Immune Modulation and Efficacy of Tixagevimab/Cilgavimab Pre-Exposure Prophylaxis in Lung Transplant Recipients During the Omicron Wave
Abstract
1. Introduction
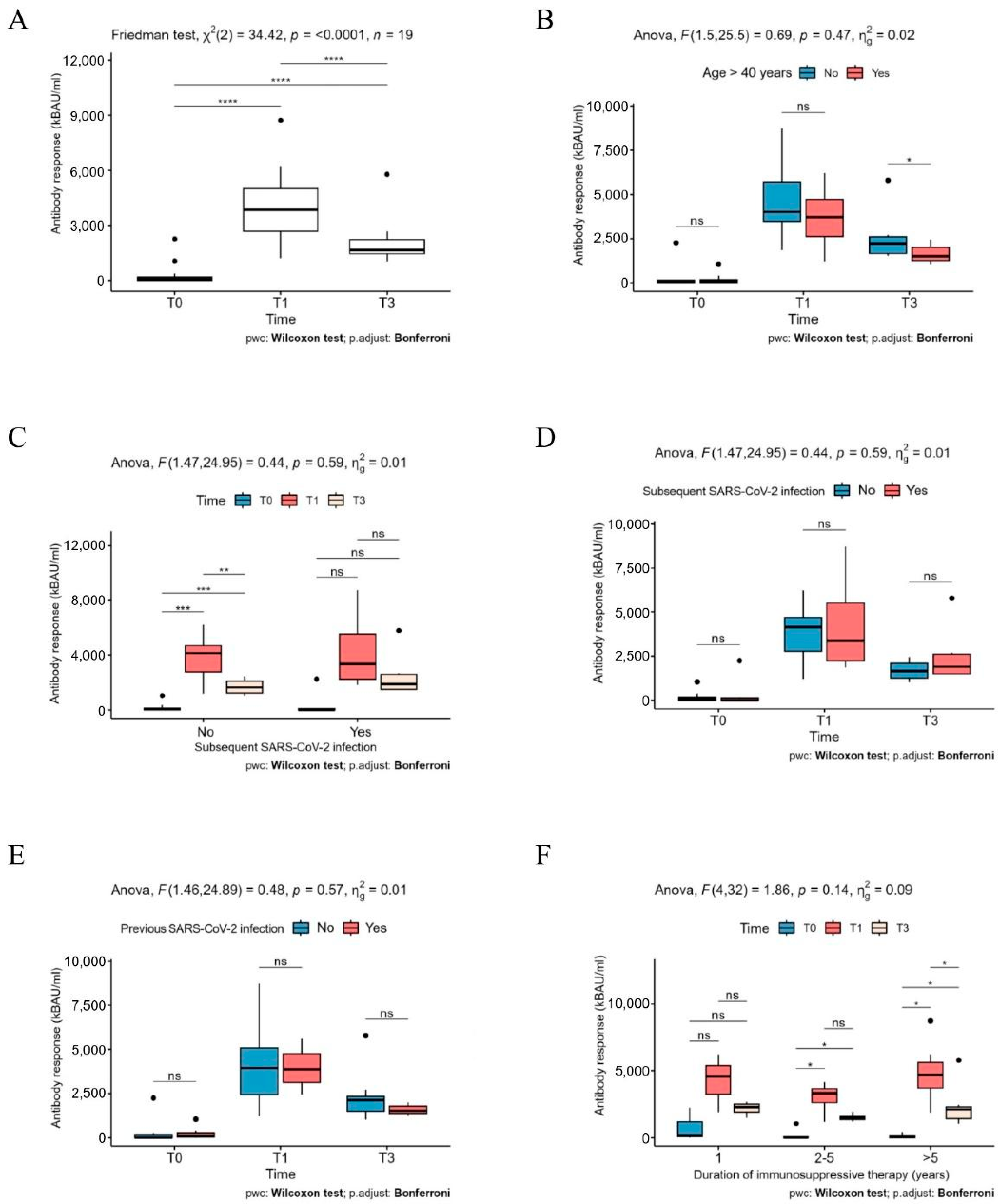
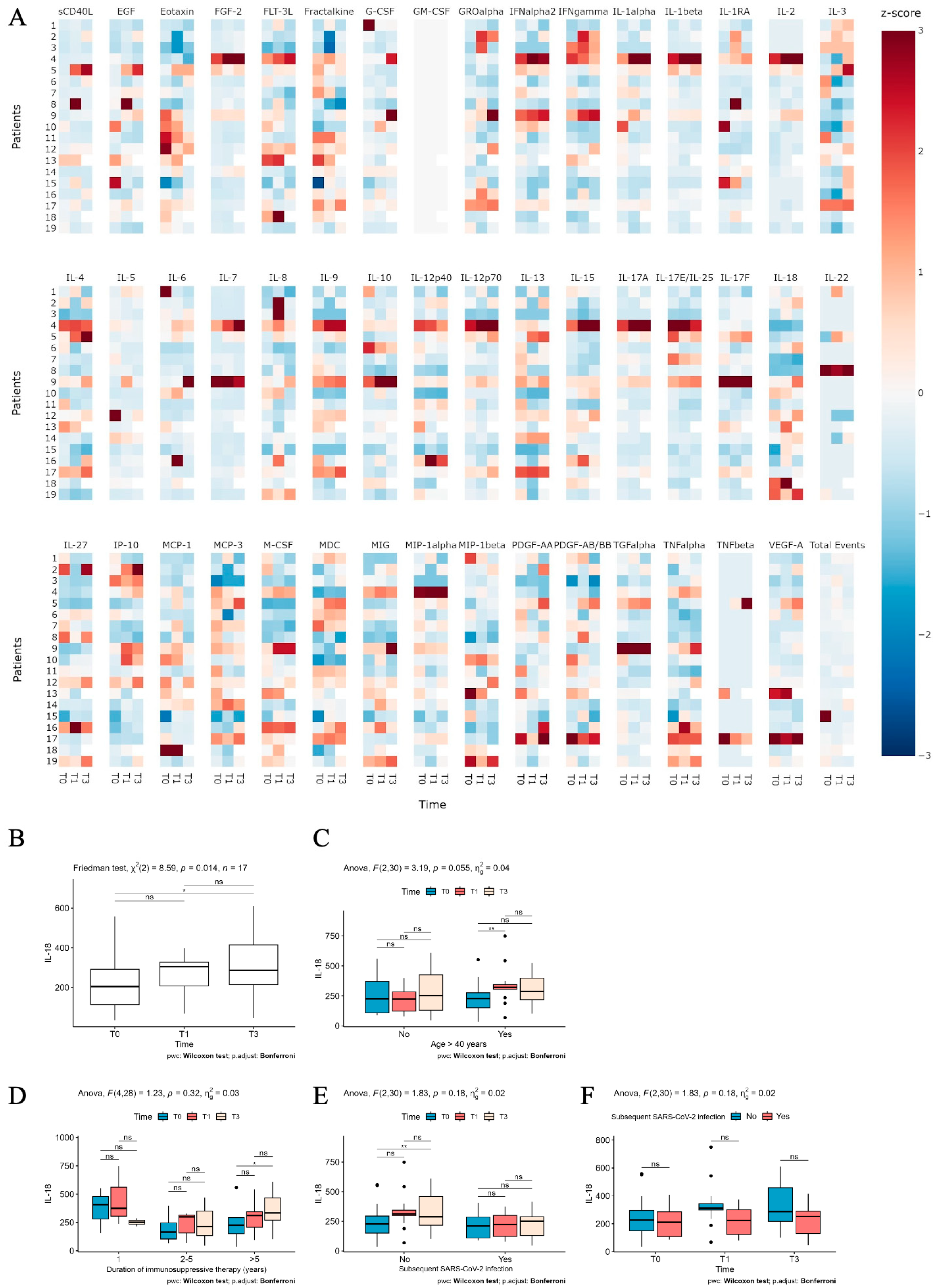
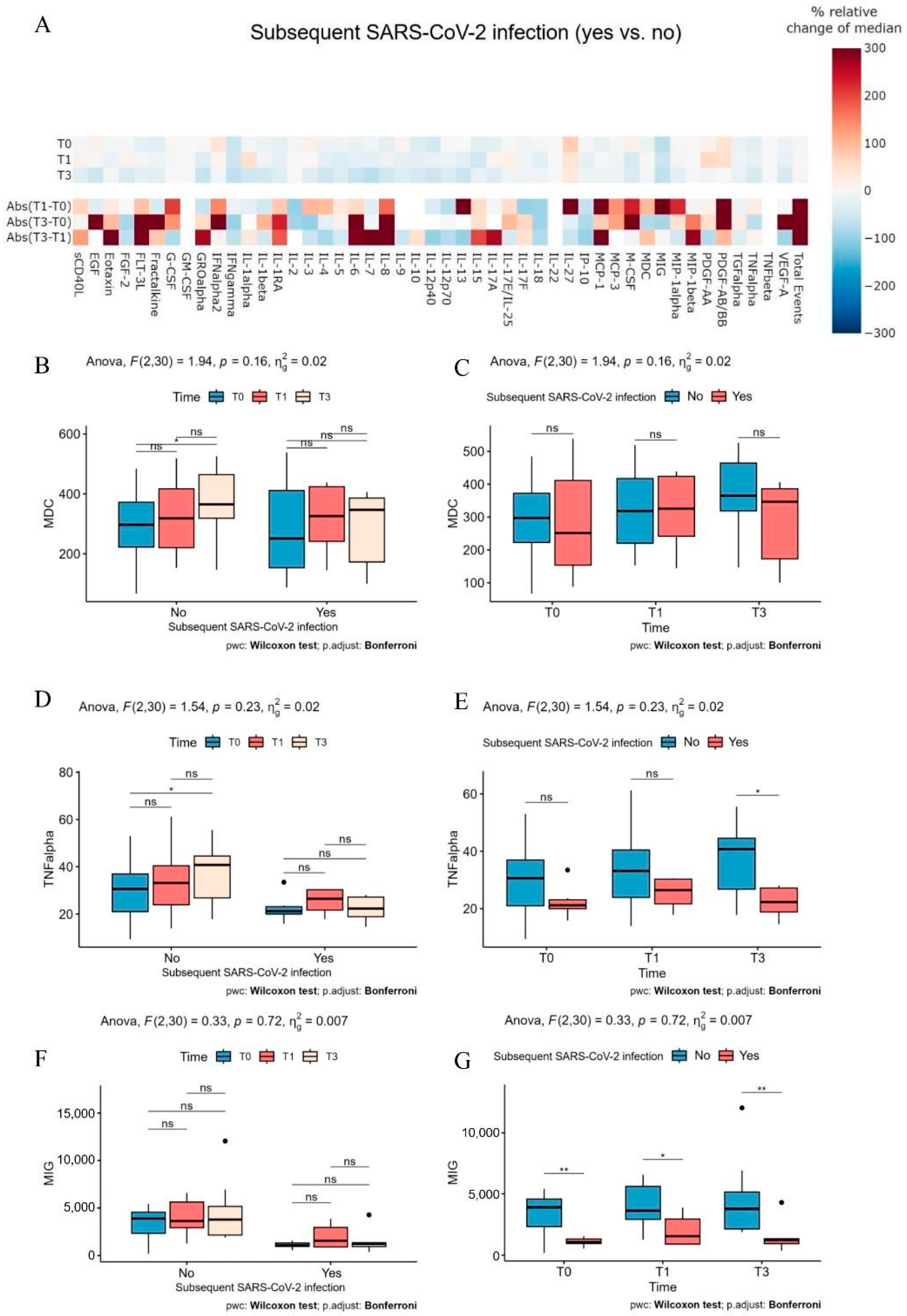
2. Results
3. Discussion
4. Materials and Methods
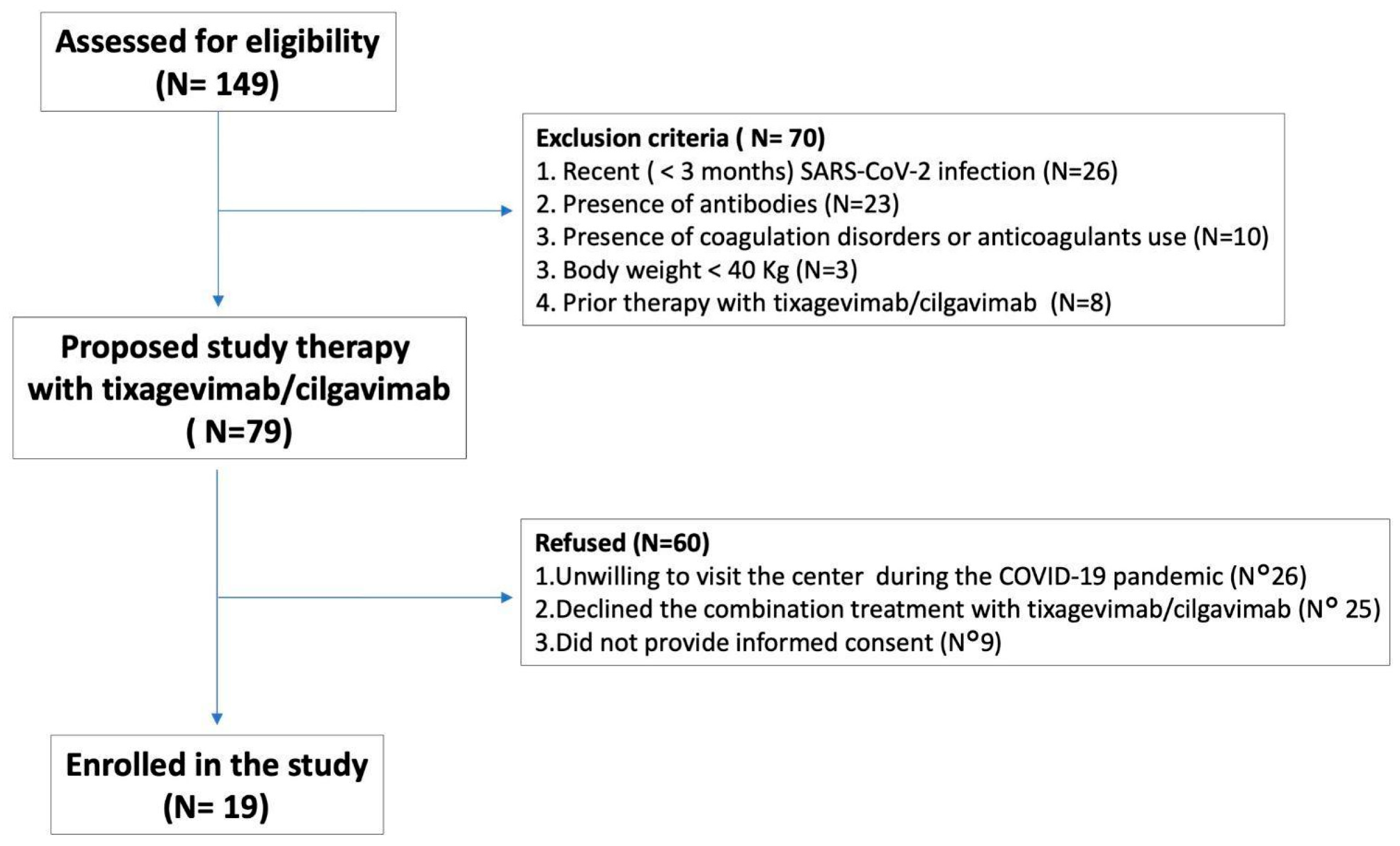
4.1. Participants, Study Design and Data Collection
4.2. Luminex and ELISA Assay
4.3. Statistical Analysis
5. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coll, E.; Fernández-Ruiz, M.; Sánchez-Álvarez, J.E.; Martínez-Fernández, J.R.; Crespo, M.; Gayoso, J.; Bada-Bosch, T.; Oppenheimer, F.; Moreso, F.; López-Oliva, M.O.; et al. COVID-19 in transplant recipients: The Spanish experience. Am. J. Transplant. 2021, 21, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Scheffert, J.L.; Raza, K. Immunosuppression in lung transplantation. J. Thorac. Dis. 2014, 6, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Dauriat, G.; Beaumont, L.; Nguyen, L.B.L.; Picard, B.R.; Penhouet, M.; Coiffard, B.; Salpin, M.; Demant, X.; Raymond, C.S.; Carlier, N.; et al. Efficacy of three COVID-19 vaccine doses in lung transplant recipients: A multicentre cohort study. Eur. Respir. J. 2023, 61, 2200502. [Google Scholar] [CrossRef] [PubMed]
- Khorramnia, S.; Navidi, Z.; Orandi, A.; Iravani, M.M.; Malekabad, E.S.; Moghadam, S.H.P. Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: A systematic review and meta-analysis. Clin. Transplant. Res. 2024, 38, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Dejenie, T.A. Protective roles and protective mechanisms of neutralizing antibodies against SARS-CoV-2 infection and their potential clinical implications. Front. Immunol. 2023, 14, 1055457. [Google Scholar] [CrossRef] [PubMed]
- Angioni, R.; Sánchez-Rodríguez, R.; Munari, F.; Bertoldi, N.; Arcidiacono, D.; Cavinato, S.; Marturano, D.; Zaramella, A.; Realdon, S.; Cattelan, A.; et al. Age-severity matched cytokine profiling reveals specific signatures in COVID-19 patients. Cell Death Dis. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Demolder, S.; Schaevers, V.; Lagrou, K.; De Munter, P.; Beeckmans, H.; Verleden, G.M.; Godinas, L.; Dupont, L.J.; Van Bleyenbergh, P.; Lorent, N.; et al. COVID-19 Outcomes in Lung Transplant Recipients Following Pre-Exposure Prophylaxis With Tixagevimab-Cilgavimab During the Omicron BA.5 Surge: A Single Center Analysis. Transpl. Int. 2024, 37, 12061. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, J.; Simon, S.; Barton, J.; Barnikel, M.; Bachmann, M.; Klingenberg, M.-S.; Veit, T.; Kneidinger, N. Efficacy of pre-exposure prophylaxis to prevent SARS-CoV-2 infection after lung transplantation: A two center cohort study during the omicron era. Infection 2023, 51, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Tavelli, A.; Agrenzano, S.; Hafeez, N.; Scianna, G.; Maria, A.; Marino, F.; De La Cruz, E.; Di Giorgio, M.; Osorio, E.; et al. Tixagevimab/Cilgavimab as SARS-CoV-2 Pre-Exposure Prophylaxis in Lung Transplant Recipients during the Omicron Wave: A Real-World Monocentric Experience. Microorganisms 2024, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Ko, J.-H.; Park, J.; Moon, H.-W.; Baek, J.Y.; Jung, S.; Lim, H.-Y.; Kim, K.-C.; Huh, K.; Cho, S.Y.; et al. Estimating the Neutralizing Effect and Titer Correlation of Semi-Quantitative Anti-SARS-CoV-2 Antibody Immunoassays. Front. Cell. Infect. Microbiol. 2022, 12, 822599. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Takamatsu, Y.; Asai, Y.; Tsuchiya, K.; Matsuda, K.; Oshiro, Y.; Inamura, N.; Terada, M.; Nemoto, T.; Kimura, M.; et al. High diagnostic accuracy of quantitative SARS-CoV-2 spike-binding-IgG assay and correlation with in vitro viral neutralizing activity. Heliyon 2024, 10, e24513. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Vaage, E.B.; Mehta, A.; Chopra, A.; Tietze, L.; Kolderup, A.; Anthi, A.; König, M.; Nygaard, G.; Lind, A.; et al. Titers of antibodies against ancestral SARS-CoV-2 correlate with levels of neutralizing antibodies to multiple variants. npj Vaccines 2022, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001, 19, 423–474. [Google Scholar] [CrossRef] [PubMed]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front. Immunol. 2017, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Althaus, T.; Tan, C.W.; Costantini, A.; Ni Chia, W.; Chau, N.V.V.; Van Tan, L.; Mattiuzzo, G.; Rose, N.J.; Voiglio, E.; et al. WHO international standard for SARS-CoV-2 antibodies to determine markers of protection. Lancet Microbe 2022, 3, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK570371/ (accessed on 3 April 2024).
| Variable | N = 19 |
|---|---|
| Sex, n (%) | |
| Male | 9 (47) |
| Female | 10 (53) |
| Age at visit, median years (IQR) | 51 (40–64) |
| Time after transplant, median years (IQR) | 5 (6 months–15 years) |
| Lung transplant procedure, n (%) | |
| Bilateral | 19 (100) |
| Unilateral | 0 (0) |
| Underlying disease, n (%) | |
| Cystic Fibrosis/Bronchiectasis | 9 (47) |
| Fibrosis/interstitial lung disease | 5 (27) |
| COPD/Emphysema | 4 (21) |
| Pulmonary vascular disease | 1 (5) |
| Immunosuppression, n (%) | |
| Cyclosporine | 10 (53) |
| Mycophenolate | 9 (47) |
| Everolimus | 8 (42) |
| Tacrolimus | 6 (32) |
| Dual Immunosuppression, n (%) | 15 (79) |
| Pre-study SARS-CoV-2 infection n (%) | 7 (37) |
| Vaccination status, n (%) | |
| No or incomplete vaccination | 1 (5) |
| Full vaccination (at least 3 doses) | 18 (95) |
| Humoral immune response after vaccine, n (%) | |
| <50 kBAU/ml | 9 (50) |
| 50–700 kBAU/ml | 8 (42) |
| >700 kBAU/ml | 2 (8) |
| Follow-up, median days after first visit during study (IQR) | 180 (172–194) |
| Humoral immune response 1 month after PrEP, n (%) | |
| <50 kBAU/ml | 0 (0) |
| 50–700 kBAU/ml | 0 (0) |
| >700 kBAU/ml | 19 (100) |
| Humoral immune response 3 month after PrEP, n (%) | |
| <50 kBAU/ml | 0 (0) |
| 50–700 kBAU/ml | 0 (0) |
| >700 kBAU/ml | 19 (100) |
| SARS-CoV-2 infection after tixagevimab/cilgavimab, n (%) | 6 (32) |
| COVID-19 severity during study period, n (%) | |
| Asymptomatic | 0 (0) |
| Mild | 5 (83) |
| Moderate | 0 (0) |
| Severe | 1 (17) |
| Critical | 0 (0) |
| Early treatment of SARS-CoV-2 infection, n (%) | |
| None/reduction in immunosuppression | 2 (33) |
| Remdesivir | 1 (17) |
| Molnupiravir | 2 (33) |
| Sotrovimab | 1 (17) |
| Hospitalization, n (%) | 1 (17) |
| Death, n (%) | 0 (0) |
| COVID-19-associated death, n (%) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasset, L.; Angioni, R.; Presa, N.; Sánchez-Rodríguez, R.; Cozzolino, C.; Bertoldi, N.; Marinello, S.; Loy, M.; Mazzitelli, M.; Rea, F.; et al. Immune Modulation and Efficacy of Tixagevimab/Cilgavimab Pre-Exposure Prophylaxis in Lung Transplant Recipients During the Omicron Wave. Int. J. Mol. Sci. 2025, 26, 3696. https://doi.org/10.3390/ijms26083696
Sasset L, Angioni R, Presa N, Sánchez-Rodríguez R, Cozzolino C, Bertoldi N, Marinello S, Loy M, Mazzitelli M, Rea F, et al. Immune Modulation and Efficacy of Tixagevimab/Cilgavimab Pre-Exposure Prophylaxis in Lung Transplant Recipients During the Omicron Wave. International Journal of Molecular Sciences. 2025; 26(8):3696. https://doi.org/10.3390/ijms26083696
Chicago/Turabian StyleSasset, Lolita, Roberta Angioni, Nicolò Presa, Ricardo Sánchez-Rodríguez, Claudia Cozzolino, Nicole Bertoldi, Serena Marinello, Monica Loy, Maria Mazzitelli, Federico Rea, and et al. 2025. "Immune Modulation and Efficacy of Tixagevimab/Cilgavimab Pre-Exposure Prophylaxis in Lung Transplant Recipients During the Omicron Wave" International Journal of Molecular Sciences 26, no. 8: 3696. https://doi.org/10.3390/ijms26083696
APA StyleSasset, L., Angioni, R., Presa, N., Sánchez-Rodríguez, R., Cozzolino, C., Bertoldi, N., Marinello, S., Loy, M., Mazzitelli, M., Rea, F., Cattelan, A., & Molon, B. (2025). Immune Modulation and Efficacy of Tixagevimab/Cilgavimab Pre-Exposure Prophylaxis in Lung Transplant Recipients During the Omicron Wave. International Journal of Molecular Sciences, 26(8), 3696. https://doi.org/10.3390/ijms26083696








