Multiomics Studies on the Effects of High-Temperature Stress on Male Sterility in Gossypium barbadense
Abstract
1. Introduction
2. Results and Analysis
2.1. Quality Assessment of the Transcriptomic and Metabolomic Sequencing Data
2.2. Effects of High-Temperature Stress on Floral Organ Development and Hormonal Regulation in G. barbadense GB150
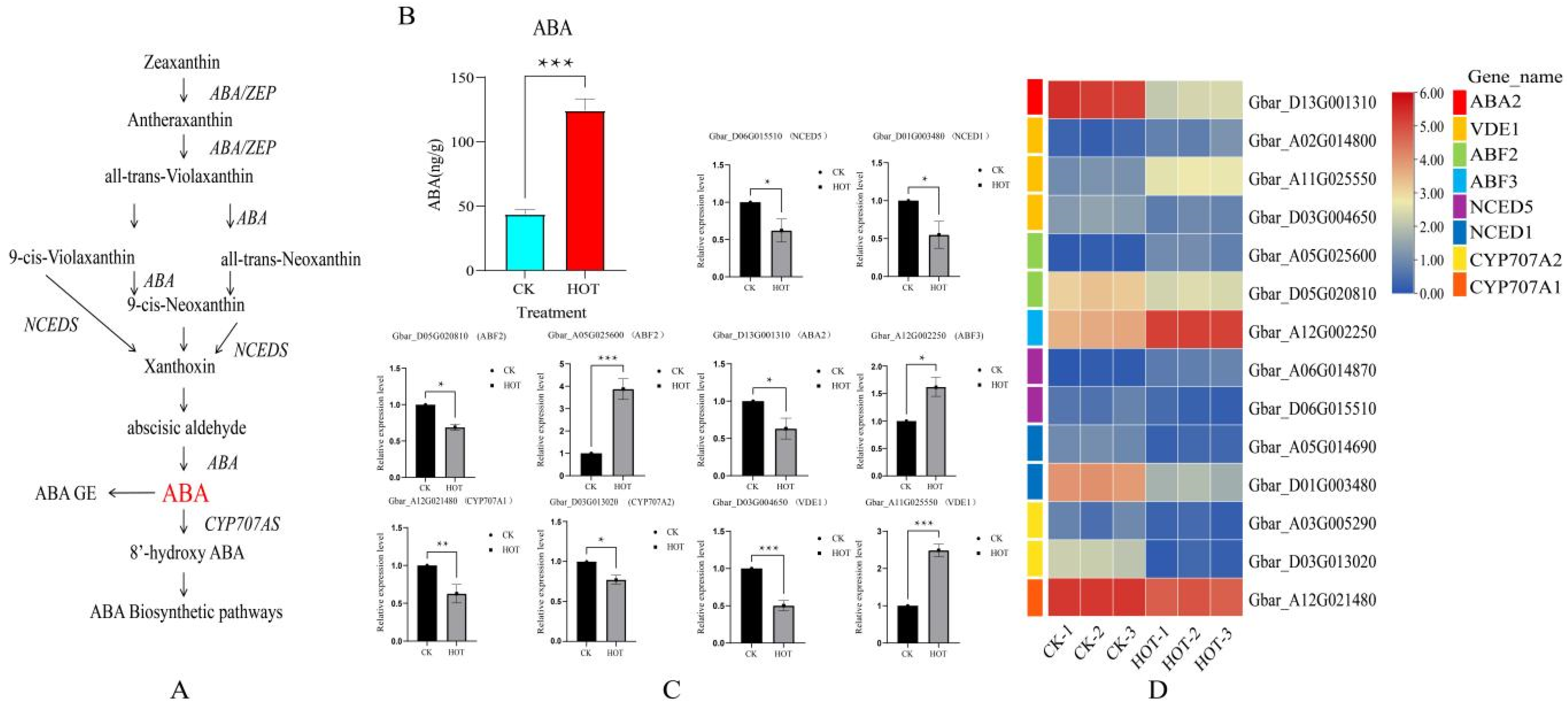
2.3. Changes in ABA Content and Expression Levels in G. barbadense Anthers Under HT Stress
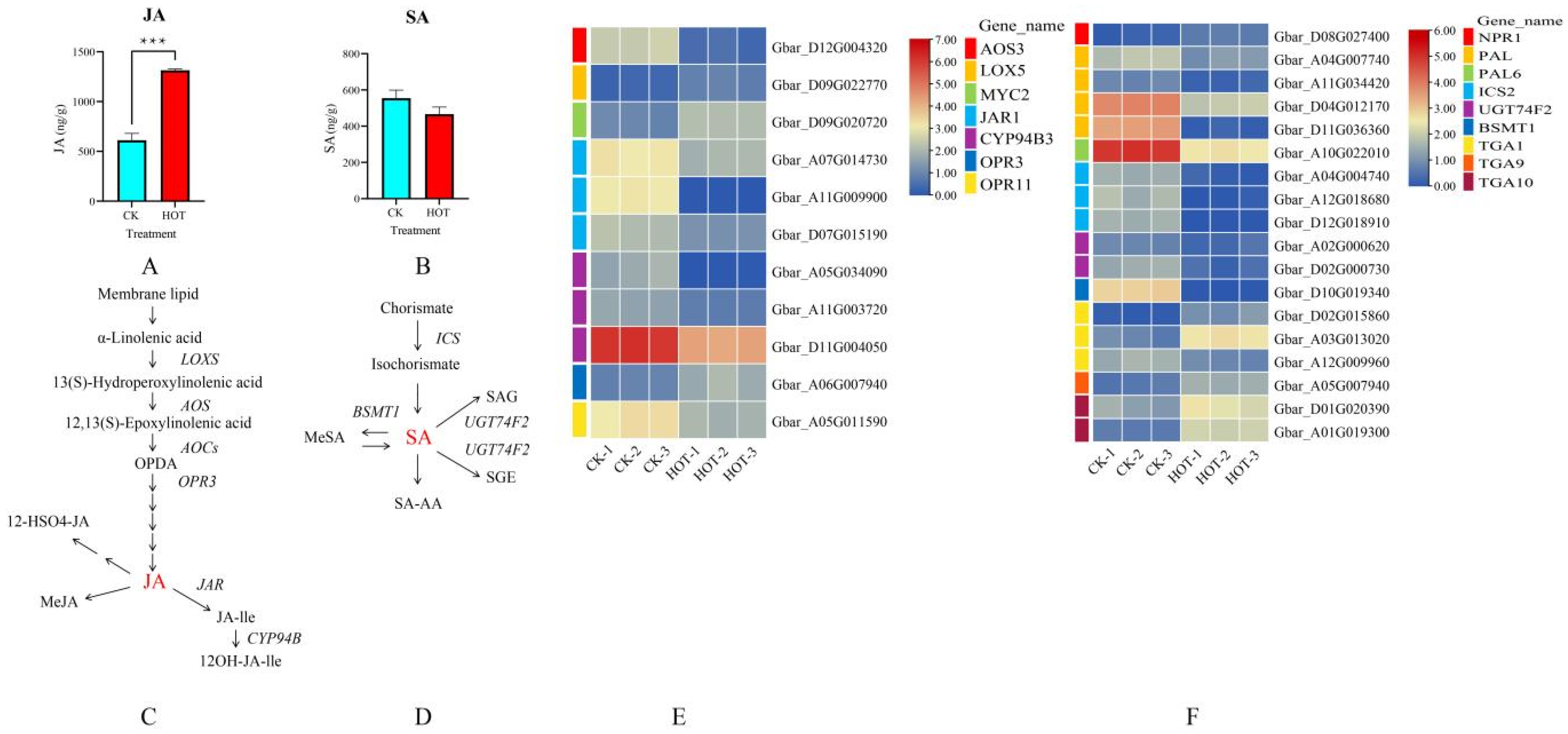
2.4. Effects of HT Stress on JA and SA Biosynthesis and Signal Transduction
2.5. Effects of HT Stress on IAA, GA20 and tZR Biosynthesis and Signal Transduction
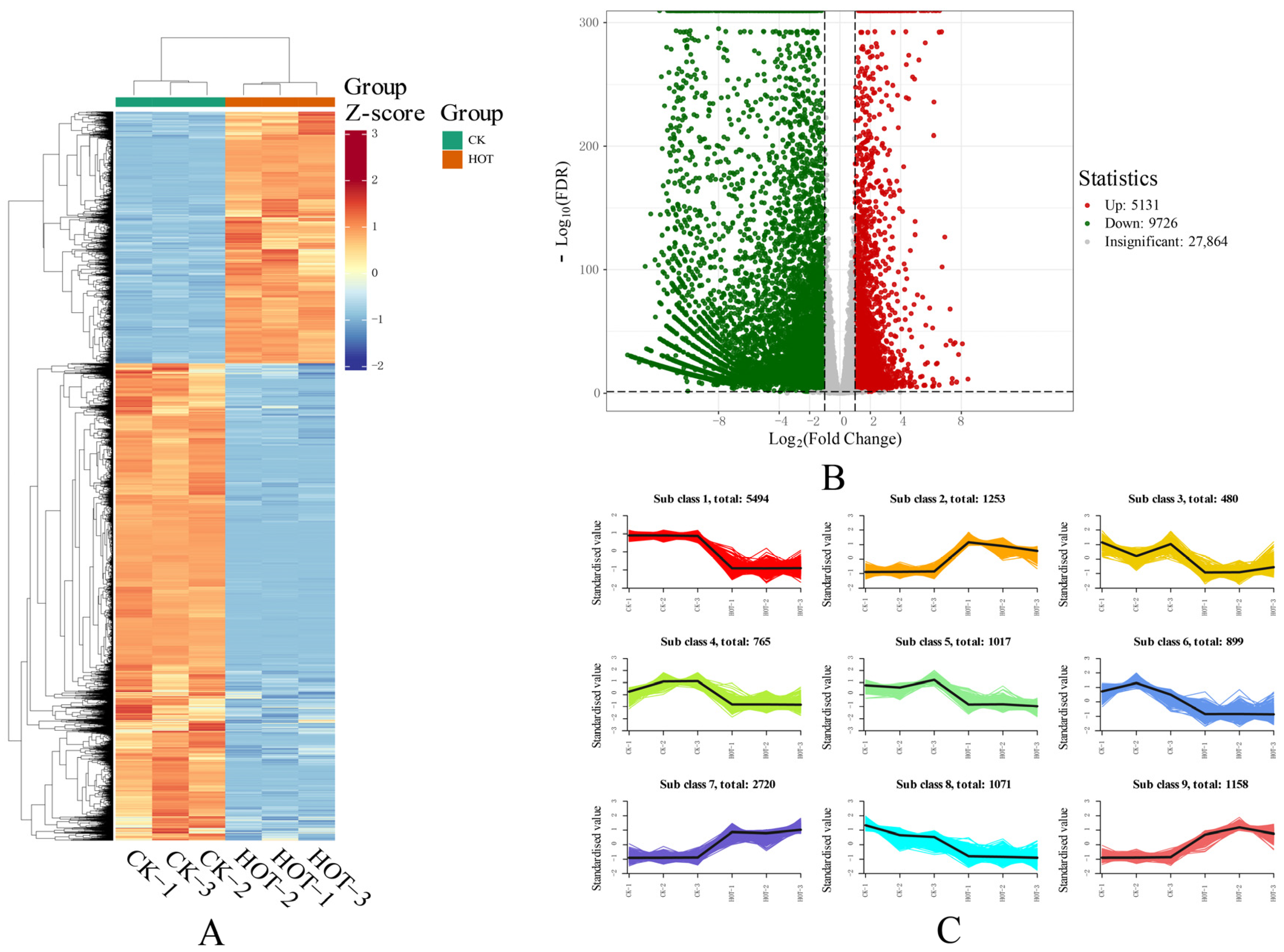
2.6. Changes in the Whole Transcriptome Under HT Stress
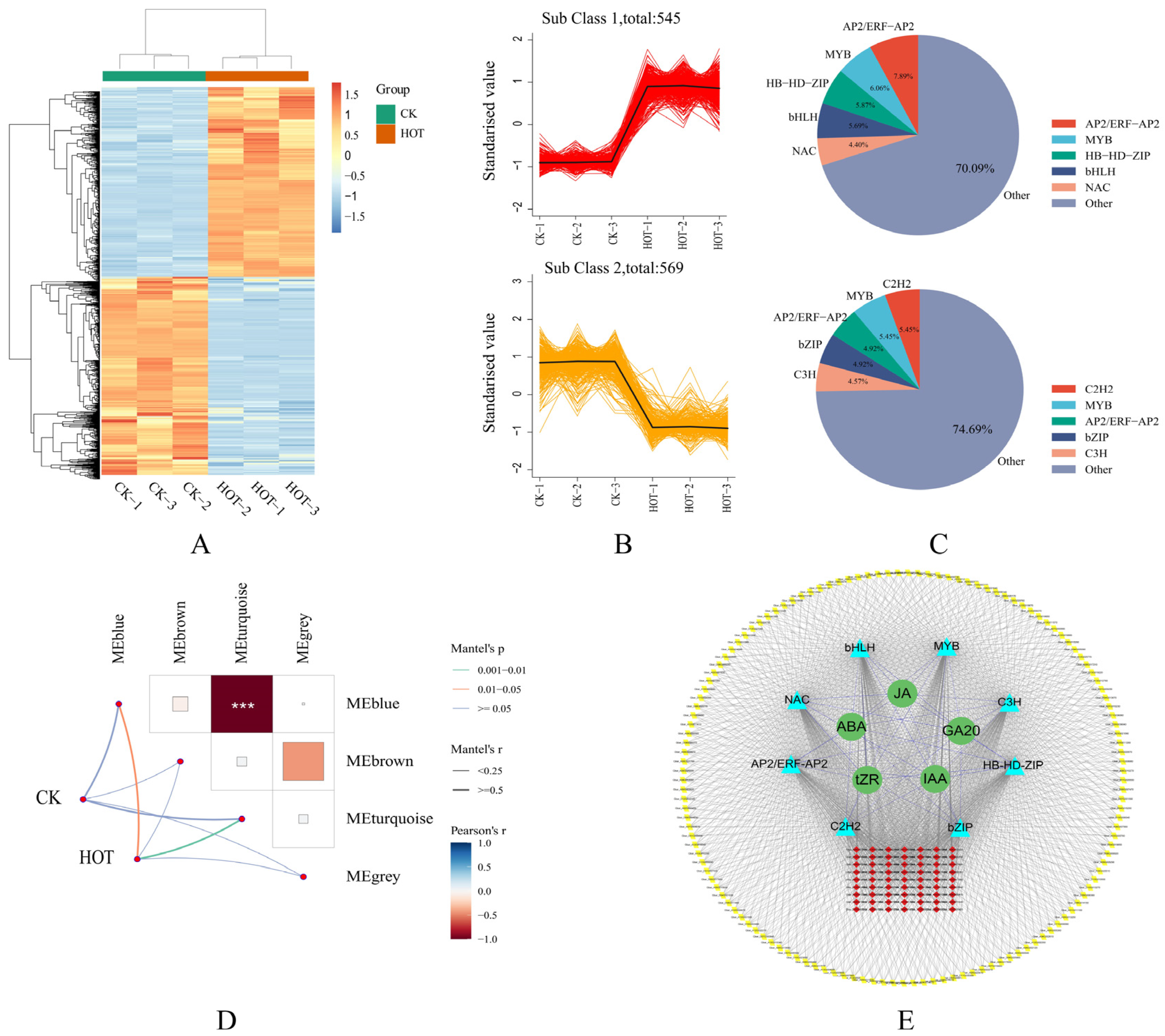
2.7. Construction and TF Analysis of the Hormone-Related Coexpression Network
2.8. Influence of HT Stress on TF Expression and Construction of a Genome-Wide Coexpression Network
2.9. Expression Validation and Genetic Variation Analysis of Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of Flower Organ Indices
4.3. Determination of Plant Hormone Contents
4.4. Transcriptomic Sequencing
4.5. Coexpression Network Construction
4.6. qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HT | High-temperature |
| G. Barbadense | Gossypium barbadense |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| DEGs | Differentially expressed genes |
| PCA | Principal components analysis |
| WGCNA | weighted gene co-expression network analysis |
| ABA | Abscisic Acid |
| JA | Jasmonic Acid |
| SA | Salicylic Acid |
| GA20 | Gibberellic Acid 20 |
| tZR | trans-Zeatin Riboside |
| IAA | 3-Indoleacetic acid |
| VED | Violaxanthin de-epoxidase |
| ABF | Abscisic Acid (ABA) Responsive Element Binding Factors |
| NCED | 9-cis-epoxycarotenoid dioxygenase gene |
| CYP | Cytochrome P450 |
| AOS | Allene Oxide Synthase |
| LOX | Lysyl oxidase |
| MYC | Transcription factor MYC |
| JAR | Jasmonate Responsive |
| OPR | 12-oxo-phytodienoic acid reductase |
| NPR | Non-expressor of pathogenesis-related |
| PAL | Phenylalanine ammonia-lyase |
| ICS | Isochorismate synthase |
| UGT | UDP-glucuronosyltransferase |
| BSMT | Benzoic acid/salicylic acid carboxyl methyltransferase |
| TGA | TGACG motif binding factor |
| ARF | Auxin Response Factors |
| AUX | Auxin/Indole-3-Acetic Acid |
| SAUR | Small Auxin Up RNA |
| ARR | Arabidopsis response regulators |
| AHP | Histidine-containing Phosphotransfer Protein |
| ZOG | Zeatin O-glucosyltransferase |
| miaA | tRNA dimethylallyltransferase |
| CKX | Cytokinin Oxidase/Dehydrogenase |
| DELLA | DELLA protein |
| GA2OX | Gibberellin 2-oxidase |
| GID | Gibberellin receptor |
| PIF | Phytochrome-interacting Factors |
References
- Cao, Z.; Tang, H.; Cai, Y.; Zeng, B.; Zhao, J.; Tang, X.; Lu, M.; Wang, H.; Zhu, X.; Wu, X.; et al. Natural variation of HTH5 from wild rice, Oryza rufipogon Griff., is involved in conferring high-temperature tolerance at the heading stage. Plant Biotechnol. J. 2022, 20, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Hodges, H.F.; McCarty, W.H.; McKinion, J.M. Weather and Cotton Growth: Present and Future; MSU-MAFES: Starkville, MS, USA, 1996. [Google Scholar]
- Pettigrew, W.T. The effect of higher temperatures on cotton lint yield production and fiber quality. Crop Sci. 2008, 48, 278–285. [Google Scholar] [CrossRef]
- Achard, P.; Renou, J.P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef]
- Zahid, K.R.; Ali, F.; Shah, F.; Younas, M.; Shah, T.; Shahwar, D.; Hassan, W.; Ahmad, Z.; Qi, C.; Lu, Y.; et al. Response and tolerance mechanism of cotton Gossypium hirsutum L. to elevated temperature stress: A review. Front. Plant Sci. 2016, 7, 937. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asner, G.P. Climate and management contributions to recent trends in US agricultural yields. Science 2003, 299, 1032. [Google Scholar] [CrossRef]
- Pan, X.; Du, L.; Tao, J.; Jiang, S.; Qian, D.; Duan, J. Dynamic changes of flavonoids in Abelmoschus manihot different organs at different growth periods by UPLC-MS/MS. J. Chromatogr. B 2017, 1059, 21–26. [Google Scholar] [CrossRef]
- Yi, D.; Zhang, H.; Lai, B.; Liu, L.; Pan, X.; Ma, Z.; Wang, Y.; Xie, J.; Shi, S.; Wei, Y. Integrative analysis of the coloring mechanism of red longan pericarp through metabolome and transcriptome analyses. J. Agric. Food Chem. 2020, 69, 1806–1815. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Mi, X.; Zhao, S.; An, Y.; Xia, X.; Guo, R.; Wei, C. Multi-omics analysis to visualize the dynamic roles of defense genes in the response of tea plants to gray blight. Plant J. 2021, 106, 862–875. [Google Scholar] [CrossRef]
- Li, S.; Deng, B.; Tian, S.; Guo, M.; Liu, H.; Zhao, X. Metabolic and transcriptomic analyses reveal different metabolite biosynthesis profiles between leaf buds and mature leaves in Ziziphus jujuba mill. Food Chem. 2021, 347, 129005. [Google Scholar] [CrossRef]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Q.; Wang, Y.; Xu, Y.; Li, J.; Zhao, S.; Wang, D.; Ma, Z.; Yan, F.; Liu, Y. Combined transcriptomic and metabolomic analysis reveals the role of phenylpropanoid biosynthesis pathway in the salt tolerance process of Sophora alopecuroides. Int. J. Mol. Sci. 2021, 22, 2389–2399. [Google Scholar] [CrossRef]
- Wang, X.; Shan, X.; Wu, Y.; Su, S.; Li, S.; Liu, H.; Han, J.; Xue, C.; Yuan, Y. iTRAQ-based quantitative proteomic analysis reveals new metabolic pathways responding to chilling stress in maize seedlings. J. Proteom. 2016, 146, 14–24. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Yoshida, T.; Tanaka, A.; Sasaki, R.; Bando, A.; Toh, S.; Lepiniec, L.; Kawakami, N. Isolation and characterization of high temperature-resistant germination mutants of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 1081–1094. [Google Scholar] [CrossRef]
- Nie, H.; Cheng, C.; Kong, J.; Li, H.; Hua, J. Plant non-coding RNAs function in pollen development and male sterility. Front. Plant Sci. 2023, 14, 1109941. [Google Scholar] [CrossRef]
- You, J.; Li, M.; Li, H.; Bai, Y.; Zhu, X.; Kong, X.; Chen, X.; Zhou, R. Integrated methylome and transcriptome analysis widen the knowledge of cytoplasmic male sterility in cotton (Gossypium barbadense L.). Front. Plant Sci. 2022, 13, 770098. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, P.; Li, N.; Xu, X.; Wang, S.; Chang, S.; Zhang, Y.; Wang, X.; Liu, W.; Ma, Y.; et al. A male-sterile mutant with necrosis-like dark spots on anthers was generated in cotton. Front. Plant Sci. 2022, 13, 1102196. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Arif, M.S.; Ahmad, R.; Hasanuzzaman, M.; Ali, B.; Hussain, A. Approaches in enhancing thermotolerance in plants: An updated review. J. Plant Growth Regul. 2020, 39, 456–480. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.O.N.; Miller, G.A.D. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.N.; Dennis, E.S.; Dolferus, R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol. 2007, 48, 1319–1330. [Google Scholar] [CrossRef]
- Qin, G.; Zhao, N.; Wang, W.; Wang, M.; Zhu, J.; Yang, J.; Lin, F.; Huang, X.; Zhang, Y.; Min, L.; et al. Glyphosate-Induced Abscisic Acid Accumulation Causes Male Sterility in Sea Island Cotton. Plants 2023, 12, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Ma, Q.; Qin, Y.; Wang, H.; Chen, P.; Ma, L.; Fu, X.; Zhu, L.; Wei, H.; et al. Deficiencies in the formation and regulation of anther cuticle and tryphine contribute to male sterility in cotton PGMS line. BMC Genom. 2020, 21, 825. [Google Scholar] [CrossRef]
- Gan, Z.; Feng, Y.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. Downregulation of the auxin transporter gene SlPIN8 results in pollen abortion in tomato. Plant Mol. Biol. 2019, 99, 561–573. [Google Scholar] [CrossRef]
- Chen, C.; Pang, Y.; Pan, X.; Zhang, L. Impacts of climate change on cotton yield in China from 1961 to 2010 based on provincial data. J. Meteorol. Res. 2015, 29, 515–524. [Google Scholar] [CrossRef]
- Fragkostefanakis, S.; Mesihovic, A.; Simm, S.; Paupière, M.J.; Hu, Y.; Paul, P.; Mishra, S.K.; Tschiersch, B.; Theres, K.; Bovy, A.; et al. HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 2016, 170, 2461–2477. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Khan, A.H.; Ma, Y.; He, X.; Yang, J.; Zhang, R.; Ma, H.; Zuo, C.; Li, Y.; et al. Histone H3 lysine 27 trimethylation suppresses jasmonate biosynthesis and signaling to affect male fertility under high temperature in cotton. Plant Commun. 2023, 4, 100660. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ma, Y.; Wu, Y.; Akbar, A.; Shaban, M.; Ullah, A.; Deng, J.; Khan, A.S.; Chi, H.; Zhu, L.; et al. High-temperature stress suppresses allene oxide cyclase 2 and causes male sterility in cotton by disrupting jasmonic acid signaling. Crop J. 2023, 11, 33–45. [Google Scholar] [CrossRef]
- Luo, P.; Di, D.; Wu, L.; Yang, J.; Lu, Y.; Shi, W. MicroRNAs are involved in regulating plant development and stress response through fine-tuning of TIR1/AFB-dependent auxin signaling. Int. J. Mol. Sci. 2022, 23, 510. [Google Scholar] [CrossRef]
- Nagpal, P.; Ellis, C.M.; Weber, H.; Ploense, S.E.; Barkawi, L.S.; Guilfoyle, T.J.; Hagen, G.; Alonso, J.M.; Cohen, J.D.; Farmer, E.E.; et al. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 2005, 132, 4107–4118. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, L.; Huang, M.; He, X.; Yang, Y.; Liu, X.; Li, Y.; Hou, X. Gibberellins play an essential role in late embryogenesis of Arabidopsis. Nat. Plants 2018, 4, 289–298. [Google Scholar] [CrossRef]
- Kawai, K.; Takehara, S.; Kashio, T.; Morii, M.; Sugihara, A.; Yoshimura, H.; Ito, A.; Hattori, M.; Toda, Y.; Kojima, M.; et al. Evolutionary alterations in gene expression and enzymatic activities of gibberellin 3-oxidase 1 in Oryza. Commun. Biol. 2022, 5, 67. [Google Scholar] [CrossRef]
- Liu, H.; Shang, H.; Yang, H.; Liu, W.; Tsugama, D.; Nonomura, K.-I.; Zhou, A.; Wu, W.; Takano, T.; Liu, S. RNA-Binding Protein MAC5A Is Required for Gibberellin-Regulated Stamen Development. Int. J. Mol. Sci. 2022, 23, 2009. [Google Scholar] [CrossRef]
- Plackett, A.R.G.; Ferguson, A.C.; Powers, S.J.; Wanchoo-Kohli, A.; Phillips, A.L.; Wilson, Z.A.; Hedden, P.; Thomas, S.G. DELLA activity is required for successful pollen development in the Columbia ecotype of Arabidopsis. New Phytol. 2014, 201, 825–836. [Google Scholar] [CrossRef]
- Yang, D.-L.; Yao, J.; Mei, C.-S.; Tong, X.-H.; Zeng, L.-J.; Li, Q.; Xiao, L.-T.; Sun, T.-P.; Li, J.; Deng, X.-W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef]
- Khan, A.H.; Min, L.; Ma, Y.; Zeeshan, M.; Jin, S.; Zhang, X. High-temperature stress in crops: Male sterility, yield loss and potential remedy approaches. Plant Biotechnol. J. 2023, 21, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Hollender, C.A.; Kang, C.; Darwish, O.; Geretz, A.; Matthews, B.F.; Slovin, J.; Alkharouf, N.; Liu, Z. Floral transcriptomes in woodland strawberry uncover developing receptacle and anther gene networks. Plant Physiol. 2014, 165, 1062–1075. [Google Scholar] [CrossRef]
- Greenham, K.; Guadagno, C.R.; Gehan, M.A.; Mockler, T.C.; Weinig, C.; Ewers, B.E.; McClung, C.R. Temporal network analysis identifies early physiological and transcriptomic indicators of mild drought in Brassica rapa. elife 2017, 6, e29655. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.J.; Michno, J.-M.; Jeffers, J.; Hoekenga, O.; Dilkes, B.; Baxter, I.; Myers, C.L. Integrating coexpression networks with GWAS to prioritize causal genes in maize. Plant Cell 2018, 30, 2922–2942. [Google Scholar] [CrossRef]
- Azam, M.; Zhang, S.; Huai, Y.; Abdelghany, A.M.; Shaibu, A.S.; Qi, J.; Feng, Y.; Liu, Y.; Li, J.; Qiu, L.; et al. Identification of genes for seed isoflavones based on bulk segregant analysis sequencing in soybean natural population. Theor. Appl. Genet. 2023, 136, 13. [Google Scholar] [CrossRef]
- Zou, J.; Yang, L.; Li, Y.; Piao, M.; Li, Y.; Yao, N.; Zhang, X.; Zhang, Q.; Hu, G.; Yang, D.; et al. Comparative Proteomics Combined with Morphophysiological Analysis Revealed Chilling Response Patterns in Two Contrasting Maize Genotypes. Cells 2022, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Pares, R.B.; Bernstein, T.; McDowell, S.C.; Brown, E.; Stubrich, J.; Rosenberg, A.; Cahoon, E.B.; Cahoon, R.E.; Poulsen, L.R.; et al. The lipid flippases ALA4 and ALA5 play critical roles in cell expansion and plant growth. Plant Physiol. 2020, 182, 2111–2125. [Google Scholar] [CrossRef]
- Raza, A.; Salehi, H.; Rahman, A.; Zahid, Z.; Haghjou, M.M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defensesunder induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef]
- Singh, S.; Praveen, A.; Dudha, N.; Bhadrecha, P. Integrating physiological and multi-omics methods to elucidate heat stress tolerance for sustainable rice production. Physiol. Olecularbiol. Plants 2024, 30, 1185–1208. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Sun, X.; Dai, X.; Zhang, P.; Ma, Y.; Xu, Y.; Wang, L.; Zhang, J. Integrated Multi-Omics Analysis to Investigate the Molecular Mechanisms Underlying the Response of Auricularia heimuer to High-Temperature Stress. J. Fungi 2025, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]




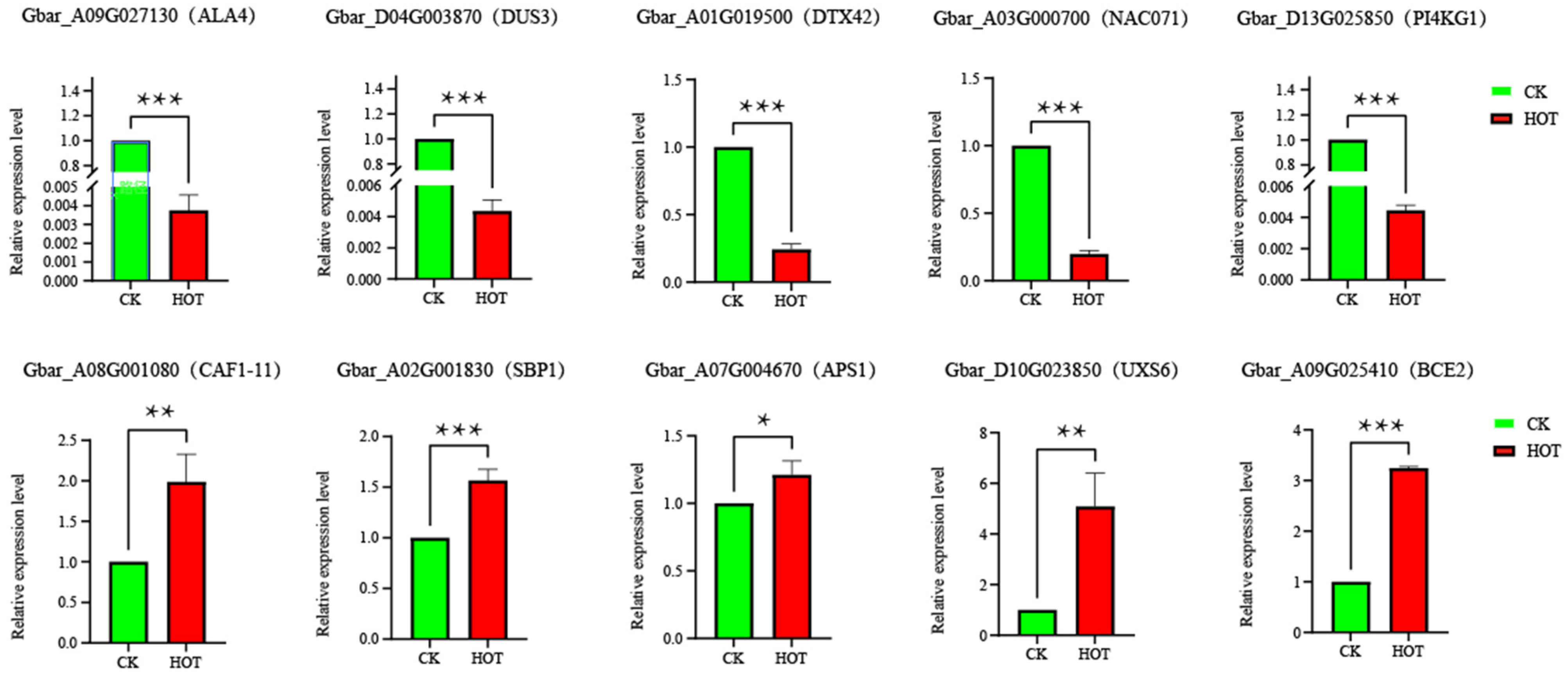
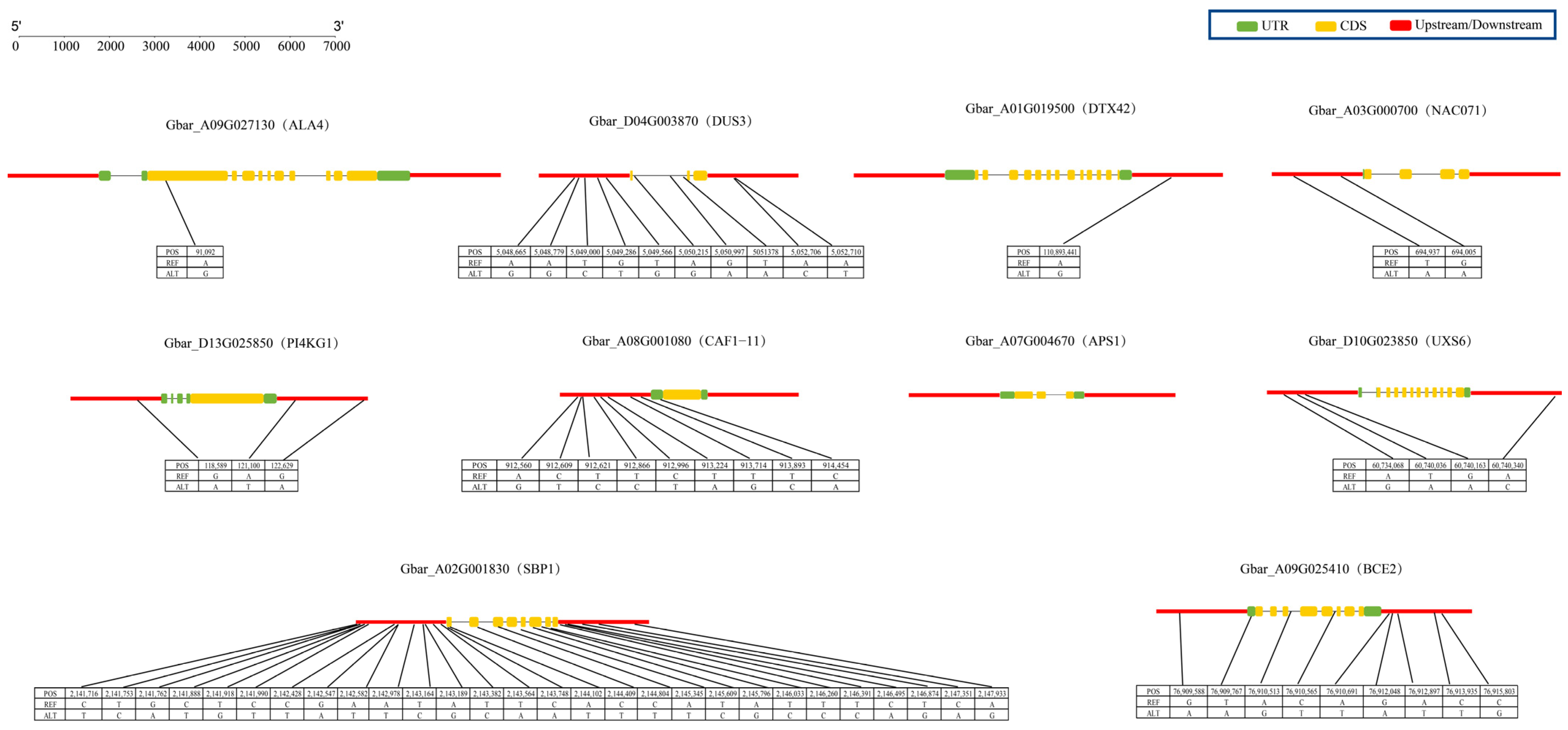
| Sample | Raw Reads | Reads Mapped | Clean Reads | Clean Base (G) | Error Rate (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|
| CK-1 | 47,820,474 | 40,368,138 (94.21%) | 42,847,734 | 6.43 | 0.01 | 95.2 | 44.46 |
| CK-2 | 74,758,782 | 65,763,208 (93.82%) | 70,097,786 | 10.51 | 0.02 | 94.28 | 44.44 |
| CK-3 | 88,691,976 | 77,567,651 (94.88%) | 81,755,950 | 12.26 | 0.01 | 96.38 | 44.29 |
| HOT-1 | 99,523,708 | 87,674,236 (95.02%) | 92,264,390 | 13.84 | 0.01 | 97.19 | 43.76 |
| HOT-2 | 87,482,908 | 77,555,852 (95.87%) | 80,895,212 | 12.13 | 0.01 | 97.12 | 43.86 |
| HOT-3 | 49,351,338 | 41,977,255 (95.83%) | 43,803,982 | 6.57 | 0.01 | 96.2 | 44.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Deng, X.; Gao, M.; Lv, T.; Cai, Y.; Qu, Y.; Chen, Q.; Zheng, K. Multiomics Studies on the Effects of High-Temperature Stress on Male Sterility in Gossypium barbadense. Int. J. Mol. Sci. 2025, 26, 3693. https://doi.org/10.3390/ijms26083693
Li J, Deng X, Gao M, Lv T, Cai Y, Qu Y, Chen Q, Zheng K. Multiomics Studies on the Effects of High-Temperature Stress on Male Sterility in Gossypium barbadense. International Journal of Molecular Sciences. 2025; 26(8):3693. https://doi.org/10.3390/ijms26083693
Chicago/Turabian StyleLi, Jiangbo, Xiaojuan Deng, Man Gao, Tao Lv, Yongsheng Cai, Yanying Qu, Quanjia Chen, and Kai Zheng. 2025. "Multiomics Studies on the Effects of High-Temperature Stress on Male Sterility in Gossypium barbadense" International Journal of Molecular Sciences 26, no. 8: 3693. https://doi.org/10.3390/ijms26083693
APA StyleLi, J., Deng, X., Gao, M., Lv, T., Cai, Y., Qu, Y., Chen, Q., & Zheng, K. (2025). Multiomics Studies on the Effects of High-Temperature Stress on Male Sterility in Gossypium barbadense. International Journal of Molecular Sciences, 26(8), 3693. https://doi.org/10.3390/ijms26083693






