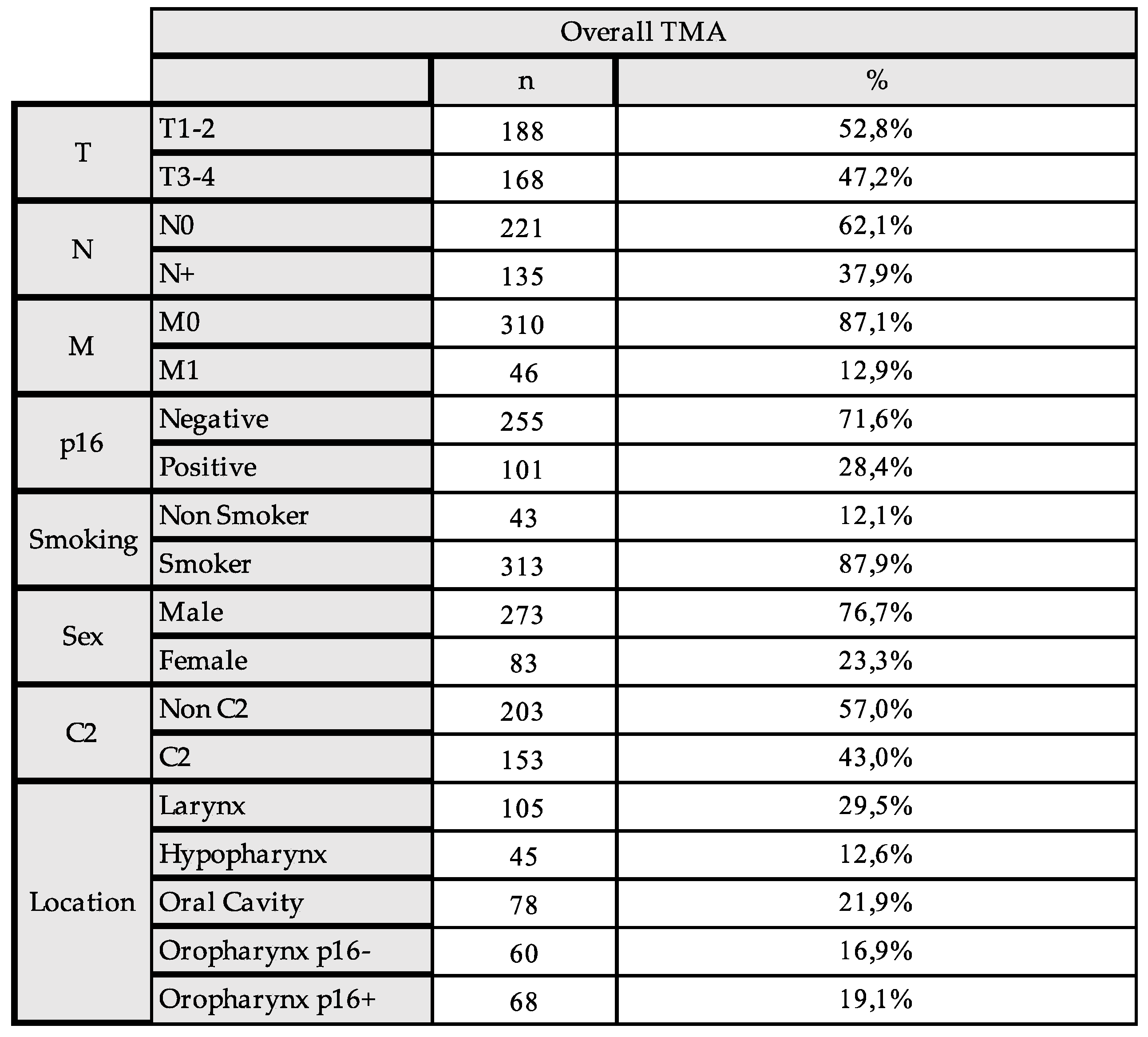
2.1. The Expression of IDO1 and IL4I1 and Association with Clinicopathologic Characteristics
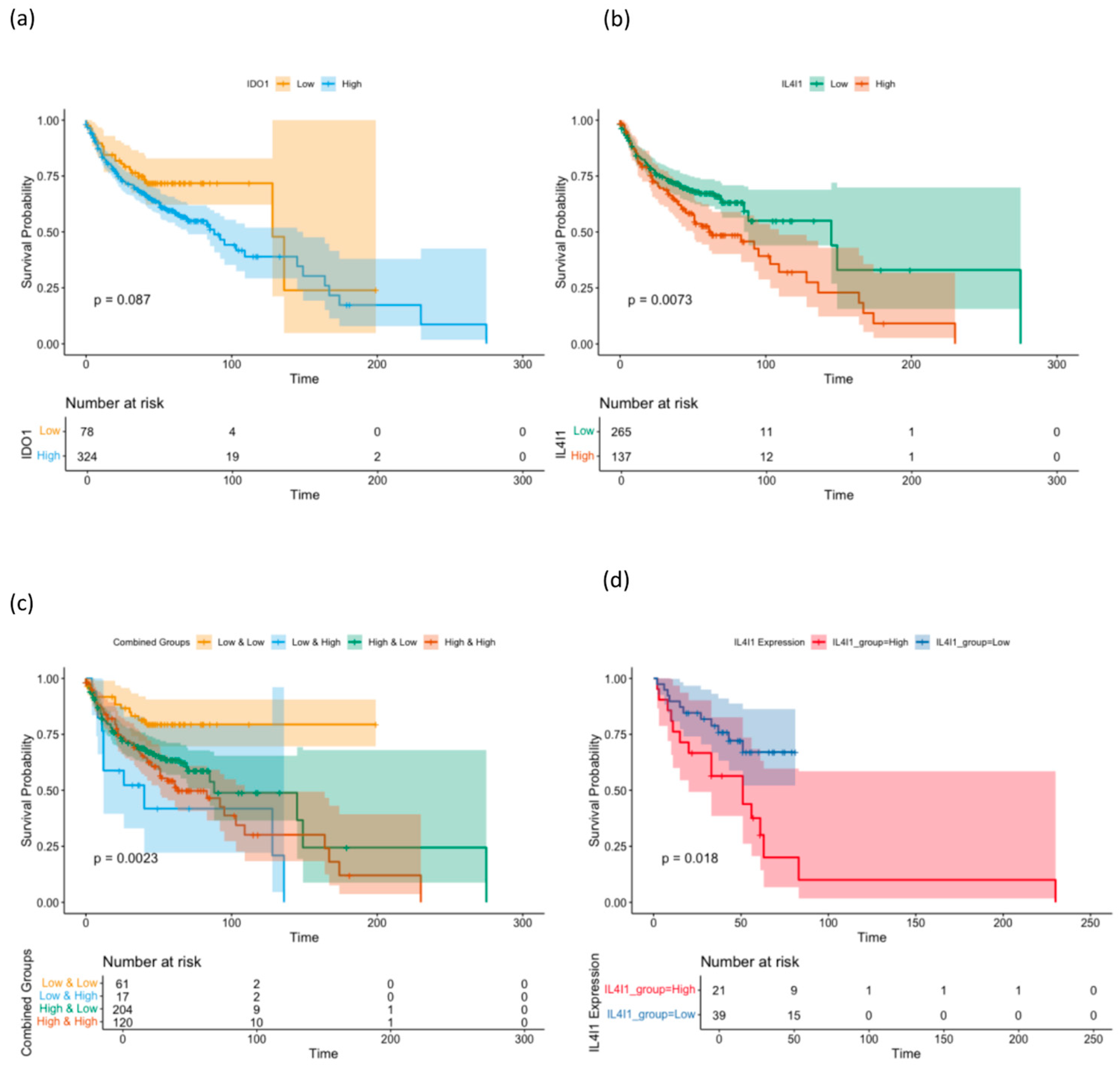
2.2. Prognostic Impact of the Expression of IDO1 and IL4I1
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Immunohistochemistry and the Assessment of the IDO1 and IL4I1 Expression
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nature reviews. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Della Corte, C.M.; Di Liello, R.; Viscardi, G.; Sparano, F.; Iacovino, M.L.; Paragliola, F.; Piccolo, A.; Napolitano, S.; Martini, G.; et al. Immunotherapy for head and neck cancer: Present and future. Crit. Rev. Oncol. 2022, 174, 103679. [Google Scholar] [CrossRef]
- Zeitler, L.; Murray, P.J. IL4i1 and IDO1: Oxidases that control a tryptophan metabolic nexus in cancer. J. Biol. Chem. 2023, 299, 104827. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.-L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef]
- Bhatia, A.; Burtness, B. Treating Head and Neck Cancer in the Age of Immunotherapy: A 2023 Update. Drugs 2023, 83, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.B.; King, A.J.; Warner, E.L.; Aneja, S.; Kann, B.H.; Bylund, C.L. Using ChatGPT to evaluate cancer myths and misconceptions: Artificial intelligence and cancer information. JNCI Cancer Spectr. 2023, 7, pkad015. [Google Scholar] [CrossRef]
- Le, X.; Ferrarotto, R.; Wise-Draper, T.; Gillison, M. Evolving Role of Immunotherapy in Recurrent Metastatic Head and Neck Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 899–906. [Google Scholar] [CrossRef]
- Lee, Y.S.; Johnson, D.E.; Grandis, J.R. An update: Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin. Emerg. Drugs 2018, 23, 283–299. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J.; Jin, S.; Wang, R.; Li, M.; Zhang, Z.; Yang, X.; Ma, H. Metabolic landscape of head and neck squamous cell carcinoma informs a novel kynurenine/Siglec-15 axis in immune escape. Cancer Commun. 2024, 44, 670–694. [Google Scholar] [CrossRef]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front. Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; A Wainwright, D. IDO1 in cancer: A Gemini of immune checkpoints. Cell. Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Spranger, S.; Binder, D.C.; Gritsina, G.; Lauing, K.L.; Giles, F.J.; Wainwright, D.A. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin. Cancer Res. 2015, 21, 5427–5433. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, N.; Zhang, X.; Han, X.; Zhai, X.; Lu, Y. IDO and TDO as a potential therapeutic target in different types of depression. Metab. Brain Dis. 2018, 33, 1787–1800. [Google Scholar] [CrossRef]
- Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. Role of IDO and TDO in Cancers and Related Diseases and the Therapeutic Implications. J. Cancer 2019, 10, 2771–2782. [Google Scholar] [CrossRef]
- Zakharia, Y.; McWilliams, R.R.; Rixe, O.; Drabick, J.; Shaheen, M.F.; Grossmann, K.F.; Kolhe, R.; Pacholczyk, R.; Sadek, R.; Tennant, L.L.; et al. Phase II trial of the IDO pathway inhibitor indoximod plus pembrolizumab for the treatment of patients with advanced melanoma. J. Immunother. Cancer 2021, 9, e002057. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; Jiang, L.; et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 2021, 184, 792–809.e23. [Google Scholar] [CrossRef]
- Sadik, A.; Patterson, L.F.S.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef] [PubMed]
- Gkountana, G.V.; Wang, L.; Giacomini, M.; Hyytiäinen, A.; Juurikka, K.; Salo, T.; Al-Samadi, A. IDO1 correlates with the immune landscape of head and neck squamous cell carcinoma: A study based on bioinformatics analyses. Front. Oral Heal. 2024, 5, 1335648. [Google Scholar] [CrossRef]
- Struckmeier, A.-K.; Radermacher, A.; Fehrenz, M.; Bellin, T.; Alansary, D.; Wartenberg, P.; Boehm, U.; Wagner, M.; Scheller, A.; Hess, J.; et al. IDO1 is highly expressed in macrophages of patients in advanced tumour stages of oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2022, 149, 3623–3635. [Google Scholar] [CrossRef]
- Hasegawa, M.; Amano, Y.; Kihara, A.; Matsubara, D.; Fukushima, N.; Takahashi, H.; Chikamatsu, K.; Nishino, H.; Mori, Y.; Yoshida, N.; et al. Guanylate binding protein 5 is an immune-related biomarker of oral squamous cell carcinoma: A retrospective prognostic study with bioinformatic analysis. Cancer Med. 2024, 13, e7431. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Wu, Y.-H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, B.J.; van Baren, N.; Baurain, J.-F. Is There a Clinical Future for IDO1 Inhibitors After the Failure of Epacadostat in Melanoma? Annu. Rev. Cancer Biol. 2020, 4, 241–256. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kladi-Skandali, A.; Strati, A.; Koytsodontis, G.; Kirodimos, E.; Giotakis, E.; Maragoudakis, P.; Gagari, E.; Maratou, E.; Dimitriadis, G.; et al. Prognostic impact of indoleamine 2,3-dioxygenase 1 (IDO1) mRNA expression on circulating tumour cells of patients with head and neck squamous cell carcinoma. ESMO Open 2020, 5, e000646. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef]
- Mazzoni, A.; Capone, M.; Ramazzotti, M.; Vanni, A.; Locatello, L.G.; Gallo, O.; De Palma, R.; Cosmi, L.; Liotta, F.; Annunziato, F.; et al. IL4I1 Is Expressed by Head–Neck Cancer-Derived Mesenchymal Stromal Cells and Contributes to Suppress T Cell Proliferation. J. Clin. Med. 2021, 10, 2111. [Google Scholar] [CrossRef]
- Tan, X.; Li, G.; Deng, H.; Xiao, G.; Wang, Y.; Zhang, C.; Chen, Y. Obesity enhances the response to neoadjuvant anti-PD1 therapy in oral tongue squamous cell carcinoma. Cancer Med. 2024, 13, e7346. [Google Scholar] [CrossRef]
- Hoos, A.; Cordon-Cardo, C. Tissue Microarray Profiling of Cancer Specimens and Cell Lines: Opportunities and Limitations. Mod. Pathol. 2001, 81, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, N.M. Tissue Microarray: A rapidly evolving diagnostic and research tool. Ann. Saudi Med. 2009, 29, 123–127. [Google Scholar] [CrossRef]
- Ferrandino, R.M.; Chen, S.; Kappauf, C.; Barlow, J.; Gold, B.S.; Berger, M.H.; Westra, W.H.; Teng, M.S.; Khan, M.N.; Posner, M.R.; et al. Performance of Liquid Biopsy for Diagnosis and Surveillance of Human Papillomavirus–Associated Oropharyngeal Cancer. Arch. Otolaryngol. Neck Surg. 2023, 149, 971–977. [Google Scholar] [CrossRef]
- Huber, L.T.; Kraus, J.M.; Ezić, J.; Wanli, A.; Groth, M.; Laban, S.; Hoffmann, T.K.; Wollenberg, B.; Kestler, H.A.; Brunner, C. Liquid biopsy: An examination of platelet RNA obtained from head and neck squamous cell carcinoma patients for predictive molecular tumor markers. Explor. Target. Anti-tumor Ther. 2023, 4, 422–446. [Google Scholar] [CrossRef]
- Hothorn, T.; Zeileis, A. Generalized Maximally Selected Statistics. Biometrics 2008, 64, 1263–1269. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidl, B.; Lauterbach, M.; Stögbauer, F.; Mogler, C.; Ribbat-Idel, J.; Perner, S.; Wollenberg, B. Immunohistochemical Profiling of IDO1 and IL4I1 in Head and Neck Squamous Cell Carcinoma: Interplay for Metabolic Reprogramming? Int. J. Mol. Sci. 2025, 26, 3719. https://doi.org/10.3390/ijms26083719
Schmidl B, Lauterbach M, Stögbauer F, Mogler C, Ribbat-Idel J, Perner S, Wollenberg B. Immunohistochemical Profiling of IDO1 and IL4I1 in Head and Neck Squamous Cell Carcinoma: Interplay for Metabolic Reprogramming? International Journal of Molecular Sciences. 2025; 26(8):3719. https://doi.org/10.3390/ijms26083719
Chicago/Turabian StyleSchmidl, Benedikt, Maren Lauterbach, Fabian Stögbauer, Carolin Mogler, Julika Ribbat-Idel, Sven Perner, and Barbara Wollenberg. 2025. "Immunohistochemical Profiling of IDO1 and IL4I1 in Head and Neck Squamous Cell Carcinoma: Interplay for Metabolic Reprogramming?" International Journal of Molecular Sciences 26, no. 8: 3719. https://doi.org/10.3390/ijms26083719
APA StyleSchmidl, B., Lauterbach, M., Stögbauer, F., Mogler, C., Ribbat-Idel, J., Perner, S., & Wollenberg, B. (2025). Immunohistochemical Profiling of IDO1 and IL4I1 in Head and Neck Squamous Cell Carcinoma: Interplay for Metabolic Reprogramming? International Journal of Molecular Sciences, 26(8), 3719. https://doi.org/10.3390/ijms26083719






