Strategies for Signal Amplification of Thyroid Hormones via Electromigration Techniques Coupled with UV Detection and Laser-Induced Fluorescence
Abstract
1. Introduction
2. Results and Discussions
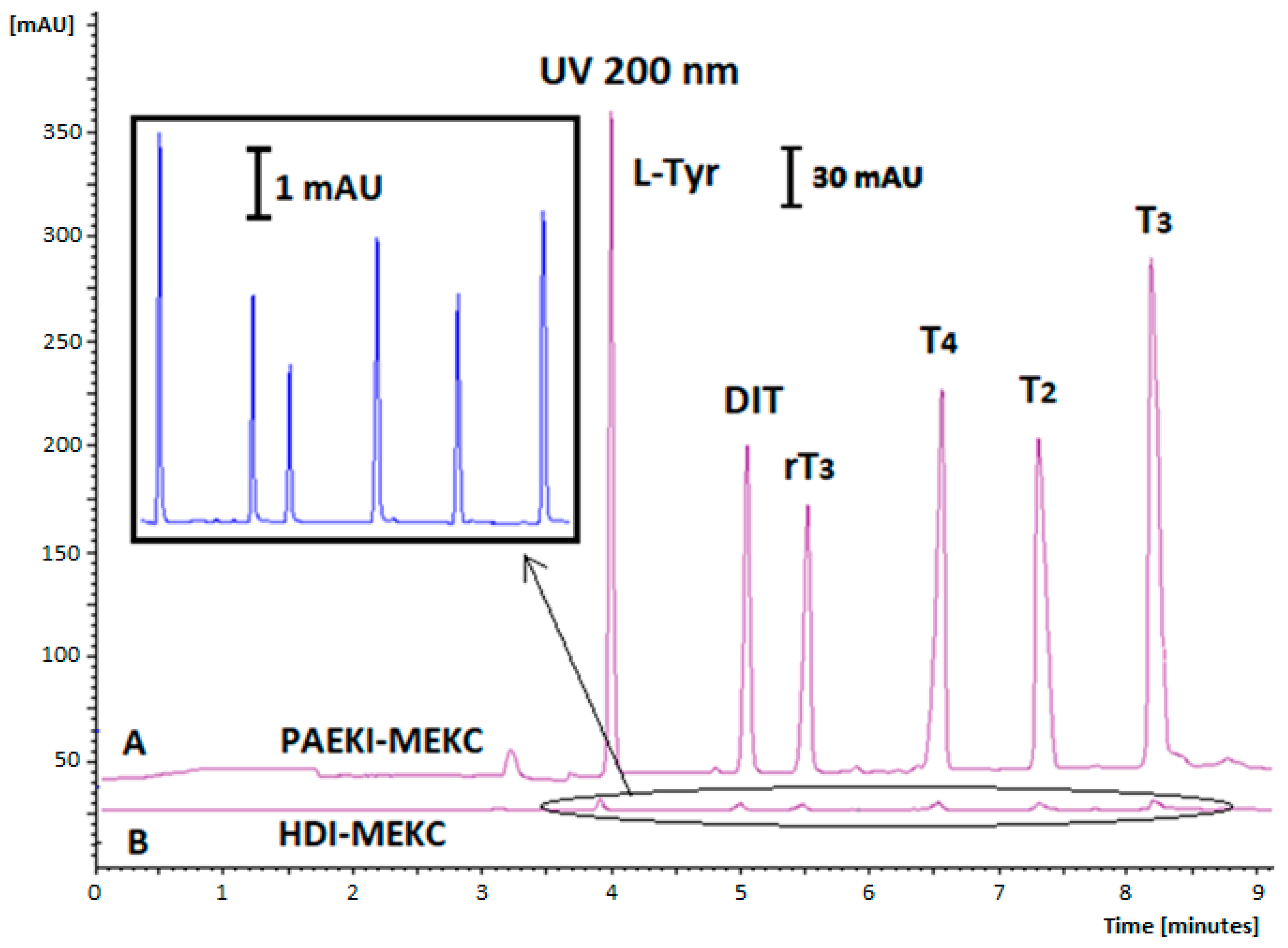
2.1. Optimization of the Derivatization Procedures
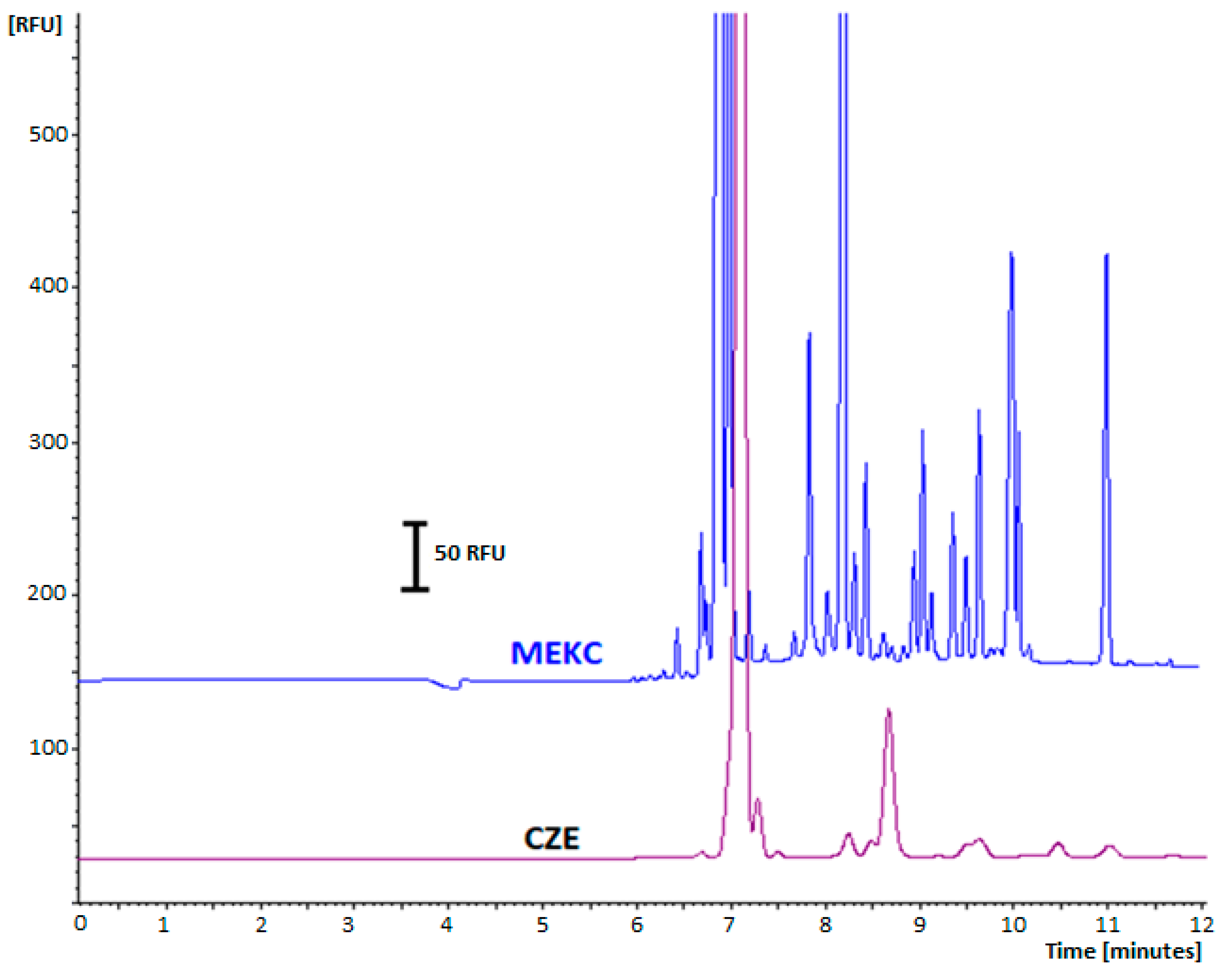
2.2. Buffer Optimization
2.3. The Effect of Surfactant Addition to BGE on the Fluorescence Signal Enhancement
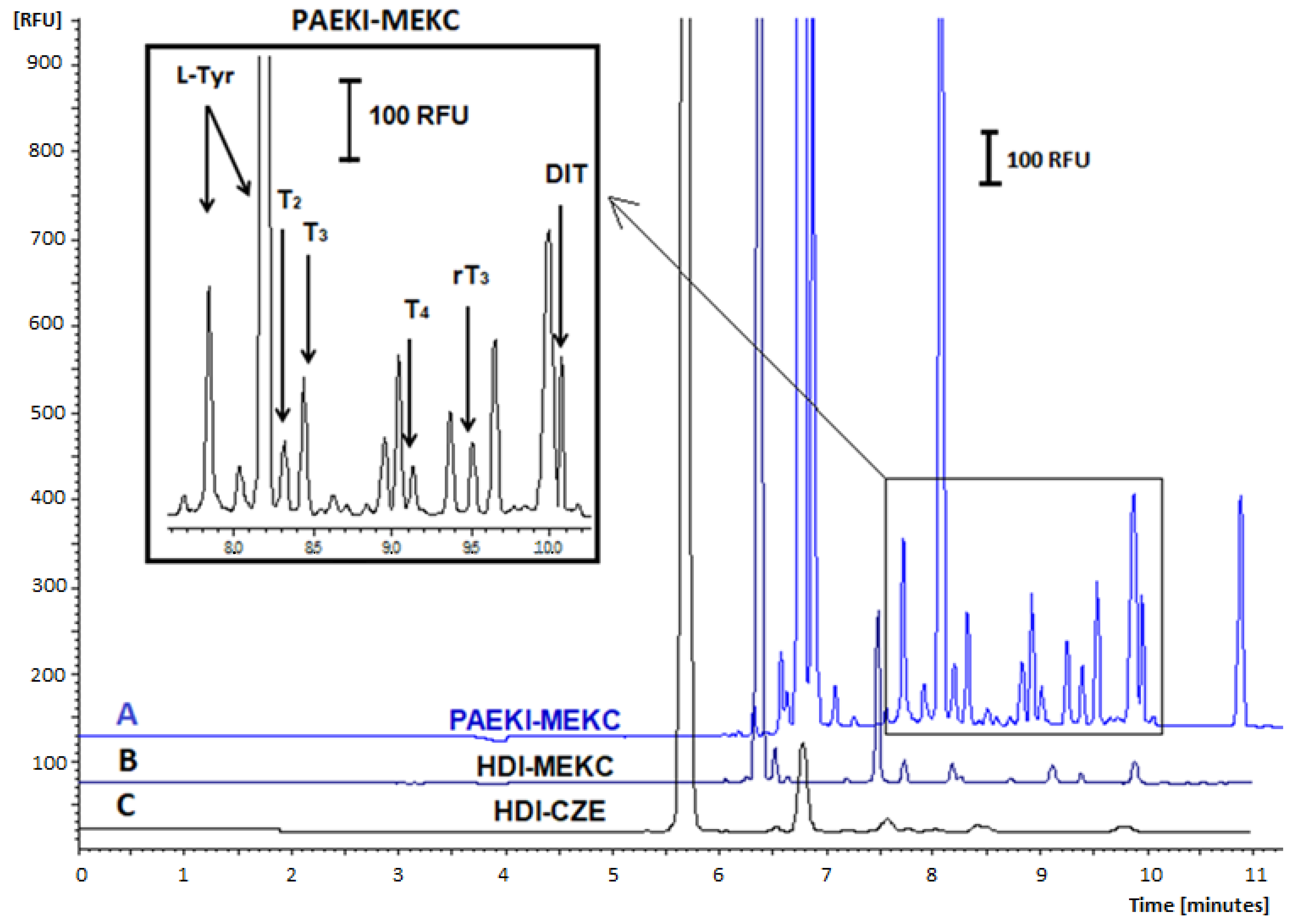
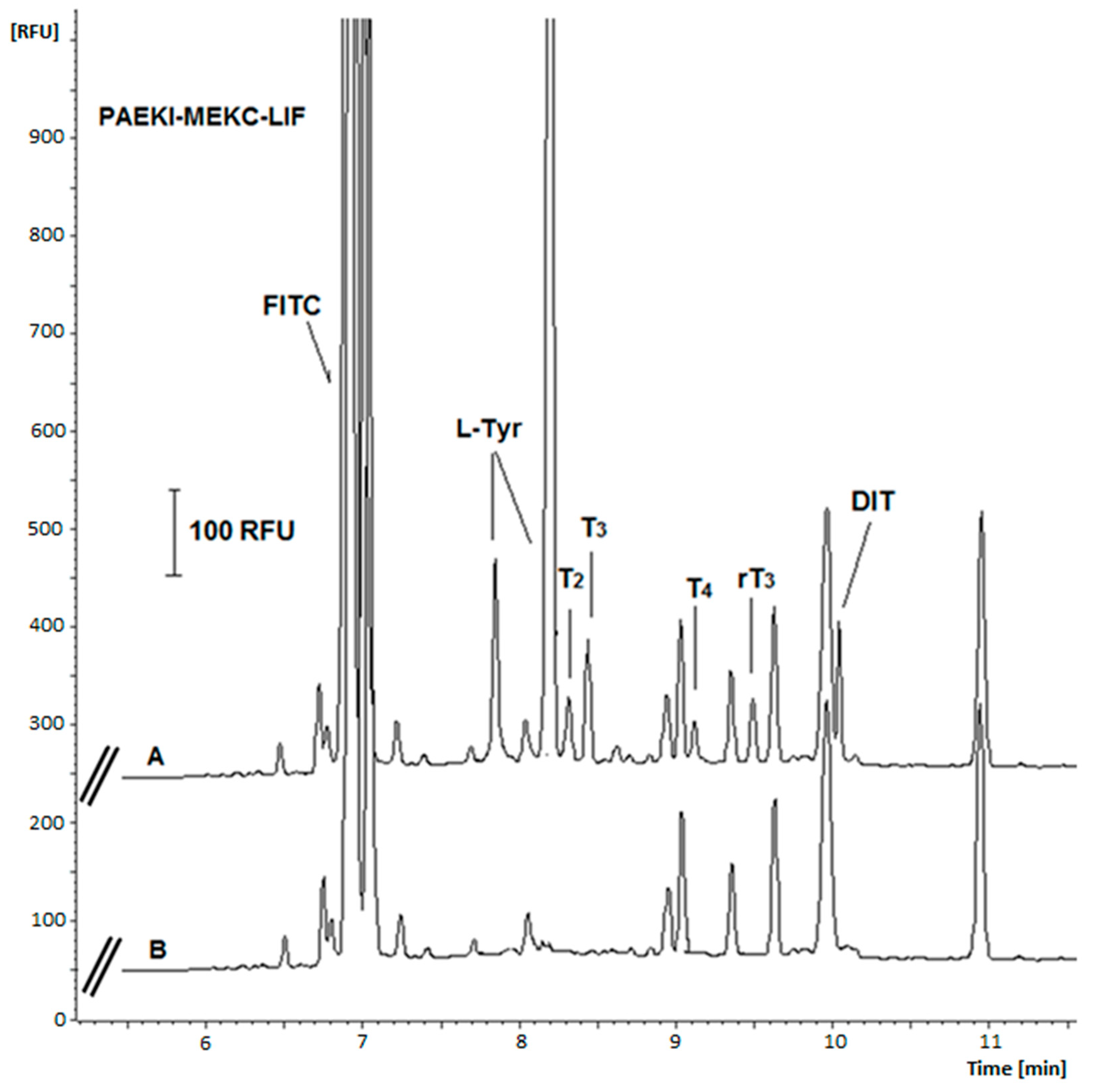
2.4. Signal Amplification Using the Modification of Injection Parameters
2.5. Validation Study
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. CE Instrumentation
3.3. Derivatization Procedure
3.4. Extraction Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunisue, T.; Fisher, J.W.; Kannan, K. Determination of six thyroid hormones in the brain and thyroid gland using isotope-dilution liquid chromatography/tandem mass spectrometry. Anal. Chem. 2011, 83, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Svanfelt, J.; Eriksson, J.; Kronberg, L. Analysis of thyroid hormones in raw and treated wastewater. J. Chromatogr. A 2010, 1217, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [PubMed]
- van der Spek, A.H.; Fliers, E.; Boelen, A. The classic pathways of thyroid hormone metabolism. Mol. Cell. Endocrinol. 2017, 458, 29–38. [Google Scholar] [CrossRef]
- Köhrle, J. The Colorful Diversity of Thyroid Hormone Metabolites. Eur. Thyroid J. 2019, 8, 115–129. [Google Scholar] [CrossRef]
- Nappi, A.; Moriello, C.; Morgante, M.; Fusco, F.; Crocetto, F.; Miro, C. Effects of thyroid hormones in skeletal muscle protein turnover. J. Basic Clin. Physiol. Pharmacol. 2024, 35, 253–264. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Hassan, I.; El-Masri, H.; Ford, J.; Brennan, A.; Handa, S.; Paul Friedman, K.; Gilbert, M.E. Extrapolating In Vitro Screening Assay Data for Thyroperoxidase Inhibition to Predict Serum Thyroid Hormones in the Rat. Toxicol. Sci. Off. J. Soc. Toxicol. 2020, 173, 280–292. [Google Scholar] [CrossRef]
- Chen, Z.; Meima, M.E.; Peeters, R.P.; Visser, W.E. Thyroid Hormone Transporters in Pregnancy and Fetal Development. Int. J. Mol. Sci. 2022, 23, 15113. [Google Scholar] [CrossRef]
- Kim, S.M.; Ryu, V.; Miyashita, S.; Korkmaz, F.; Lizneva, D.; Gera, S.; Latif, R.; Davies, T.F.; Iqbal, J.; Yuen, T.; et al. Thyrotropin, Hyperthyroidism, and Bone Mass. J. Clin. Endocrinol. Metab. 2021, 106, e4809–e4821. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, W.; Zhang, Y.; Zhang, M.; Huan, Z.; Yu, C.; Zhang, X.; Wang, Y.; Xu, J. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr. Disord. 2021, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tu, Y.; Rong, R.; Zhang, Z.; Chen, W.; Long, L.; Zhang, Y.; Wang, C.; Pan, B.; Wu, X.; et al. Association of thyroid hormone with osteoarthritis: From mendelian randomization and RNA sequencing analysis. J. Orthop. Surg. Res. 2024, 19, 429. [Google Scholar] [CrossRef] [PubMed]
- Pagnin, M.; Kondos-Devcic, D.; Chincarini, G.; Cumberland, A.; Richardson, S.J.; Tolcos, M. Role of thyroid hormones in normal and abnormal central nervous system myelination in humans and rodents. Front. Neuroendocrinol. 2021, 61, 100901. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Davis, P.J.; Lin, H.Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid Hormones Interaction with Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front. Cell Dev. Biol. 2021, 8, 614030. [Google Scholar] [CrossRef]
- Karbownik-Lewińska, M.; Stępniak, J.; Iwan, P.; Lewiński, A. Iodine as a potential endocrine disruptor-a role of oxidative stress. Endocrine 2022, 78, 219–240. [Google Scholar] [CrossRef]
- Mazzilli, R.; Medenica, S.; Di Tommaso, A.M.; Fabozzi, G.; Zamponi, V.; Cimadomo, D.; Rienzi, L.; Ubaldi, F.M.; Watanabe, M.; Faggiano, A.; et al. The role of thyroid function in female and male infertility: A narrative review. J. Endocrinol. Investig. 2023, 46, 15–26. [Google Scholar] [CrossRef]
- Jonklaas, J. Optimal Thyroid Hormone Replacement. Endocr. Rev. 2022, 43, 366–404. [Google Scholar] [CrossRef]
- D’Aurizio, F.; Kratzsch, J.; Gruson, D.; Petranović Ovčariček, P.; Giovanella, L. Free thyroxine measurement in clinical practice: How to optimize indications, analytical procedures, and interpretation criteria while waiting for global standardization. Crit. Rev. Clin. Lab. Sci. 2023, 60, 101–140. [Google Scholar] [CrossRef]
- Teixeira, P.F.D.S.; Dos Santos, P.B.; Pazos-Moura, C.C. The role of thyroid hormone in metabolism and metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820917869. [Google Scholar] [CrossRef]
- Gnanasekar, R.; Sarnaik, J.; Joseph, N.C.; Kadwad, V.; Mathur, A. Development of two-step radioimmunoassay (RIA) for the measurement of free triiodothyronine in human serum based on antibody coated tubes. J. Radioanal. Nucl. Chem. 2021, 329, 71–76. [Google Scholar] [CrossRef]
- Gant Kanegusuku, A.; Araque, K.A.; Nguyen, H.; Wei, B.; Hosseini, S.; Soldin, S.J. The effect of specific binding proteins on immunoassay measurements of total and free thyroid hormones and cortisol. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821989240. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. The Free Hormone Hypothesis: When, why, and How to Measure the Free Hormone Levels to Assess Vitamin D, Thyroid, Sex Hormone, and Cortisol Status. JBMR Plus 2020, 5, e10418. [Google Scholar] [CrossRef] [PubMed]
- Basolo, A.; Matrone, A.; Elisei, R.; Santini, F. Effects of tyrosine kinase inhibitors on thyroid function and thyroid hormone metabolism. Semin. Cancer Biol. 2022, 79, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.; Vassalle, C.; Iervasi, G.; Sabatino, L. Main Factors Involved in Thyroid Hormone Action. Molecules 2021, 26, 7337. [Google Scholar] [CrossRef]
- Wang, D.; Stapleton, H.M. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 397, 1831–1839. [Google Scholar] [CrossRef]
- Yue, B.; Rockwood, A.L.; Sandrock, T.; La’ulu, S.L.; Kushnir, M.M.; Meikle, A.W. Free thyroid hormones in serum by direct equilibrium dialysis and online solid-phase extraction--liquid chromatography/tandem mass spectrometry. Clin. Chem. 2008, 54, 642–651. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Gika, H.G.; Papadoyannis, I.N. Rapid HPLC analysis of thyroid gland hormones tri-iodothyronine (T3) and thyroxine (T4) in human biological fluids after SPE. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 681–692. [Google Scholar] [CrossRef]
- Gika, H.G.; Samanidou, V.F.; Papadoyannis, I.N. Development of a validated HPLC method for the determination of iodotyrosines and iodothyronines in pharmaceuticals and biological samples using solid phase extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 814, 163–172. [Google Scholar] [CrossRef]
- Brown, A.K.; Wong, C.S. Measurement of thyroxine and its glucuronide in municipal wastewater and solids using weak anion exchange solid phase extraction and ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1525, 71–78. [Google Scholar] [CrossRef]
- Ackermans, M.T.; Kettelarij-Haas, Y.; Boelen, A.; Endert, E. Determination of thyroid hormones and their metabolites in tissue using SPE UPLC-tandem MS. Biomed. Chromatogr. BMC 2012, 26, 485–490. [Google Scholar] [CrossRef]
- Simon, E.; Bytingsvik, J.; Jonker, W.; Leonards, P.E.; de Boer, J.; Jenssen, B.M.; Lie, E.; Aars, J.; Hamers, T.; Lamoree, M.H. Blood plasma sample preparation method for the assessment of thyroid hormone-disrupting potency in effect-directed analysis. Environ. Sci. Technol. 2011, 45, 7936–7944. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, B.; He, M.; Chen, B. Ion pair hollow fiber liquid-liquid-liquid microextraction combined with capillary electrophoresis-ultraviolet detection for the determination of thyroid hormones in human serum. J. Chromatogr. A 2014, 1356, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, X.; Li, P.; Shi, G.; Zhou, T. Simultaneous determination of thyroxine and triiodothyronine in pharmaceutical formulations using capillary electrophoresis with amperometric detection. J. Sep. Sci. 2010, 33, 2417–2422. [Google Scholar] [CrossRef]
- Michalke, B.; Schramel, P. Iodine speciation in biological samples by capillary electrophoresis-inductively coupled plasma mass spectrometry. Electrophoresis 1999, 20, 2547–2553. [Google Scholar] [CrossRef]
- Molina, M.; Silva, M. Analytical potential of fluorescein analogues for ultrasensitive determinations of phosphorus-containing amino acid herbicides by micellar electrokinetic chromatography with laser-induced fluorescence detection. Electrophoresis 2002, 23, 1096–1103. [Google Scholar] [CrossRef]
- Magdy, G.; Belal, F.F.; Abdel-Megied, A.M.; Abdel Hakiem, A.F. Micelle-Enhanced conventional and synchronous spectrofluorimetric methods for the simultaneous determination of lesinurad and febuxostat: Application to human plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119239. [Google Scholar] [CrossRef]
- Walash, M.I.; Belal, F.; Tolba, M.M.; Halawa, M.I. Micelle-enhanced spectrofluorimetric determination of amlexanox in bioadhesive buccal tablets: Application to content uniformity testing. Lumin. J. Biol. Chem. Lumin. 2015, 30, 823–829. [Google Scholar] [CrossRef]
- Aydin, B.; Acar, M.; Arik, M.; Onganer, Y. The fluorescence resonance energy transfer between dye compounds in micellar media. Dye. Pigment. Int. J. 2009, 81, 156–160. [Google Scholar] [CrossRef]
- Attia, T.Z.; Boushra, J.M.; Abdel Hakiem, A.F.; Lashien, A.S.; Noureldeen, D.A.M. Spectrofluorimetric determination of the anti-Covid 19 agent, remdesivir, in vials and spiked human plasma. Lumin. J. Biol. Chem. Lumin. 2022, 37, 1192–1199. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis Step5. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 20 March 2025).
| Analytes | Separation Technique | LOD | Extraction Technique | Sample Matrix | Reference |
|---|---|---|---|---|---|
| T4, T3, rT3, T2, rT2, T1 | isotope dilution LC-MS/MS | 0.6–1.1 ng mL−1 | SPE | Rat brain and thyroid gland | [1] |
| T4, T3, rT3, T2, rT2 | LC-MS/MS | 0.3–3.9 ng L−1 | SPE | Waste water | [2] |
| T4, T3, rT3, T2 | LC-ESI-MS/MS | 0.34–1.4 ng mL−1 | SPE | Serum | [25] |
| T4, T3 | LC-MS | 1.0 ng L−1 | LLE, SPE | Plasma | [26] |
| T4, T3 | HPLC-UV | 1–2 ng | SPE | Urine, plasma | [27] |
| T4, T3, T2, L-Tyr, DIT, MIT | HPLC-UV | 0.02–0.1 ng µL−1 | SPE | Urine, plasma | [28] |
| T4 | UHPLC-MS/MS | 0.64–0.79 ng L−1 | SPE | Waste water | [29] |
| T4, T3, rT3, T2, rT2 | UPLC-MS/MS | 0.16–0.59 pg | LLE, SPE | Human and animal tissues | [30] |
| T4 | GC/LC-MS | 0.16 µM | LLE, SPE | Plasma | [31] |
| T4, T3, rT3, T2, MIT, DIT | CE-UV | 0.54–1.43 µg L−1 | IP-HF-LLLME | Serum | [32] |
| T4, T3 | CE-AD | 0.085–0.1 µM | - | Pharmaceuticals | [33] |
| T4, T3, Iodides | CE-ICP-MS | 0.08–3.5 µg L−1 | - | Serum, urine | [34] |
| L-Tyr, T2, T3, rT3, T4, DIT | PAEKI-LIF | * 3.0–15.2 pg mL−1 | SPE | Urine | This study |
| Derivatization Agents | Parameter of Derivatization Procedure | |||||||
|---|---|---|---|---|---|---|---|---|
| Derivatization Agent | Buffer Solution (pH 9.5) | Analytes Volume (µL) | Time (h) | Temp. (°C) | ||||
| Solvent | Concentration (mM) | Volume (µL) | Concentration and Type of Buffer | Volume (µL) | ||||
| 5-DTAF | DMSO | 5 | 5 | 0.5 M Na2CO3 | 20 | 75 | 1 | 40 |
| CFSE | Acetone | 5 | 5 | 0.1 M Na2B4O7 | 20 | 75 | 24 | 20 |
| FITC | 1.5 | 10 | 0.2 M Na2HPO4 | 40 | 50 | 2 | 40 | |
| Dilution prior the electrophoretic separation 1000 times (or 10,000 times for L-Thy) with deionized water for all samples. Na2CO3-sodium carbonate, Na2HPO4-disodium hydrogen phosphate | ||||||||
| Analyte | LOD | EF | ||||
|---|---|---|---|---|---|---|
| UV [ng mL−1] | LIF [pg mL−1] | UV PAEKI/HDI | LIF-PAEKI/ UV-HDI | |||
| HDI | PAEKI | HDI * | PAEKI * | |||
| L-Tyr | 45.5 | 3.6 | 80.5 | 3.0 | 26.8 | 15,149 |
| T2 | 78.1 | 7.2 | 306.1 | 14.7 | 23.3 | 7467 |
| T3 | 56.8 | 4.8 | 116.2 | 8.5 | 29.6 | 4685 |
| rT3 | 113.6 | 8.9 | 355.2 | 15.2 | 20.9 | 5322 |
| T4 | 62.5 | 6.3 | 394.6 | 13.3 | 13.7 | 6702 |
| DIT | 78.1 | 7.5 | 117.8 | 7.6 | 15.5 | 10,300 |
| Analyte | PAEKI-MEKC-LIF | HDI-UV | ||||||
|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | R2 | LOD [pg mL−1] | LOQ [pg mL−1] | Precision (%RSD) | |||
| Migration Time | Peak Hight Signal | Peak Hight Signal | ||||||
| L-Tyr * | 2090.0 | −836.8 | 0.9997 | 3.0 | 9.9 | 2.1 | 7.7 | 4.1 |
| T2 | 512.5 | −1811.2 | 0.9985 | 14.7 | 48.4 | 2.4 | 8.4 | 4.8 |
| T3 | 1057.4 | −4785.4 | 0.9991 | 8.5 | 28 | 2.5 | 9.4 | 5.3 |
| rT3 | 595.5 | −9599.2 | 0.9984 | 15.2 | 49.8 | 2.7 | 10.1 | 5.8 |
| T4 | 396.1 | −17,551 | 0.9989 | 13.3 | 44 | 2.8 | 9.2 | 5.1 |
| DIT | 2025.8 | 10,167 | 0.9990 | 7.6 | 25 | 2.4 | 8.2 | 4.3 |
| Analyte | Nominal Concentration [pg mL−1] | Intra-Day (n = 6) | Inter-Day (n = 6) | ||||
|---|---|---|---|---|---|---|---|
| Observed Concentration [pg mL−1] (mean ± SD) | Accuracy [%] | Precision [CV%] | Observed Concentration [pg mL−1] (mean ± SD) | Accuracy [%] | Precision [CV%] | ||
| L-Tyr | LQC | 98.4 ± 3.2 | 98.4 | 8.4 | 97.8 ± 4.4 | 97.8 | 9.5 |
| MQC | 1003.2 ± 19.2 | 100.3 | 5.3 | 1004.5 ± 25.4 | 100.4 | 7.7 | |
| HQC | 9993.1 ± 87.5 | 99.3 | 3.2 | 9991.7 ± 89.8 | 99.9 | 4.4 | |
| T2 | LQC | 102.2 ± 5.2 | 102.2 | 11.0 | 104.9 ± 6.9 | 104.9 | 12.7 |
| MQC | 1004.9 ± 19.8 | 100.5 | 7.9 | 1008.1 ± 22.4 | 100.8 | 8.3 | |
| HQC | 9988.6 ± 84.3 | 99.9 | 5.1 | 9985.6 ± 98.3 | 99.9 | 4.5 | |
| T3 | LQC | 97.2 ± 5.2 | 97.2 | 9.8 | 97.0 ± 5.9 | 97.0 | 10.7 |
| MQC | 995.0 ± 24.4 | 99.5 | 8.9 | 993.3 ± 28.3 | 99.3 | 9.2 | |
| HQC | 9989.7 ± 89.7 | 99.9 | 4.4 | 9978.0 ± 90.2 | 99.8 | 7.5 | |
| rT3 | LQC | 103.8 ± 7.2 | 103.8 | 9.9 | 104.2 ± 8.8 | 104.2 | 11.8 |
| MQC | 1005.8 ± 26.7 | 100.6 | 8.0 | 1007.8 ± 29.3 | 100.8 | 8.7 | |
| HQC | 9982.0 ± 83.2 | 99.8 | 4.3 | 9964.0 ± 91.6 | 99.6 | 6.2 | |
| T4 | LQC | 103.7 ± 5.6 | 103.7 | 9.1 | 104.5 ± 7.6 | 104.5 | 10.4 |
| MQC | 985.9 ± 30.6 | 98.6 | 6.5 | 983.4 ± 32.7 | 98.3 | 7.8 | |
| HQC | 10,021.6 ± 91.6 | 100.2 | 3.2 | 10,044.6 ± 97.0 | 100.5 | 4.1 | |
| DIT | LQC | 97.9 ± 3.2 | 97.9 | 9.0 | 96.7 ± 5.2 | 96.7 | 9.8 |
| MQC | 997.9 ± 17.6 | 99.8 | 7.2 | 995.8 ± 21.9 | 99.6 | 8.4 | |
| HQC | 10,037.5 ± 68.3 | 100.4 | 4.0 | 10,049.3 ± 75.3 | 100.5 | 6.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieckowski, M.; Olędzka, I.; Bączek, T.; Kowalski, P. Strategies for Signal Amplification of Thyroid Hormones via Electromigration Techniques Coupled with UV Detection and Laser-Induced Fluorescence. Int. J. Mol. Sci. 2025, 26, 3708. https://doi.org/10.3390/ijms26083708
Pieckowski M, Olędzka I, Bączek T, Kowalski P. Strategies for Signal Amplification of Thyroid Hormones via Electromigration Techniques Coupled with UV Detection and Laser-Induced Fluorescence. International Journal of Molecular Sciences. 2025; 26(8):3708. https://doi.org/10.3390/ijms26083708
Chicago/Turabian StylePieckowski, Michał, Ilona Olędzka, Tomasz Bączek, and Piotr Kowalski. 2025. "Strategies for Signal Amplification of Thyroid Hormones via Electromigration Techniques Coupled with UV Detection and Laser-Induced Fluorescence" International Journal of Molecular Sciences 26, no. 8: 3708. https://doi.org/10.3390/ijms26083708
APA StylePieckowski, M., Olędzka, I., Bączek, T., & Kowalski, P. (2025). Strategies for Signal Amplification of Thyroid Hormones via Electromigration Techniques Coupled with UV Detection and Laser-Induced Fluorescence. International Journal of Molecular Sciences, 26(8), 3708. https://doi.org/10.3390/ijms26083708







