Elevated Serum Protein Induced by Vitamin K Absence or Antagonist II Levels in Patients with Hepatic Hemangiomas
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patients and Hemangiomas
2.2. Comparison of Patients and Control Subjects
2.3. Associations Among Hemangioma Size and Clinical Parameters Including Coagulation Factors
2.4. Comparison of Clinical Parameters at the First Examination with Those at the Last Examination in the Follow-Up Period by Changes in Hemangioma Size
2.5. Comparison of Clinical Parameters in Patients with and Without Chronic Liver Disease as an Underlying Disease
2.6. Correlations Between PIVKA-II and Clinical Parameters
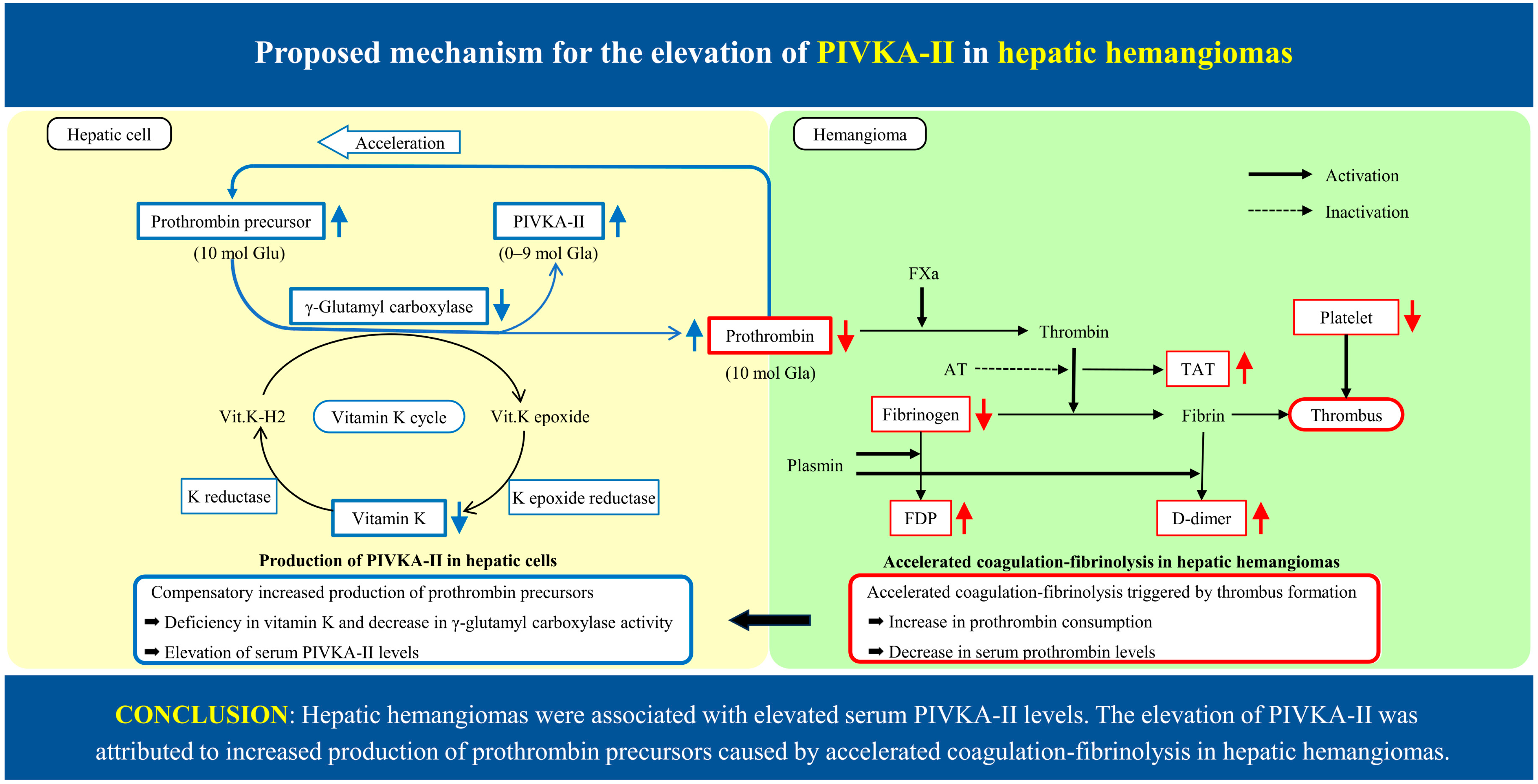
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PIVKA-II | Protein induced by vitamin K absence or antagonist-II |
| AFP | α-Fetoprotein |
| PT | Prothrombin time |
| TAT | Thrombin–antithrombin III complex |
| FDP | Fibrin and fibrinogen degradation products |
| US | Ultrasonography |
| CT | Computed tomography |
| M2BPGi | Mac-2 binding protein glycosylation isomer |
References
- Inagaki, Y.; Tang, W.; Makuuchi, M.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Clinical and molecular insights into the hepatocellular carcinoma tumor marker des-γ-carboxy-prothrombin. Liver Int. 2010, 31, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Kim, J.H.; Kang, S.H.; Lee, B.J.; Seo, Y.S.; Yim, H.J.; Yeon, J.J.P.; Park, J.J.; Kim, J.S.; Bak, Y.T.; et al. The influence of alcoholic liver disease on serum PIVKA-II levels in patients without hepatocellular carcinoma. Gut Liver. 2015, 9, 224–230. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Li, S.; Lin, M.; Xie, X.; Shi, H.; Jiang, Y.; Zheng, S.; Shao, H.; Yang, N.; et al. Changes and clinical significance of PIVKA-II in hepatitis E patients. Front. Public Health 2022, 9, 784718. [Google Scholar] [CrossRef]
- Ji, J.; Liu, L.; Jiang, F.; Wen, X.; Zhang, Y.; Li, S.; Lou, J.; Wang, Y.; Liu, N.; Guo, Q.; et al. The clinical application of PIVKA-II in hepatocellular carcinoma and chronic liver diseases: A multi-center study in China. J. Clin. Lab. Anal. 2021, 35, e24013. [Google Scholar] [CrossRef]
- Jang, T.Y.; Dai, C.Y. Cutoff values of protein induced by vitamin K absence or antagonist II for diagnosing hepatocellular carcinoma. Medicine 2022, 101, e30936. [Google Scholar] [CrossRef] [PubMed]
- Matsueda, K.; Yamamoto, H.; Yoshida, Y.; Notohara, M. Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist II (PIVKA-II) and α-fetoprotein (AFP). J. Gastroenterol. 2006, 41, 1011–1019. [Google Scholar] [CrossRef]
- Farina, A.; Tartaglione, S.; Preziosi, A.; Mancini, P.; Angeloni, A.; Anastasi, E. PANC-1 cell line as an experimental model for characterizing PIVKA-II production, distribution, and molecular mechanisms leading to protein release in PDAC. Int. J. Mol. Sci. 2024, 25, 3498. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Koda, M.; Matono, T.; Isomoto, H. Association of tumor size and internal echo pattern with coagulopathy associated with hepatic hemangioma. Mol. Clin. Oncol. 2021, 83, 14. [Google Scholar] [CrossRef]
- Maruyama, S.; Matono, T.; Koda, M. Prevalence and characteristics of hepatic hemangioma associated with coagulopathy and its predictive risk factors. J. Clin. Med. 2022, 11, 4347. [Google Scholar] [CrossRef]
- Maruyama, S.; Matono, T.; Koda, M. The natural history and management of hepatic hemangioma. J. Clin. Med. 2023, 12, 5703. [Google Scholar] [CrossRef]
- Liebman, H.A.; Furie, B.C.; Tong, M.J.; Blanchard, R.A.; Lo, K.J.; Lee, S.D.; Coleman, M.S.; Furie, B. Des-γ-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 1984, 310, 1427–1431. [Google Scholar] [CrossRef]
- Ono, M.; Ohta, H.; Ohhira, M.; Sekiya, C.; Namiki, M. Measurement of immunoreactive prothrombin, des-γ-carboxy prothrombin, and vitamin K in human liver tissue: Overproduction of immunoreactive prothrombin in hepatocellular carcinoma. Am. J. Gastroenterol. 1990, 85, 1149–1154. [Google Scholar]
- Nadano, S.; Tanimoto, K.; Onji, M.; Michitaka, K.; Horike, N.; Ohta, Y. Immunohistochemical study of PIVKA-II in hepatocellular carcinoma. Acta Hepatol. Jpn. 1990, 31, 475–476. [Google Scholar] [CrossRef][Green Version]
- Yamagata, H.; Nakanishi, T.; Furukawa, M.; Okuda, H.; Hobata, H. Levels of vitamin K, immunoreactive prothrombin, des-γ-carboxy prothrombin and γ-glutamyl carboxylase activity in hepatocellular carcinoma tissue. J. Gastroenterol. Hepatol. 1995, 10, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Kajiwara, Y.; Shirahata, A.; Okamoto, K.; Itoh, H.; Ohsata, K. Vitamin K contents in liver tissue of hepatocellular carcinoma patients. Jpn. J. Cancer Res. 2000, 91, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Miki, K.; Kokudo, N.; Sugawara, Y.; Imamura, H.; Minagawa, M. Des-γ-carboxy prothrombin in cancer and non-cancer liver tissue of patients with hepatocellular carcinoma. Int. J. Oncol. 2003, 22, 969–975. [Google Scholar] [CrossRef]
- Shah, D.V.; Engelke, J.A.; Suttie, J.W. Abnormal prothrombin in the plasma of rats carrying hepatic tumors. Blood 1987, 69, 850–854. [Google Scholar] [CrossRef]
- Shah, D.V.; Zhang, P.; Engelke, J.A.; Bach, A.U.; Suttie, J.W. Vitamin K-dependent carboxylase activity, prothrombin mRNA, and prothrombin production in two cultured rat hepatoma cell lines. Thromb. Res. 1993, 70, 365–373. [Google Scholar] [CrossRef]
- Okuda, H.; Obata, H.; Nakanishi, T.; Furukawa, R.; Hashimoto, E. Production of abnormal prothrombin (des-γ-carboxy prothrombin) by hepatocellular carcinoma: A clinical and experimental study. J. Hepatol. 1987, 4, 357–363. [Google Scholar] [CrossRef]
- Sakon, M.; Monden, M.; Gotoh, M.; Kobayashi, K.; Kanai, T.; Umeshita, K. The effects of vitamin K on the generation of des-γ-carboxy prothrombin (PIVKA-II) in patients with hepatocellular carcinoma. Am. J. Gastroenterol. 1991, 86, 339–345. [Google Scholar]
- Huisse, M.G.; Leclercq, M.; Belghiti, J.; Flejou, J.F.; Suttie, J.W.; Bezeaud, A.; Stafford, D.W.; Guillin, M.C. Mechanism of the abnormal vitamin K-dependent γ-carboxylation process in human hepatocellular carcinomas. Cancer 1994, 74, 1533–1541. [Google Scholar] [CrossRef]
- Okuda, H.; Nakanishi, T.; Furukawa, R.; Furukawa, M.; Obata, H. Production of abnormal prothrombin (PIVKA-II) by human hepatoma cells in culture. Acta Hepatol. Jpn. 1988, 29, 47–51. [Google Scholar] [CrossRef]
- Okuda, H.; Nakanishi, T.; Furukawa, M.; Obata, H. Vitamin K-dependent proteins and hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1991, 6, 392–399. [Google Scholar] [CrossRef]
- Blanchard, R.A.; Furie, B.C.; Jorgensen, M.; Kruger, S.F.; Furie, B. Acquired vitamin K-dependent carboxylation deficiency in liver disease. N. Engl. J. Med. 1981, 305, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, B.; Li, Z.; Wei, Q.; Ren, L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Z.; Yin, X.; Qi, X.; Lu, B.; Liu, Y.; Hou, J. Prognostic value of PIVKA-II in hepatocellular carcinoma patients receiving curative ablation: A systematic review and meta-analysis. Int. J. Biol. Markers. 2018, 33, 266–274. [Google Scholar] [CrossRef]
- Wang, G.Y.; Jiang, N.; Yi, H.M.; Wang, G.S.; Zhang, J.W.; Li, H.; Zhang, J.; Zhang, Q.; Yang, Y.; Chen, G.H. Pretransplant elevated plasma fibrinogen level is a novel prognostic predictor for hepatocellular carcinoma recurrence and patient survival following liver transplantation. Ann. Transplant. 2016, 21, 125–130. [Google Scholar] [CrossRef]
- Suzuki, H.; Nimura, Y.; Kamiya, J.; Kondo, S.; Nagino, M.; Kanai, M.; Miyachi, M. Preoperative transcatheter arterial embolization for giant cavernous hemangioma of the liver with consumption coagulopathy. Am. J. Gastroenterol. 1997, 92, 688–691. [Google Scholar]
- Carlo, I.D.; Koshy, R.; Mudares, S.A.; Ardiri, A.; Bertino, G.; Toro, A. Giant cavernous liver hemangiomas: Is it the time to change the size categories? Hepatobiliary Pancreat. Dis. Int. 2016, 15, 21–29. [Google Scholar] [CrossRef]
- Gandolfi, L.; Leo, P.; Solmi, L.; Vitelli, E.; Verros, G.; Colecchia, A. Natural history of hepatic hemangiomas: Clinical and ultrasound study. Gut 1991, 32, 677–680. [Google Scholar] [CrossRef]
- Tarao, K.; Nozaki, A.; Komatsu, H.; Komatsu, T.; Taguri, M.; Tanaka, K.; Chuma, M.; Numata, K.; Maeda, S. Real impact of tumor marker AFP and PIVKA-II in detecting very small hepatocellular carcinoma (≤2 cm, Barcelona stage 0)-assessment with large number of cases. World J. Hepatol. 2020, 12, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Abate, M.L.; Troshina, G.; Garucci, P.; Rolle, E.; Risso, A.; Burlone, M.E.; Albe, A.; Crevola, M.; Musso, E.C.; et al. Identification of the best cut-off value of PIVKA-II for the surveillance of patients at risk of hepatocellular carcinoma development. Biology 2023, 12, 94. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Value |
|---|---|
| Age (years) | 54 ± 15 |
| Male/female (n) | 122/213 |
| Biochemistry | |
| Total bilirubin (mg/dL) | 0.6 ± 0.2 |
| Albumin (g/dL) | 4.2 ± 0.2 |
| ALT (U/L) | 20 ± 14 |
| GGT (U/L) | 38 ± 40 |
| ALP (U/L) | 234 ± 74 |
| BUN (mg/dL) | 14.2 ± 3.7 |
| Cr (mg/dL) | 0.70 ± 0.17 |
| Serology | |
| PIVKA-II (mAU/mL) | 21.6 ± 8.7 |
| AFP (ng/mL) | 3.6 ± 1.4 |
| M2BPGi (COI) | 0.57 ± 0.32 |
| Hematology | |
| Hemoglobin (g/dL) | 13.6 ± 1.3 |
| Platelet (104/μL) | 22.1 ± 5.3 |
| Coagulation | |
| PT (%) | 95.2 ± 12.5 |
| Fibrinogen (mg/dL) | 285 ± 73 |
| TAT (ng/mL) | 1.42 ± 1.04 |
| D-dimer (μg/mL) | 0.68 ± 0.70 |
| FDP (μg/mL) | 1.69 ± 1.05 |
| Echo findings | |
| Size of hemangioma (mm) | 20.2 ± 16.3 |
| Small < 20 (n) | 215 (64.2%) |
| Medium 20–40 (n) | 87 (26.0%) |
| Large > 40 (n) | 33 (9.8%) |
| Location of hemangioma (n) | |
| Right lobe | 285 (85.1%) |
| Left lobe | 48 (14.3%) |
| Bilateral lobe | 2 (0.6%) |
| Number of hemangioma (n) | |
| Single | 290 (86.6%) |
| Multiple | 45 (13.4%) |
| Associated liver diseases (n) | |
| Hepatitis B | 12 (3.6%) |
| Hepatitis C | 7 (2.1%) |
| Autoimmune hepatitis | 7 (2.1%) |
| Primary biliary cholangitis | 3 (0.9%) |
| Alcoholic liver disease | 16 (4.8%) |
| Nonalcoholic steatohepatitis | 9 (2.7%) |
| Parameters | Control (n = 50) | Patient (n = 335) | p Value |
|---|---|---|---|
| Age (years) | 56 ± 13 | 54 ± 15 | 0.1941 |
| Male/female (n) | 18/32 | 122/213 | 0.6225 |
| Biochemistry | |||
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.9903 |
| Albumin (g/dL) | 4.3 ± 0.2 | 4.2 ± 0.2 | 0.0045 |
| ALT (U/L) | 19 ± 8 | 20 ± 14 | 0.3531 |
| GGT (U/L) | 30 ± 15 | 38 ± 40 | 0.0053 |
| ALP (U/L) | 216 ± 47 | 234 ± 74 | 0.0286 |
| BUN (mg/dL) | 13.3 ± 4.0 | 14.2 ± 3.7 | 0.1250 |
| Cr (mg/dL) | 0.69 ± 0.15 | 0.70 ± 0.17 | 0.5640 |
| Serology | |||
| PIVKA-II (mAU/mL) | 17.4 ± 2.8 | 21.6 ± 8.7 | <0.0001 |
| AFP (ng/mL) | 3.6 ± 1.5 | 3.6 ± 1.4 | 0.8921 |
| M2BPGi (COI) | 0.45 ± 0.18 | 0.57 ± 0.32 | 0.0001 |
| Hematology | |||
| Hemoglobin (g/dL) | 13.7 ± 1.2 | 13.6 ± 1.3 | 0.6254 |
| Platelet (104/μL) | 22.1 ± 4.7 | 22.1 ± 5.3 | 0.9903 |
| Coagulation | |||
| PT (%) | 96.0 ± 11.1 | 95.2 ± 12.5 | 0.6087 |
| Fibrinogen (mg/dL) | 298 ± 87 | 285 ± 73 | 0.3067 |
| TAT (ng/mL) | 1.05 ± 0.48 | 1.42 ± 1.04 | <0.0001 |
| D-dimer (μg/mL) | 0.46 ± 0.26 | 0.68 ± 0.66 | <0.0001 |
| FDP (μg/mL) | 1.36 ± 0.25 | 1.69 ± 0.99 | <0.0001 |
| Echo findings | |||
| Portal vein diameter (mm) | 9.4 ± 1.6 | 10.7 ± 2.2 | <0.0001 |
| Spleen index (mm2) | 1045 ± 370 | 1302 ± 537 | <0.0001 |
| Parameters | Control (n = 50) | Small (n = 215) | Medium (n = 87) | Large (n = 33) | p Value |
|---|---|---|---|---|---|
| Age (years) | 56 ± 13 | 52 ± 15 | 55 ± 14 | 59 ± 13 | 0.0164 |
| Male/female (n) | 18/32 | 72/143 | 35/52 | 15/18 | 0.6782 |
| Serology | |||||
| PIVKA-II (mAU/mL) | 17.4 ± 2.8 | 19.1 ± 5.1 | 22.3 ± 7.7 **,## | 34.0 ± 9.5 **,##,$$ | <0.0001 |
| AFP (ng/mL) | 3.6 ± 1.5 | 3.4 ± 1.3 | 3.8 ± 1.5 | 3.4 ± 1.7 | 0.0564 |
| M2BPGi (COI) | 0.45 ± 0.18 | 0.48 ± 0.25 | 0.62 ± 0.34 **,## | 0.92 ± 0.35 **,##,$$ | <0.0001 |
| Hematology | |||||
| Hemoglobin (g/dL) | 13.7 ± 1.2 | 13.6 ± 1.3 | 13.8 ± 1.3 | 13.1 ± 1.7 | 0.2114 |
| Platelet (104/μL) | 22.1 ± 5.4 | 23.1 ± 4.9 | 21.4 ± 5.4 # | 17.9 ± 4.1 **,##,$$ | <0.0001 |
| Coagulation | |||||
| PT (%) | 96.0 ± 11.1 | 94.5 ± 12.5 | 95.6 ± 13.3 | 95.5 ± 8.7 | 0.7671 |
| Fibrinogen (mg/dL) | 298 ± 87 | 294 ± 74 | 275 ± 66 | 246 ± 67 *,## | 0.0016 |
| TAT (ng/mL) | 1.05 ± 0.48 | 1.12 ± 0.65 | 1.43 ± 0.77 # | 3.39 ± 1.65 **,##,$$ | <0.0001 |
| D-dimer (μg/mL) | 0.46 ± 0.26 | 0.46 ± 0.27 | 0.64 ± 0.36 ## | 2.19 ± 1.16 **,##,$$ | <0.0001 |
| FDP (μg/mL) | 1.36 ± 0.25 | 1.36 ± 0.34 | 1.57 ± 0.58 # | 4.09 ± 1.69 **,##,$$ | <0.0001 |
| Biochemistry | |||||
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.9474 |
| Albumin (g/dL) | 4.3 ± 0.2 | 4.2 ± 0.2 | 4.3 ± 0.2 | 4.0 ± 0.3 **,##,$$ | 0.0002 |
| ALT (U/L) | 19 ± 8 | 21 ± 16 | 20 ± 10 | 16 ± 6 | 0.7291 |
| GGT (U/L) | 30 ± 15 | 43 ± 59 | 43 ± 40 | 30 ± 29 | 0.1987 |
| ALP (U/L) | 216 ± 47 | 230 ± 73 | 234 ± 68 | 236 ± 82 | 0.4517 |
| Echo findings | |||||
| Portal vein diameter (mm) | 9.4 ± 1.6 | 10.1 ± 2.1 | 11.3 ± 1.7 ** | 12.8 ± 2.3 **,##,$$ | <0.0001 |
| Spleen index (mm2) | 1045 ± 370 | 1231 ± 499 | 1326 ± 494 | 1674 ± 714 **,##,$ | <0.0001 |
| Increase (n = 71) | No Change (n = 74) | Decrease (n = 87) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | First | Last | p Value | First | Last | p Value | First | Last | p Value |
| Tumor size (mm) | 18.9 ± 10.4 | 25.0 ± 13.4 | <0.001 | 14.2 ± 7.9 | 14.0 ± 8.2 | 0.220 | 32.1 ± 22.8 | 23.6 ± 20.0 | <0.001 |
| Serology | |||||||||
| PIVKA-II (mAU/mL) | 20.5 ± 6.7 | 25.9 ± 9.0 | <0.001 | 20.4 ± 5.1 | 21.1 ± 5.1 | 0.622 | 25.9 ± 9.1 | 21.3 ± 7.0 | <0.001 |
| AFP (ng/mL) | 3.5 ± 1.5 | 3.7 ± 1.7 | 0.297 | 3.5 ± 1.6 | 3.8 ± 1.7 | 0.154 | 3.6 ± 1.5 | 3.8 ± 1.8 | 0.171 |
| M2BPGi (COI) | 0.58 ± 0.29 | 0.51 ± 0.25 | 0.003 | 0.49 ± 0.21 | 0.55 ± 0.27 | 0.014 | 0.74 ± 0.37 | 1.06 ± 0.48 | <0.001 |
| Hematology | |||||||||
| Hemoglobin (g/dL) | 13.7 ± 1.3 | 13.7 ± 1.5 | 0.726 | 13.8 ± 1.1 | 13.9 ± 1.1 | 0.965 | 13.4 ± 1.3 | 13.2 ± 1.3 | 0.115 |
| Platelet (104/μL) | 23.4 ± 4.7 | 22.1 ± 4.7 | 0.006 | 22.3 ± 5.4 | 21.2 ± 4.7 | 0.021 | 20.4 ± 5.4 | 20.6 ± 5.4 | 0.422 |
| Coagulation | |||||||||
| PT (%) | 93.7 ± 11.2 | 96.5 ± 10.1 | 0.393 | 93.3 ± 12.8 | 91.8 ± 10.1 | 0.168 | 96.1 ± 12.5 | 96.5 ± 10.5 | 0.487 |
| Fibrinogen (mg/dL) | 279 ± 68 | 267 ± 55 | 0.015 | 284 ± 69 | 273 ± 69 | 0.175 | 264 ± 63 | 281 ± 54 | 0.001 |
| TAT (ng/mL) | 1.32 ± 0.86 | 1.78 ± 1.14 | 0.001 | 1.05 ± 0.50 | 1.09 ± 0.67 | 0.669 | 1.89 ± 1.38 | 1.38 ± 1.01 | <0.001 |
| D-dimer (μg/mL) | 0.57 ± 0.35 | 0.72 ± 0.39 | <0.001 | 0.49 ± 0.33 | 0.45 ± 0.31 | 0.411 | 1.07 ± 0.97 | 0.69 ± 0.59 | <0.001 |
| FDP (μg/mL) | 1.49 ± 0.52 | 1.65 ± 0.69 | 0.001 | 1.36 ± 0.38 | 1.43 ± 0.49 | 0.254 | 2.09 ± 1.43 | 1.69 ± 0.95 | <0.001 |
| Biochemistry | |||||||||
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.068 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.624 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.141 |
| Albumin (g/dL) | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.092 | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.141 | 4.1 ± 0.3 | 4.1 ± 0.3 | 0.415 |
| ALT (U/L) | 19 ± 10 | 21 ± 10 | 0.066 | 19 ± 9 | 21 ± 10 | 0.146 | 22 ± 18 | 21 ± 10 | 0.835 |
| GGT (U/L) | 46 ± 41 | 49 ± 45 | 0.347 | 33 ± 27 | 38 ± 32 | 0.011 | 45 ± 69 | 47 ± 58 | 0.494 |
| ALP (U/L) | 215 ± 57 | 227 ± 57 | 0.187 | 233 ± 87 | 241 ± 85 | 0.040 | 244 ± 66 | 265 ± 65 | 0.002 |
| Echo findings | |||||||||
| Portal vein diameter (mm) | 11.2 ± 1.8 | 11.2 ± 2.1 | 0.877 | 10.4 ± 2.1 | 10.4 ± 1.9 | 0.891 | 11.0 ± 2.2 | 11.2 ± 2.3 | 0.298 |
| Spleen index (mm2) | 1449 ± 549 | 1430 ± 506 | 0.715 | 1151 ± 469 | 1138 ± 472 | 0.670 | 1274 ± 530 | 1312 ± 518 | 0.213 |
| Parameters | Liver Disease (+) n = 54 | Liver Disease (−) n = 281 | p Value |
|---|---|---|---|
| Age (years) | 58 ± 12 | 53 ± 15 | 0.0067 |
| Male/female (n) | 28/26 | 94/187 | 0.6220 |
| Serology | |||
| PIVKA-II (mAU/mL) | 22.6 ± 7.8 | 21.4 ± 8.9 | 0.2676 |
| AFP (ng/mL) | 3.5 ± 1.7 | 3.6 ± 1.4 | 0.7923 |
| M2BPGi (COI) | 0.69 ± 0.34 | 0.54 ± 0.31 | 0.0035 |
| Hematology | |||
| Hemoglobin (g/dL) | 14.1 ± 1.5 | 13.5 ± 1.3 | 0.0161 |
| Platelet (104/μL) | 20.3 ± 5.5 | 22.8 ± 5.1 | 0.0035 |
| Coagulation | |||
| PT (%) | 95.9 ± 13.8 | 95.1 ± 12.2 | 0.6980 |
| Fibrinogen (mg/dL) | 265 ± 72 | 288 ± 72 | 0.0147 |
| TAT (ng/mL) | 1.53 ± 1.34 | 1.40 ± 1.06 | 0.4532 |
| D-dimer (μg/mL) | 0.75 ± 0.67 | 0.67 ± 0.70 | 0.3879 |
| FDP (μg/mL) | 1.84 ± 1.13 | 1.66 ± 1.03 | 0.2548 |
| Biochemistry | |||
| Total bilirubin (mg/dL) | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.4179 |
| Albumin (g/dL) | 4.2 ± 0.3 | 4.2 ± 0.2 | 0.3087 |
| ALT (U/L) | 31 ± 26 | 18 ± 8 | 0.0005 |
| GGT (U/L) | 66 ± 67 | 33 ± 29 | 0.0005 |
| ALP (U/L) | 247 ± 86 | 231 ± 71 | 0.2364 |
| BUN (mg/dL) | 14.3 ± 3.4 | 14.2 ± 3.8 | 0.8496 |
| Cr (mg/dL) | 0.75 ± 0.18 | 0.69 ± 0.17 | 0.0492 |
| Echo findings | |||
| Tumor size (mm) | 23.5 ± 15.6 | 19.5 ± 16.3 | 0.0888 |
| Portal vein diameter (mm) | 11.6 ± 2.2 | 10.5 ± 2.2 | 0.0010 |
| Spleen index (mm2) | 1385 ± 498 | 1286 ± 543 | 0.2048 |
| Parameters | r | p Value |
|---|---|---|
| Tumor size (mm) | 0.4629 | <0.0001 |
| Platelet (104/μL) | −0.1883 | 0.0005 |
| PT (%) | 0.0408 | 0.4566 |
| Fibrinogen (mg/dL) | −0.1121 | 0.0403 |
| TAT (ng/mL) | 0.4328 | <0.0001 |
| D-dimer (μg/mL) | 0.5357 | <0.0001 |
| FDP (μg/mL) | 0.5303 | <0.0001 |
| AFP (ng/mL) | 0.0785 | 0.1518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, S.; Matono, T.; Koda, M. Elevated Serum Protein Induced by Vitamin K Absence or Antagonist II Levels in Patients with Hepatic Hemangiomas. Int. J. Mol. Sci. 2025, 26, 3681. https://doi.org/10.3390/ijms26083681
Maruyama S, Matono T, Koda M. Elevated Serum Protein Induced by Vitamin K Absence or Antagonist II Levels in Patients with Hepatic Hemangiomas. International Journal of Molecular Sciences. 2025; 26(8):3681. https://doi.org/10.3390/ijms26083681
Chicago/Turabian StyleMaruyama, Shigeo, Tomomitsu Matono, and Masahiko Koda. 2025. "Elevated Serum Protein Induced by Vitamin K Absence or Antagonist II Levels in Patients with Hepatic Hemangiomas" International Journal of Molecular Sciences 26, no. 8: 3681. https://doi.org/10.3390/ijms26083681
APA StyleMaruyama, S., Matono, T., & Koda, M. (2025). Elevated Serum Protein Induced by Vitamin K Absence or Antagonist II Levels in Patients with Hepatic Hemangiomas. International Journal of Molecular Sciences, 26(8), 3681. https://doi.org/10.3390/ijms26083681





