Multi-Omics Integration Analysis Revealed That miR-375-3p Is a Two-Sided Factor Regulating the Development and TUMORIGENESIS of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
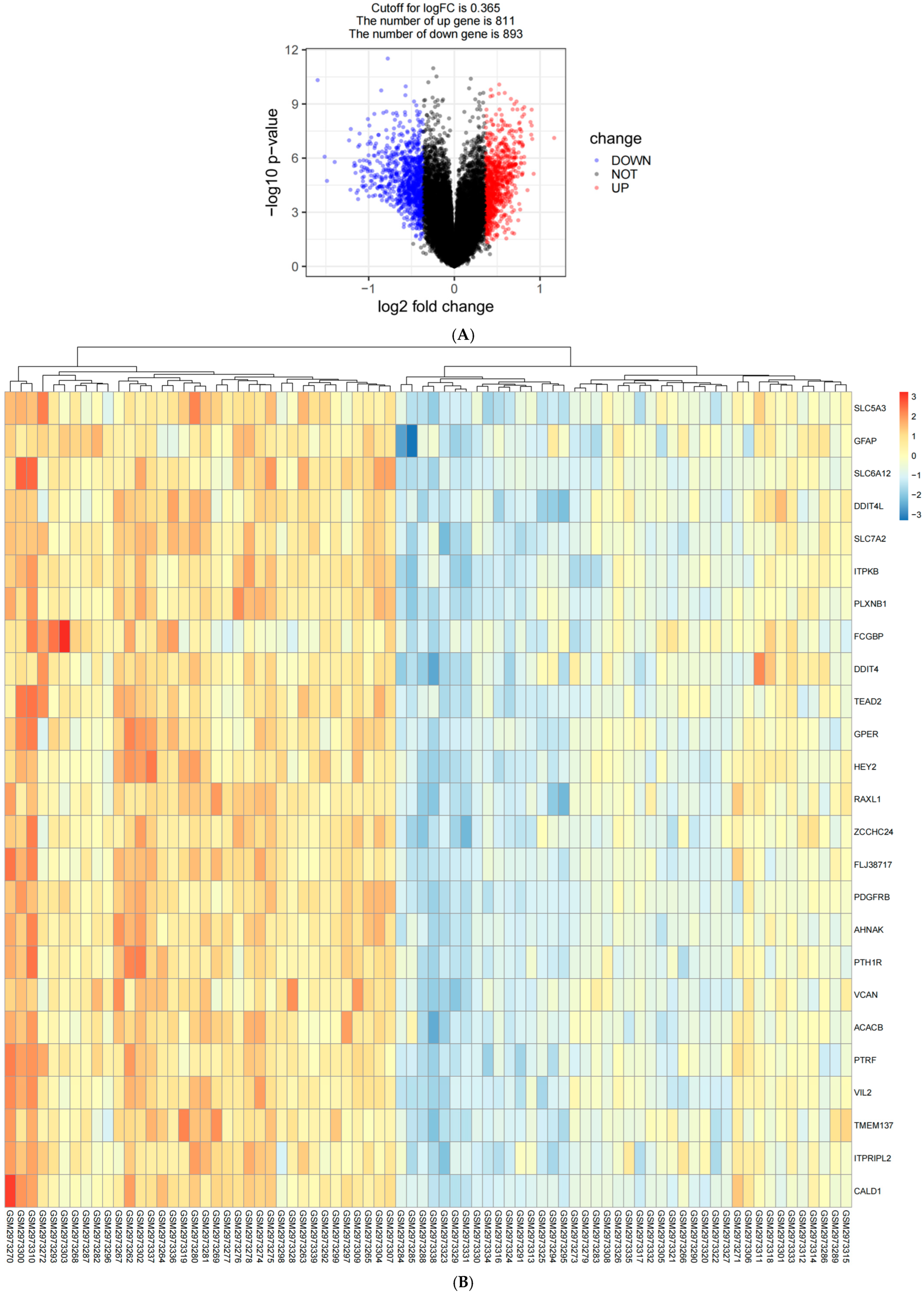
2.1. Identification of Immune Key Genes Involved in the Development of AD
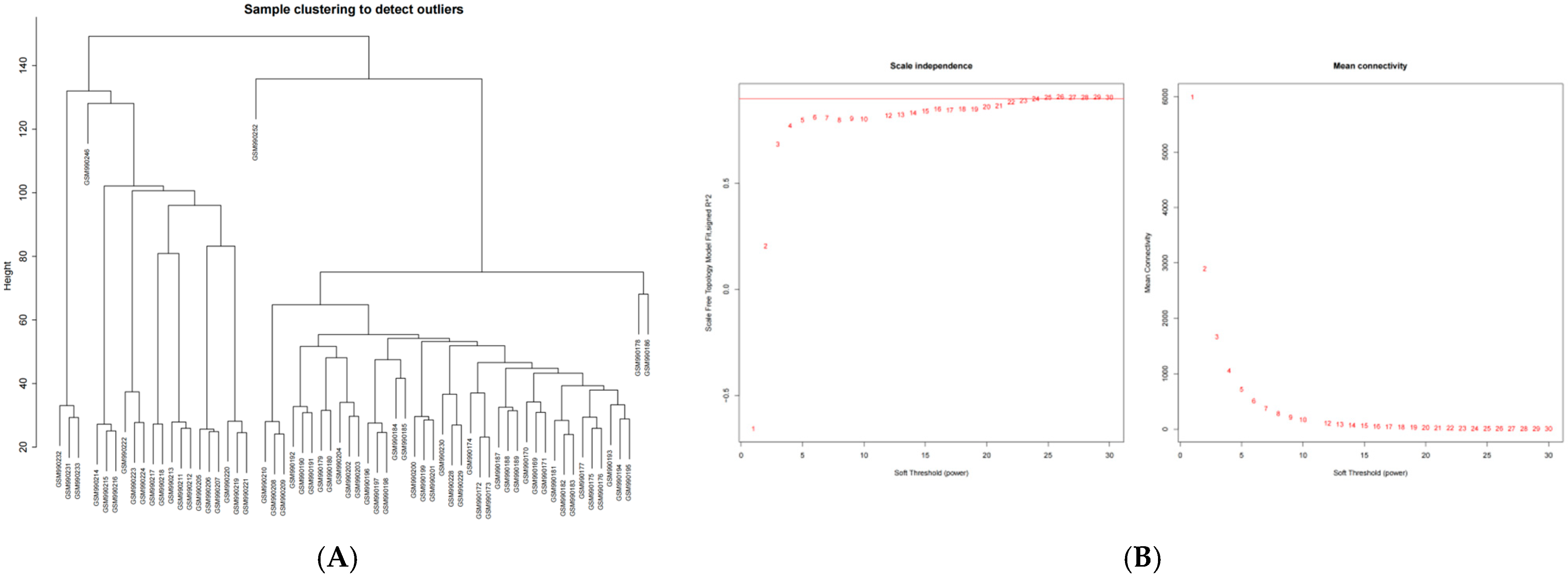
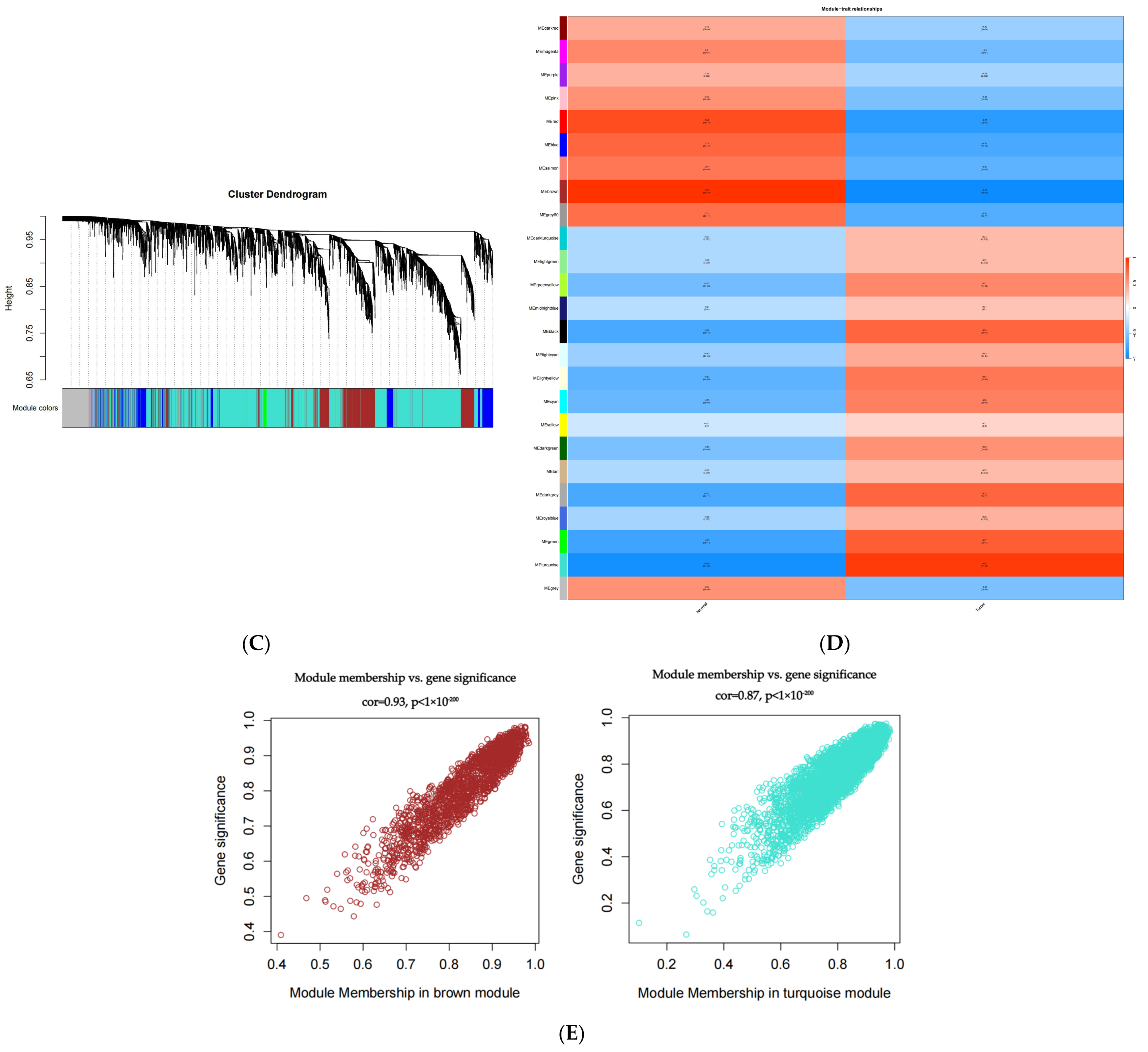
2.2. The Weighted Gene Co-Expression Network Was Constructed Using SCLC Module Genes
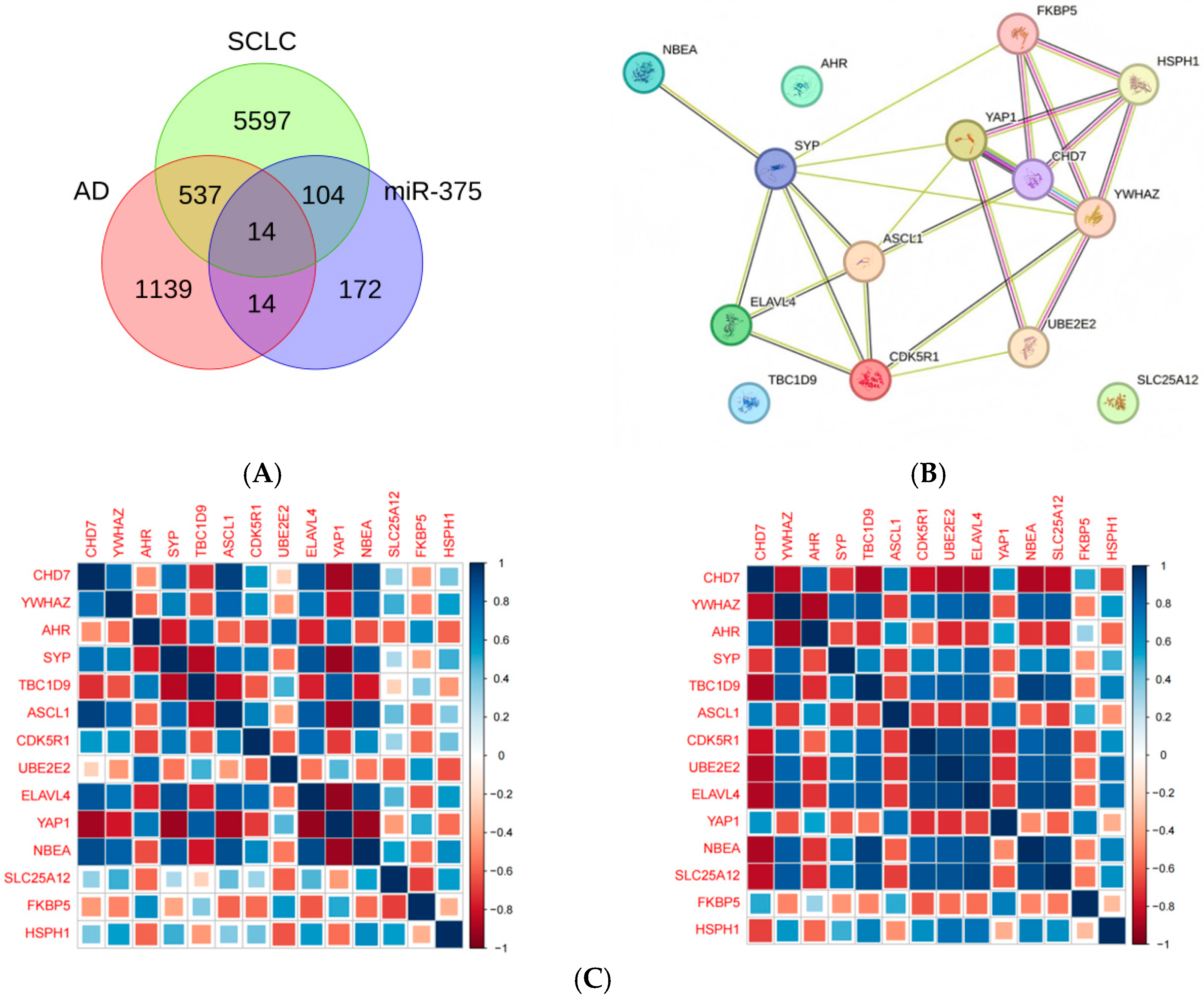
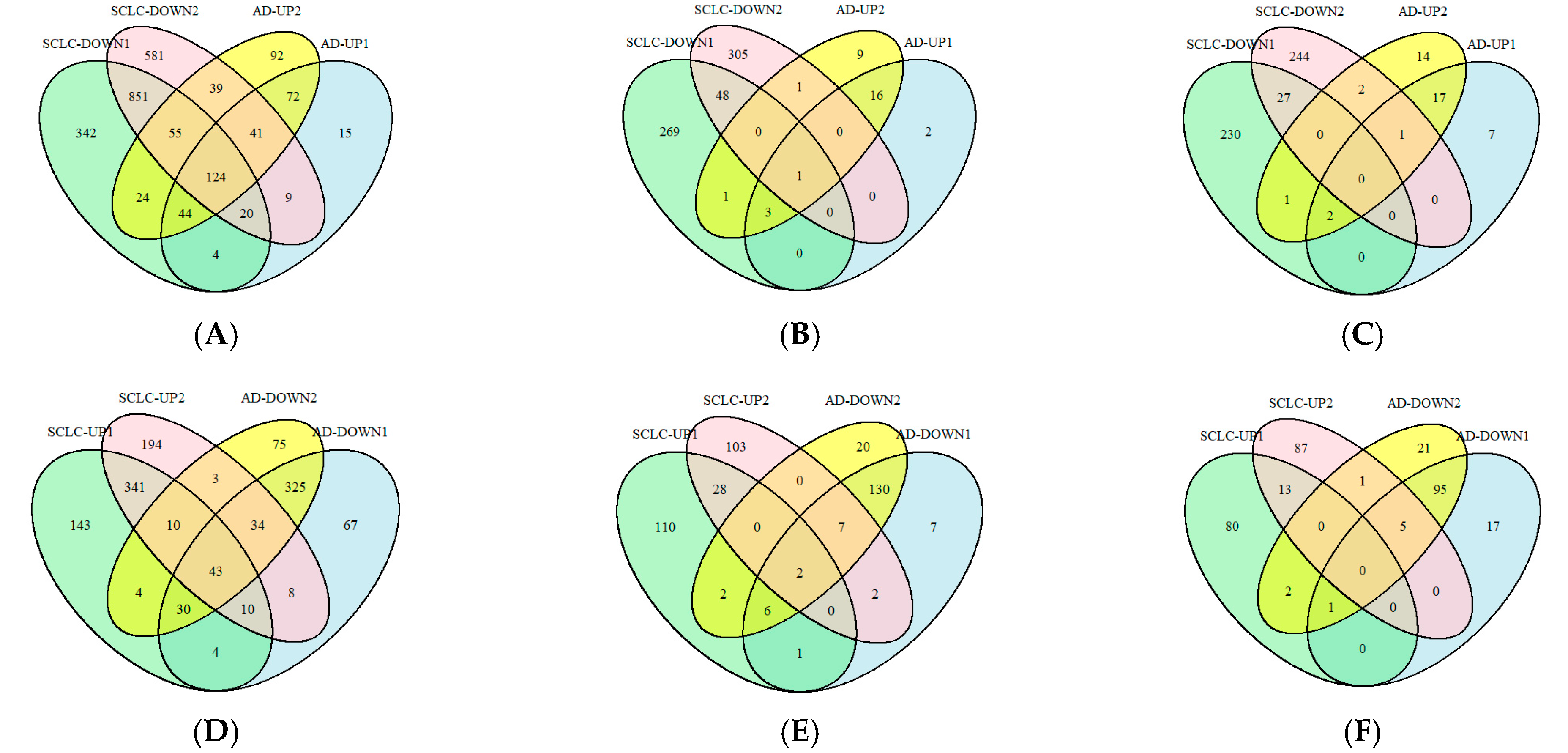
2.3. Screening for miR-375-3p Target Genes Based on AD Differential Genes and SCLC Module Genes
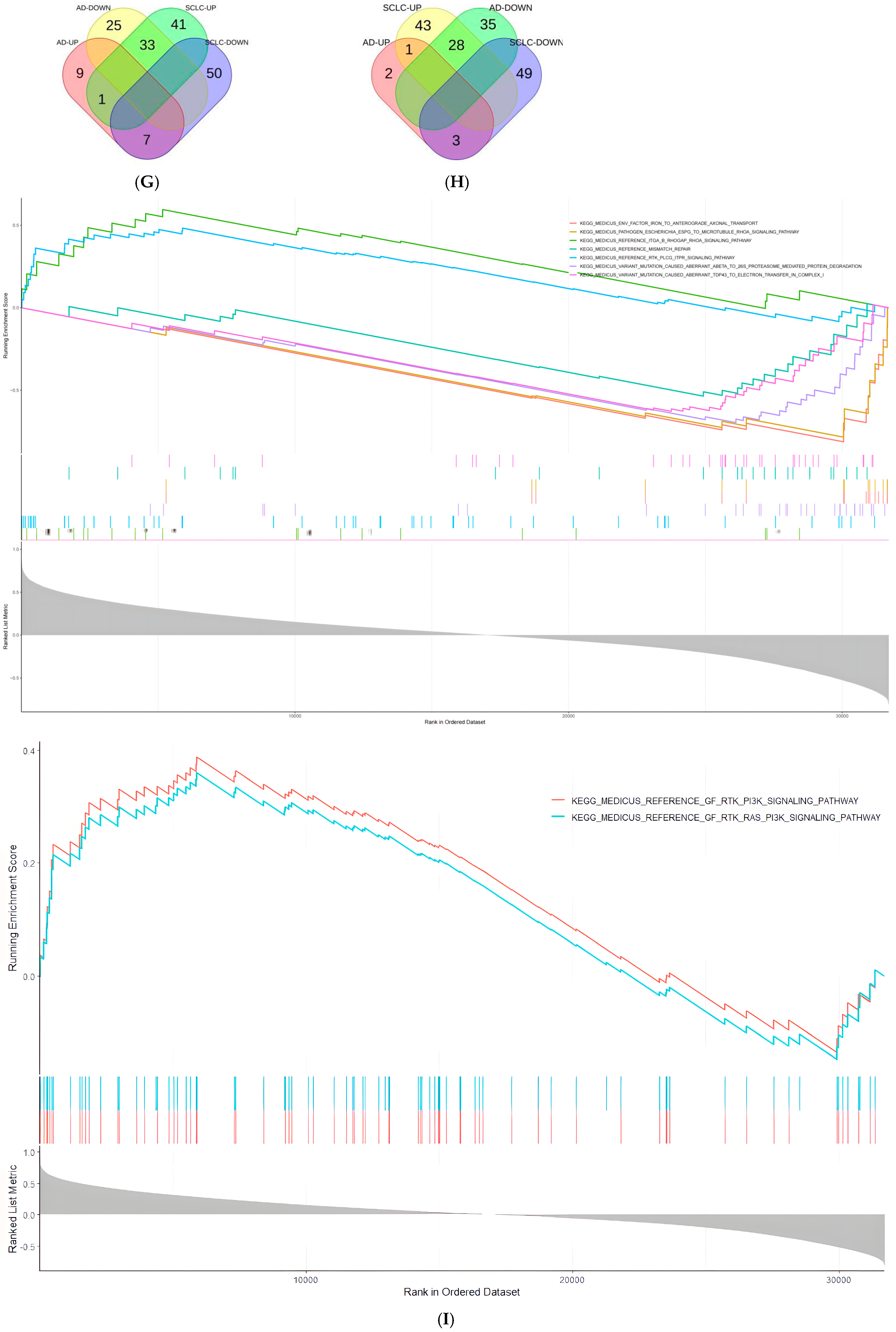
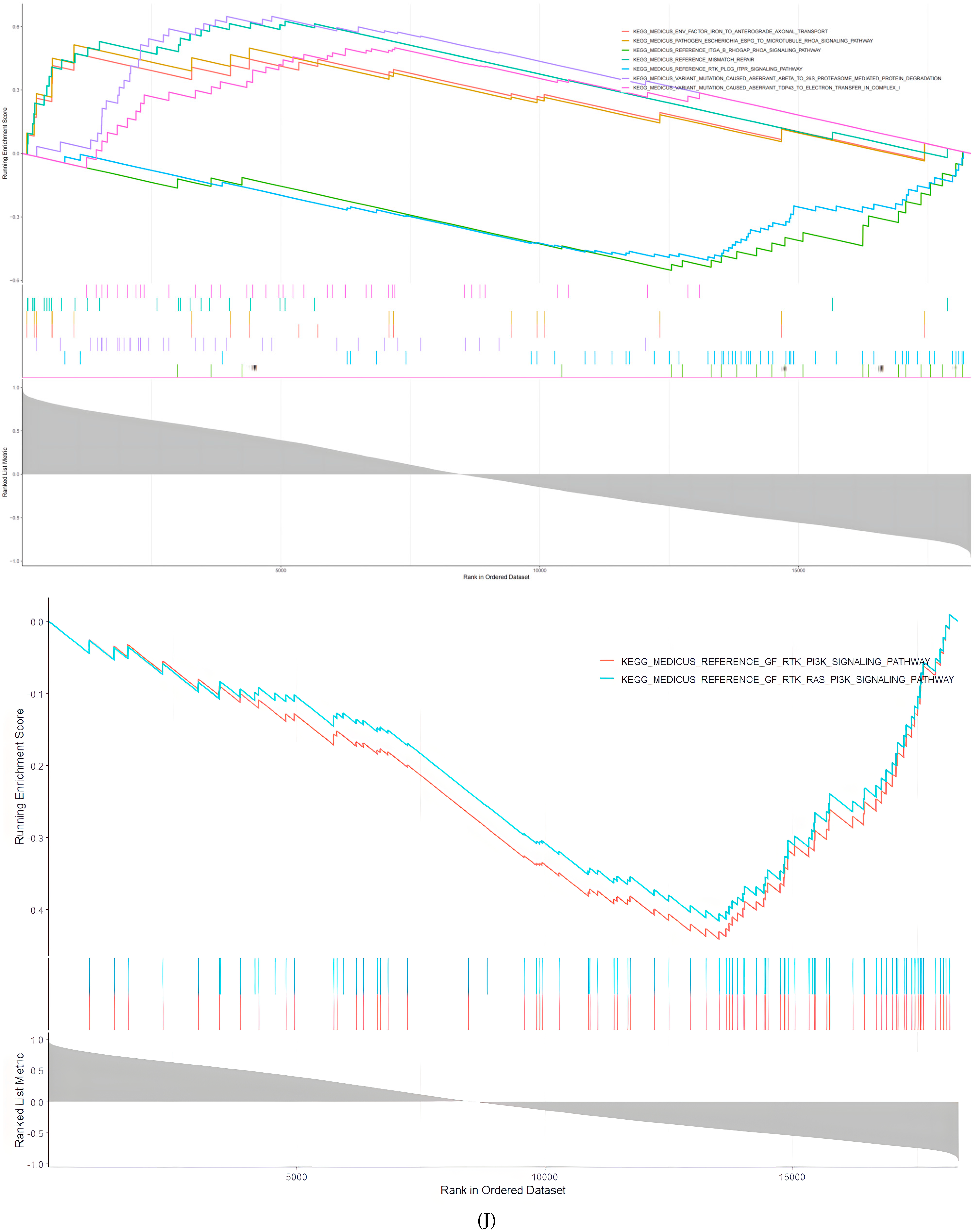
2.4. Select the Pathways in Which the Target Genes of miR-375-3p Are Differentially Regulated in Different Disease Environments
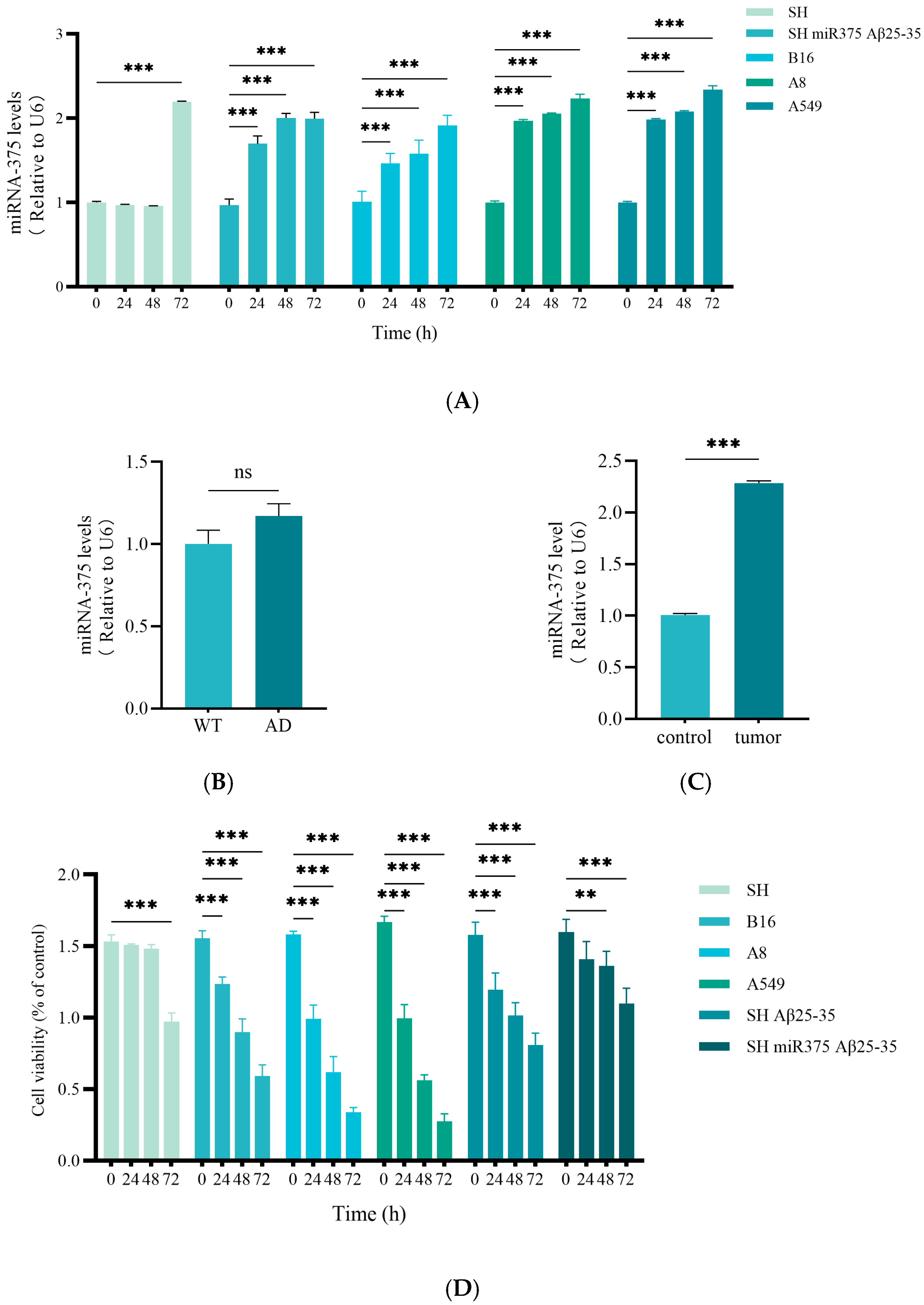
2.5. miR-375-3p in AD and SCLC Associated with Different Loops
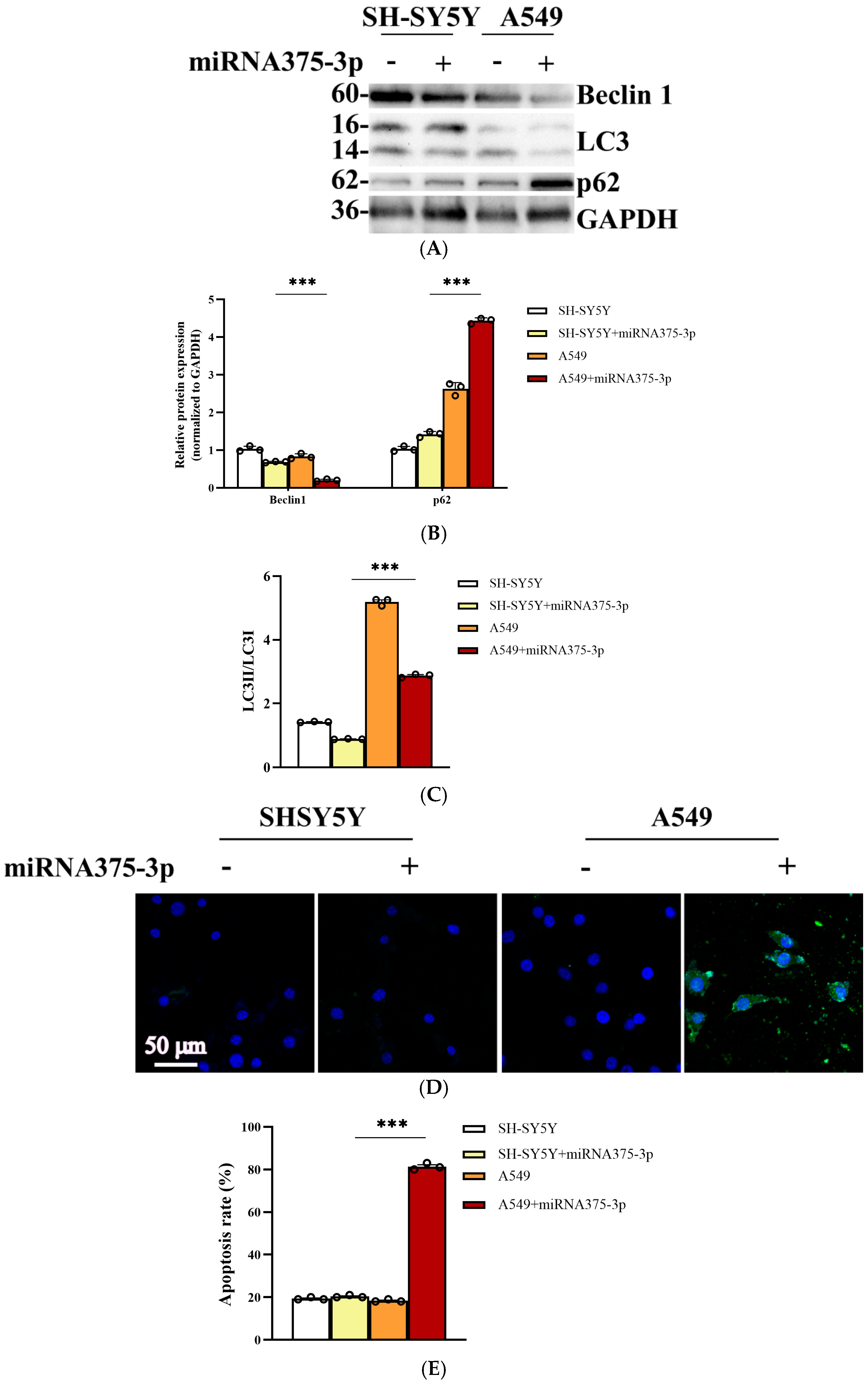
2.6. miR-375-3p Is Involved in the Differential Expression of Cell Autophagy Environment in AD and Tumor Environments
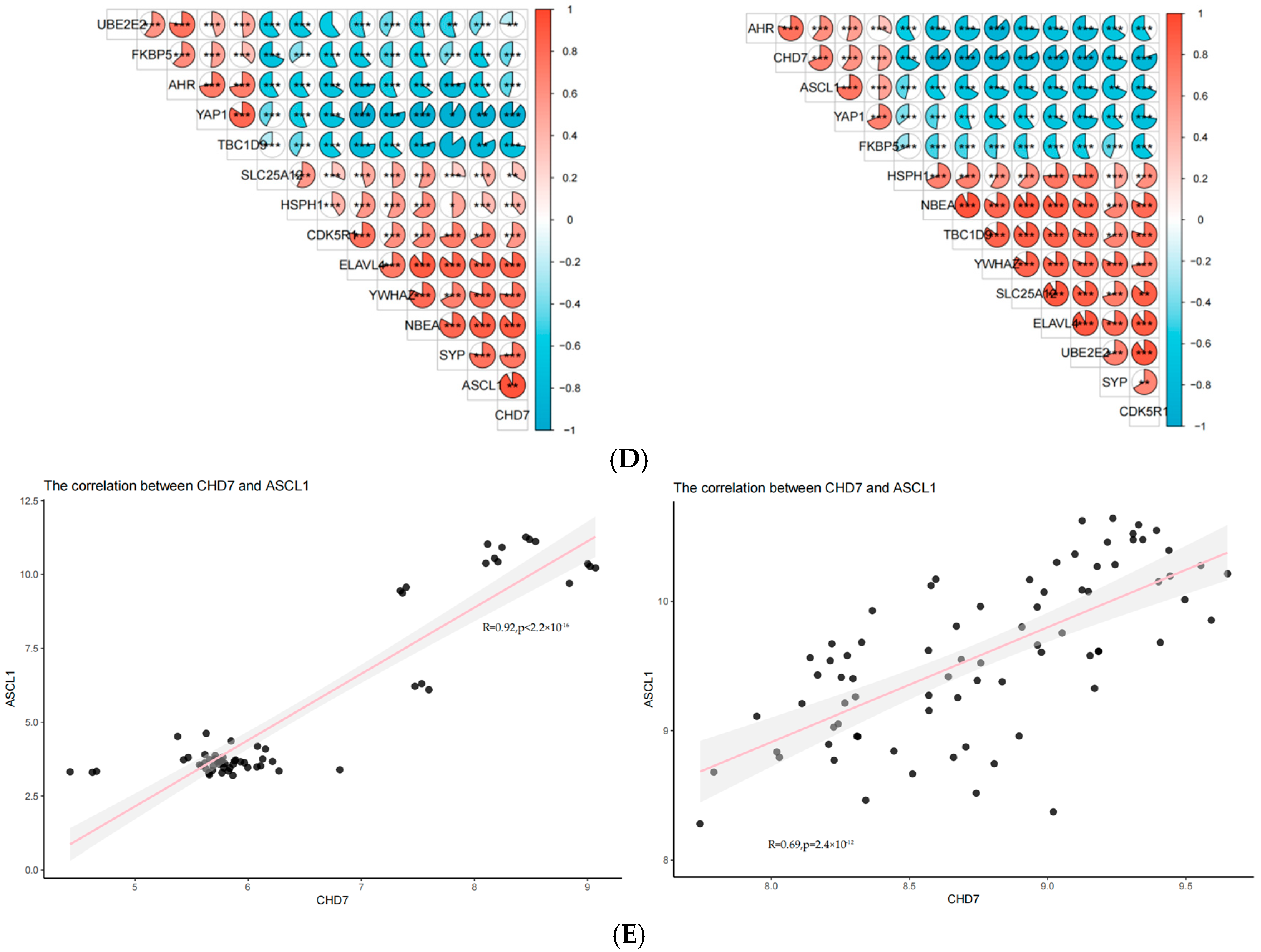
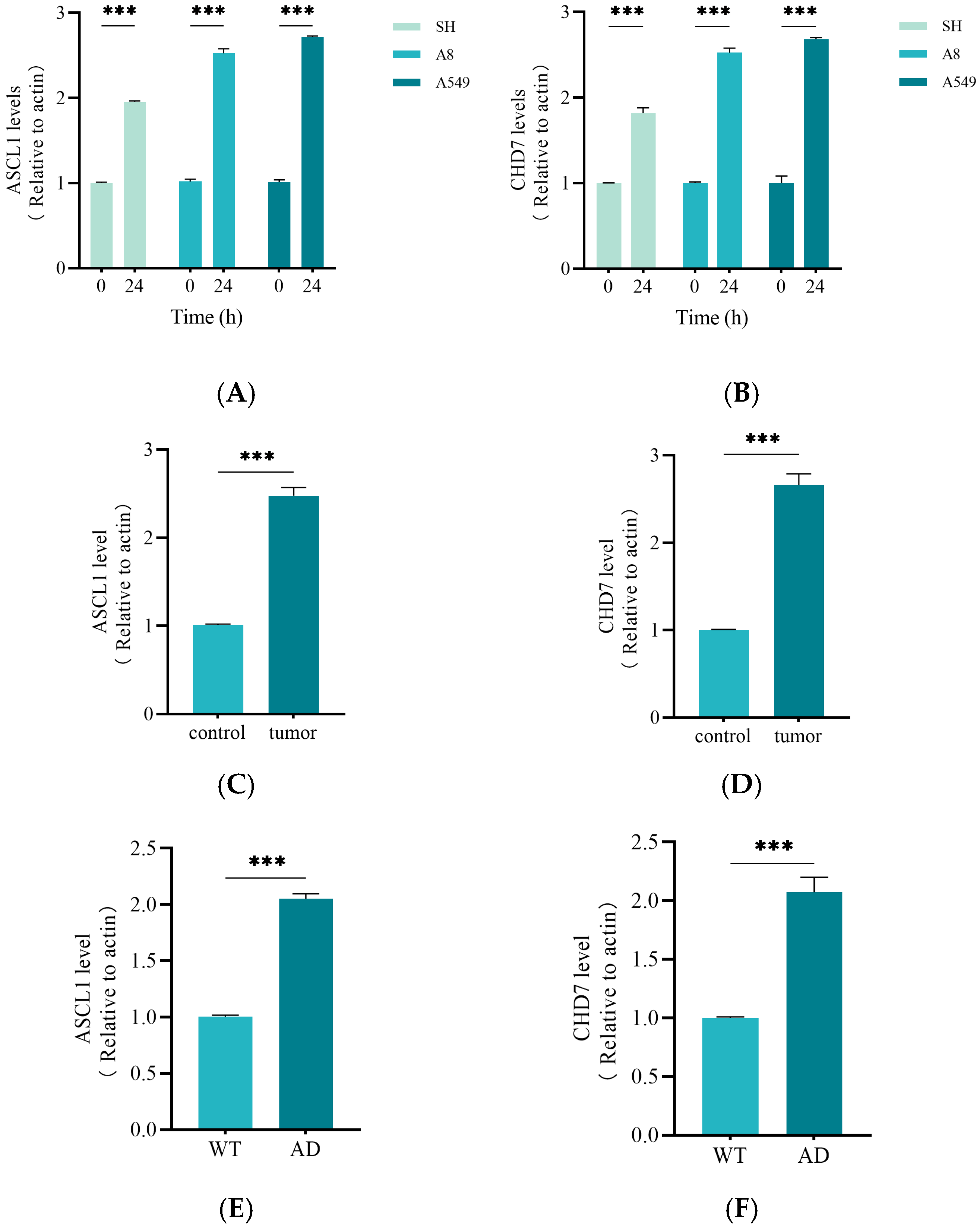
2.7. Activation of ASCL1 and CHD7 May Be the Key to the Function of miRNA375
3. Discussion
3.1. Dual Mechanism of miR-375-3p in AD and SCLC
3.2. Central Roles of ASCL1 and CHD7 in AD and SCLC
3.3. Disease-Specific Regulation of Autophagy and Apoptosis Pathways
3.4. Disease Relevance of Telomerase Activity Pathways
3.5. Research Significance and Future Direction
3.6. Summary
4. Materials and Methods
4.1. Data Collection and Preliminary Processing
4.2. WGCNA and Module Gene Selection of Differentially Expressed Genes Between Normal Group and SCLC Patients
4.3. Identification of Differentially Expressed Genes Between Samples from AD Patients and Normal Samples
4.4. Intersecting Gene Selection and PPI Construction
4.5. Enrichment Analysis and Crossover Analysis
4.6. Animal Model Groups and Drug Administry
4.7. Subcutaneous Tumor Formation Experiment in Nude Mice
4.8. The Measurement of miR-375-3p Expression Level
4.9. Total Protein Extraction and Western Blotting of Brain Tissue Samples
4.10. Tunel Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Wei, Y.; Liang, Z.; Guo, L.; Hao, X.; Zhang, Y. Review on dietary supplements as an effective improvement of Alzheimer’s disease: Focus on structures and mechanisms. Food Sci. Hum. Health (Engl.) 2024, 13, 1787–1805. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Tam, K.Y.D. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, M.; Panza, F. Impact of apolipoprotein E isoforms on sporadic Alzheimer’s disease: Beyond the role of amyloid beta. Chin. J. Regen. Med. (Engl. Ed.) 2024, 19, 80–83. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, H.; Li, R.; Wu, G.; Qin, Q.; Tang, Y. A systematic review of the role of TREM2 in Alzheimer’s disease. Chin. Med. J. 2024, 137, 1684–1694. [Google Scholar] [CrossRef]
- Ginsberg, M.S.; Grewal, R.K.; Heelan, R.T. Lung Cancer. Clin. N. Am. 2007, 45, 21–43. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef]
- Broderick, J.A.; Zamore, P.D. MicroRNA therapeutics. Gene Ther. 2011, 18, 1104–1110. [Google Scholar] [CrossRef]
- GIiri, B.; Seamon, M.; Banerjee, A. Emerging urinary alpha-synuclein and miRNA biomarkers in Parkinson’s disease. Metab. Brain Dis. 2022, 37, 1687–1696. [Google Scholar] [CrossRef]
- Vadla, G.P.; Daghat, B.; Patterson, N.; Ahmad, V.; Perez, G.; Garcia, A.; Manjunath, Y.; Kaifi, J.T.; Li, G.; Chabu, C.Y. Combining plasma extracellular vesicle Let-7b-5p, miR-184 and circulating miR-22-3p levels for NSCLC diagnosis and drug resistance prediction. Sci. Rep. 2022, 12, 6693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Z.; Lin, X.; Zhang, X.; Zhao, Q.; Sun, Y. A gene module identification algorithm and its applications to identify gene modules and key genes of hepatocellular carcinoma. Sci. Rep. 2021, 11, 5517. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M.; Robles, A.I.; Harris, C.C. Genetic variation in microRNA networks: The implications for cancer research. Nat. Rev. Cancer 2010, 10, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Z.; Yin, H.; Ren, Z.; Ma, X.; Wang, Y.; Zhang, Y.; Fu, X.; Zhang, F.; Zeng, L. miRNA375-3p/rapamycin mediates the mTOR pathway by decreasing PS1, enhances microglial cell activity to regulate autophagy in Alzheimer’s disease. Heliyon 2024, 10, e37589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, S.; Sharawat, S.K.; Ali, A.; Gaur, V.; Malik, P.S.; Kumar, S.; Mohan, A.; Guleria, R. Identification of differentially expressed circulating serum microRNA for the diagnosis and prognosis of Indian non-small cell lung cancer patients. Curr. Probl. Cancer. 2020, 44, 100540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ge, X.; Zhang, J.; Chen, L. Effect of lncRNA WT1-AS regulating WT1 on oxidative stress injury and apoptosis of neurons in Alzheimer’s disease via inhibition of the miR-375/SIX4 axis. Aging 2020, 12, 23974–23995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, S.; Saikia, J.; Sharawat, S.K.; Malik, P.S.; Kumar, S.; Mohan, A. Analysis of miR-375-3p, miR-197-3p, and miR-15a-5p Expression and Their Clinical Relevance as Biomarkers in Lung Cancer. Technol. Cancer Res. Treat. 2022, 21, 15330338221080981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soares, D.S.; Homem, C.C.F.; Castro, D.S. Function of Proneural Genes Ascl1 and Asense in Neurogenesis: How Similar Are They? Front. Cell Dev. Biol. 2022, 10, 838431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lettieri, A.; Oleari, R.; Paganoni, A.J.J.; Gervasini, C.; Massa, V.; Fantin, A.; Cariboni, A. Semaphorin Regulation by the Chromatin Remodeler CHD7: An Emerging Genetic Interaction Shaping Neural Cells and Neural Crest in Development and Cancer. Front. Cell Dev. Biol. 2021, 9, 638674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, R.; Ambasta, R.K.; Pravir, K. Autophagy and apoptosis cascade: Which is more prominent in neuronal death? Cell Mol. Life Sci. 2021, 78, 8001–8047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Lv, J.; Li, Z.; Zhang, Q.; Lu, J.; Huo, X.; Guo, M.; Liu, X.; Li, C.; Wang, J.; et al. Overcoming multi-drug resistance in SCLC: A synergistic approach with venetoclax and hydroxychloroquine targeting the lncRNA LYPLAL1-DT/BCL2/BECN1 pathway. Mol. Cancer. 2024, 23, 243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Tang, S.; Wu, Y.; Wan, X.; Zhou, M.; Li, H.; Zha, X. Upregulation of 6-phosphofructo-2-kinase (PFKFB3) by hyperactivated mammalian target of rapamycin complex 1 is critical for tumor growth in tuberous sclerosis complex. IUBMB Life 2020, 72, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, G.; Yu, R.; Lee, J.; Kim, S.; Gleason, M.R.; Qiu, K.; Montauti, E.; Wang, L.L.; Fang, D.; et al. Inhibitory co-receptor Lag3 supports Foxp3+ regulatory T cell function by restraining Myc-dependent metabolic programming. Immunity 2024, 57, 2634–2650.e5. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Horner, J.W.; Wu, C.J.; Li, J.; Lan, Z.D.; Jiang, S.; Xu, X.; Hsu, W.H.; Zal, T.; Flores, I.I.; et al. Telomerase Reverse Transcriptase Preserves Neuron Survival and Cognition in Alzheimer’s Disease Models. Nat. Aging 2021, 1, 1162–1174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eglenen-Polat, B.; Kowash, R.R.; Huang, H.-C.; Siteni, S.; Zhu, M.; Chen, K.; Bender, M.E.; Mender, I.; Stastny, V.; Drapkin, B.J.; et al. A telomere-targeting drug depletes cancer initiating cells and promotes anti-tumor immunity in small cell lung cancer. Nat. Commun. 2024, 15, 672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Zhang, C.; Ren, Z.; Wang, Y.; Zeng, L. Multi-Omics Integration Analysis Revealed That miR-375-3p Is a Two-Sided Factor Regulating the Development and TUMORIGENESIS of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 3666. https://doi.org/10.3390/ijms26083666
Bao X, Zhang C, Ren Z, Wang Y, Zeng L. Multi-Omics Integration Analysis Revealed That miR-375-3p Is a Two-Sided Factor Regulating the Development and TUMORIGENESIS of Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(8):3666. https://doi.org/10.3390/ijms26083666
Chicago/Turabian StyleBao, Xinlu, Cheng Zhang, Zhichao Ren, Yuxiang Wang, and Linlin Zeng. 2025. "Multi-Omics Integration Analysis Revealed That miR-375-3p Is a Two-Sided Factor Regulating the Development and TUMORIGENESIS of Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 8: 3666. https://doi.org/10.3390/ijms26083666
APA StyleBao, X., Zhang, C., Ren, Z., Wang, Y., & Zeng, L. (2025). Multi-Omics Integration Analysis Revealed That miR-375-3p Is a Two-Sided Factor Regulating the Development and TUMORIGENESIS of Alzheimer’s Disease. International Journal of Molecular Sciences, 26(8), 3666. https://doi.org/10.3390/ijms26083666






