Segatella copri Outer-Membrane Vesicles Are Internalized by Human Macrophages and Promote a Pro-Inflammatory Profile
Abstract
1. Introduction
2. Results
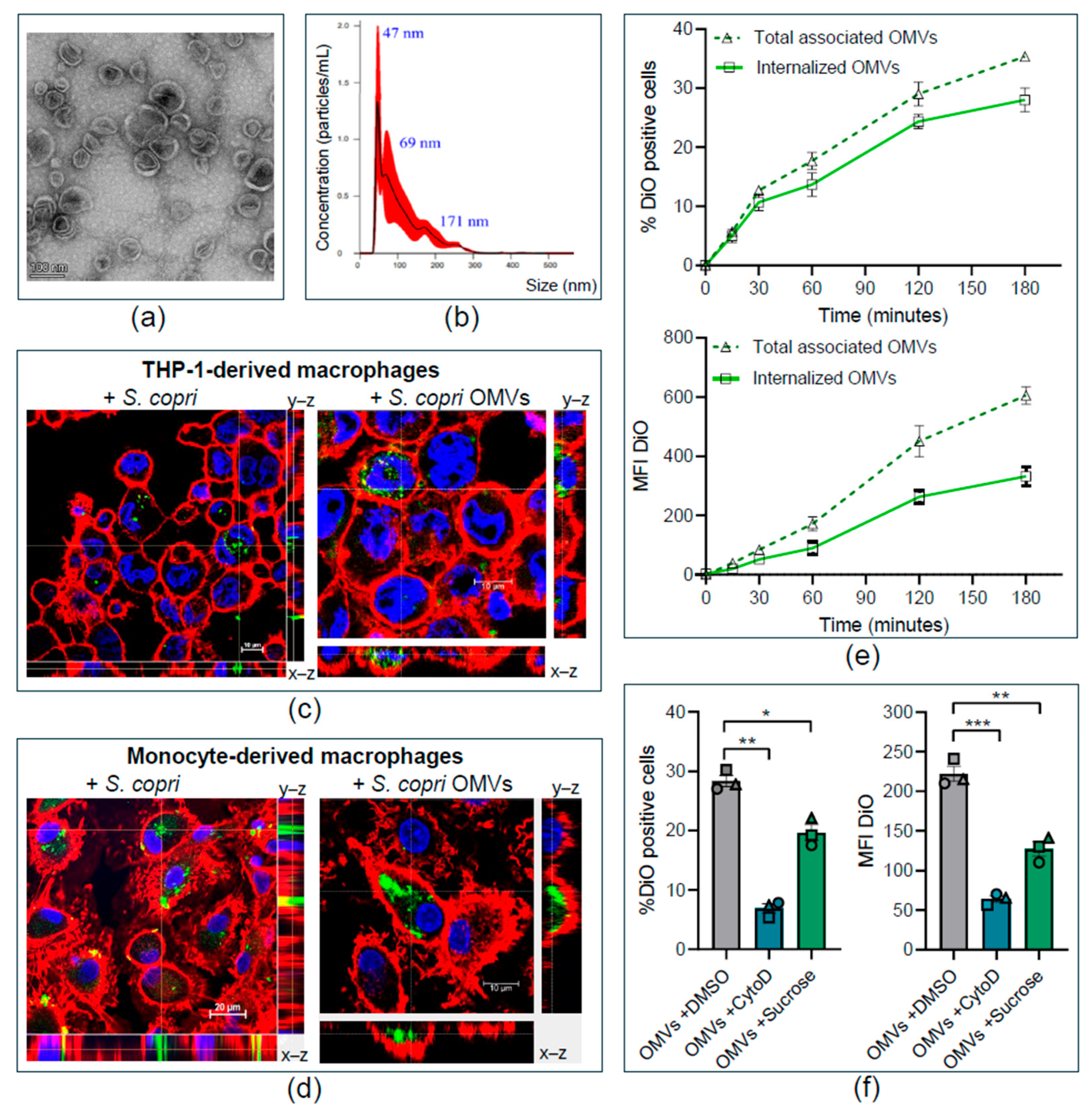
2.1. Segatella copri Secretes Outer-Membrane Vesicles That Are Internalized by Human Macrophages
2.2. Segatella copri Outer-Membrane Vesicles Enter Human Macrophages Through Macropinocytosis and Clathrin-Mediated Endocytosis
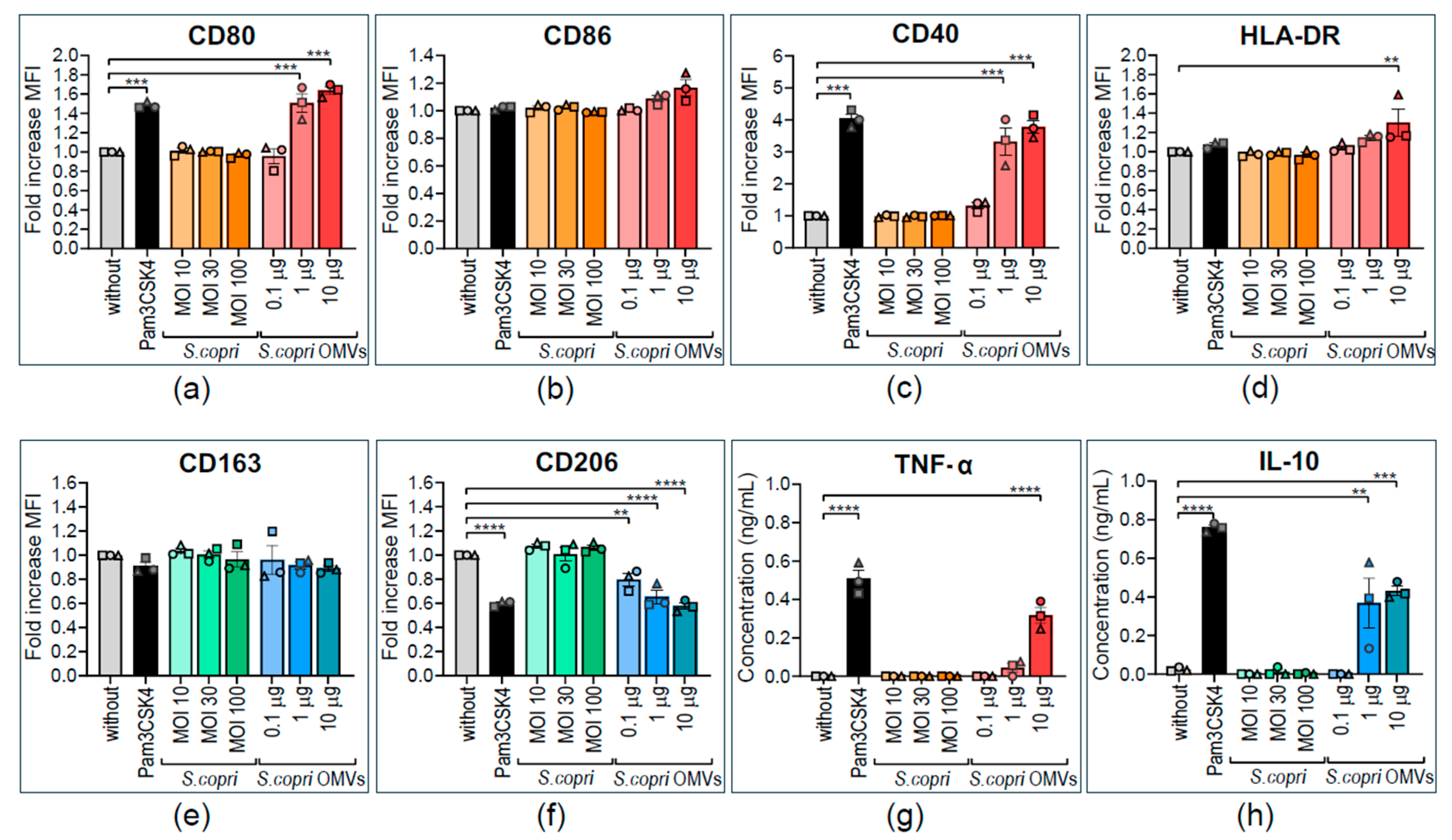
2.3. Human M0 Macrophages Acquire M1-like Profile in Response to Segatella copri Outer-Membrane Vesicles
2.4. The Pro-Inflammatory Profile of M1-Polarized Human Macrophages is Strengthened by Segatella copri-Derived Outer-Membrane Vesicles
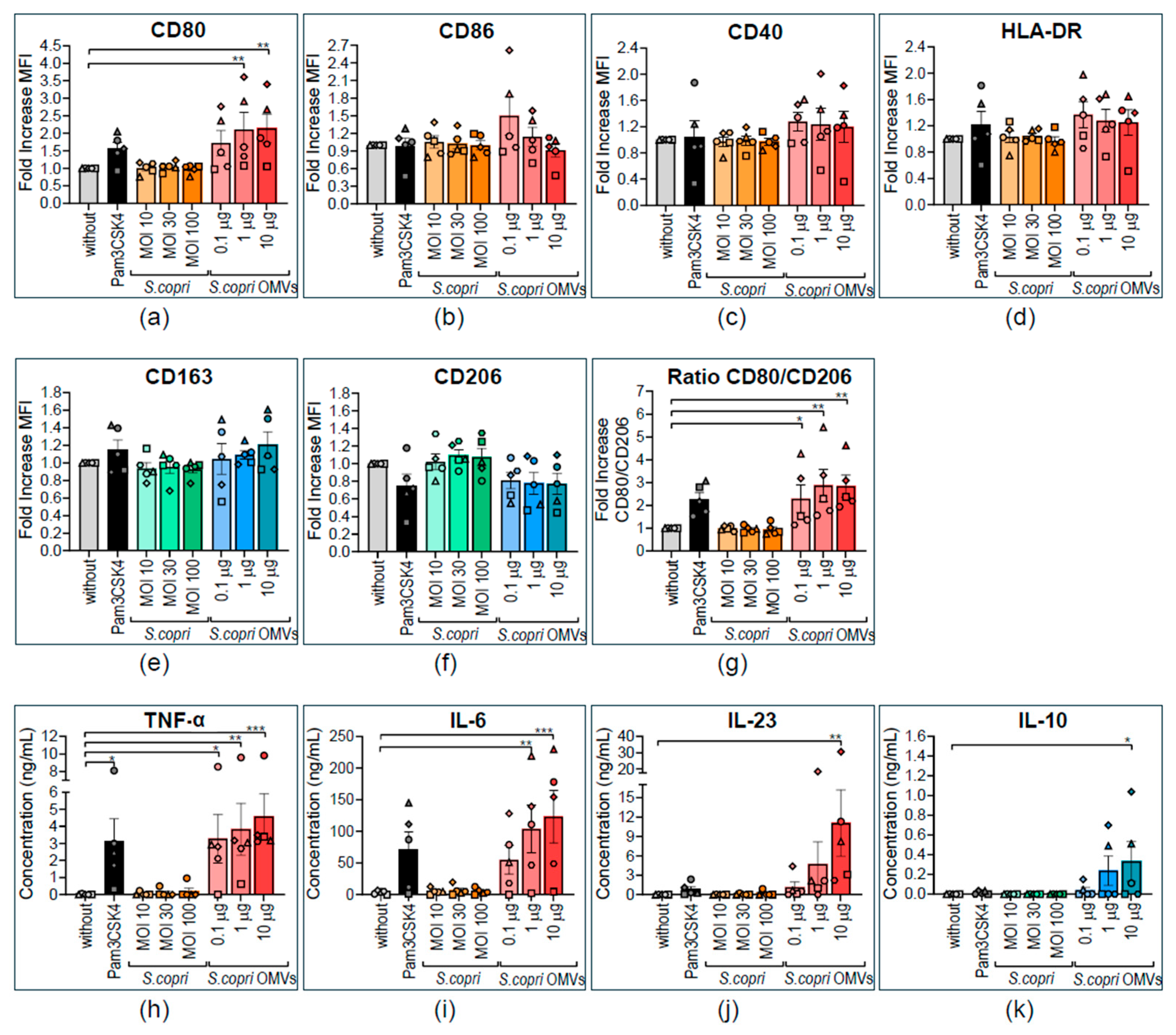
2.5. M2-Polarized Human Macrophages Are Repolarized to a M1-like Profile by Segatella copri Outer-Membrane Vesicles
3. Discussion
4. Materials and Methods
4.1. Human Blood Samples
4.2. Cultivation of Segatella copri
4.3. Purification and Characterization of Outer-Membrane Vesicles
4.4. Fluorescent Labeling of Segatella copri and OMVs
4.5. Differentiation of THP-1 Macrophages
4.6. Monocyte Isolation from Peripheral Blood and Differentiation of Monocyte-Derived Macrophages
4.7. Confocal Microscopy Analysis of the Internalization of S. copri and Its OMVs in Macrophages
4.8. Assessment of Internalization Kinetics for S. copri-OMVs in Macrophages
4.9. Inhibition of Endocytosis Pathways
4.10. Analysis of Macrophage Phenotype by Flow Cytometry
4.11. Assessment of Cytokine Secretion by ELISA
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| MD-M | Monocyte-derived macrophage |
| MHC | Major histocompatibility complex |
| NTA | Nanoparticle tracking analysis |
| OMV | Outer-membrane vesicle |
| RA | Rheumatoid arthritis |
| SCFA | Short-chain fatty acid |
| TEM | Transmission electron microscopy |
| TLR | Toll-like receptor |
References
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Tiku, V.; Tan, M.-W. Host immunity and cellular responses to bacterial outer membrane vesicles. Trends Immunol. 2021, 42, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Mozaheb, N.; Mingeot-Leclercq, M.-P. Membrane Vesicle Production as a Bacterial Defense Against Stress. Front. Microbiol. 2020, 11, 600221. [Google Scholar] [CrossRef] [PubMed]
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderón, I.L.; Fuentes, J.A. “One for All”: Functional Transfer of OMV-Mediated Polymyxin B Resistance from Salmonella enterica sv. Typhi ΔtolR and ΔdegS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. 2022, 86, e0003222. [Google Scholar] [CrossRef]
- Stentz, R.; Jones, E.; Juodeikis, R.; Wegmann, U.; Guirro, M.; Goldson, A.J.; Brion, A.; Booth, C.; Sudhakar, P.; Brown, I.R.; et al. The Proteome of Extracellular Vesicles Produced by the Human Gut Bacteria Bacteroides thetaiotaomicron In Vivo Is Influenced by Environmental and Host-Derived Factors. Appl. Environ. Microbiol. 2022, 88, e0053322. [Google Scholar] [CrossRef]
- Charpentier, L.A.; Dolben, E.F.; Hendricks, M.R.; Hogan, D.A.; Bomberger, J.M.; Stanton, B.A. Bacterial Outer Membrane Vesicles and Immune Modulation of the Host. Membranes 2023, 13, 752. [Google Scholar] [CrossRef]
- Nonaka, S.; Kadowaki, T.; Nakanishi, H. Secreted gingipains from Porphyromonas gingivalis increase permeability in human cerebral microvascular endothelial cells through intracellular degradation of tight junction proteins. Neurochem. Int. 2022, 154, 105282. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, Z.; Liu, H.; Zheng, S.; Zhang, F.; Zhu, J.; Shi, H.; Ye, H.; Chou, Z.; Gao, L.; et al. Fusobacterium nucleatum aggravates rheumatoid arthritis through FadA-containing outer membrane vesicles. Cell Host Microbe 2023, 31, 798–810.e7. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef]
- Yang, X.; Chang, Y.; Wei, W. Emerging role of targeting macrophages in rheumatoid arthritis: Focus on polarization, metabolism and apoptosis. Cell Prolif. 2020, 53, e12854. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.A.; Hegazy, S.M.; Aziz, R.K. The curious case of Prevotella copri. Gut Microbes 2023, 15, 2249152. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Fehlner-Peach, H.; Magnabosco, C.; Raghavan, V.; Scher, J.U.; Tett, A.; Cox, L.M.; Gottsegen, C.; Watters, A.; Wiltshire-Gordon, J.D.; Segata, N.; et al. Distinct Polysaccharide Utilization Profiles of Human Intestinal Prevotella copri Isolates. Cell Host Microbe 2019, 26, 680–690.e5. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Gálvez, E.J.; Pasolli, E.; De Filippis, F.; Amend, L.; Huang, K.D.; Manghi, P.; Lesker, T.-R.; Riedel, T.; Cova, L.; et al. Extension of the Segatella copri complex to 13 species with distinct large extrachromosomal elements and associations with host conditions. Cell Host Microbe 2023, 31, 1804–1819.e9. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Cerqueda-García, D.; López-Contreras, B.; Larrieta-Carrasco, E.; Villamil-Ramírez, H.; Molina-Cruz, S.; Torres, N.; Sánchez-Tapia, M.; Hernández-Pando, R.; Aguilar-Salinas, C.; et al. A metagenomic study identifies a Prevotella copri enriched microbial profile associated with non-alcoholic steatohepatitis in subjects with obesity. J. Gastroenterol. Hepatol. 2023, 38, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, Q.; Hu, R.; Yang, X.; Fang, C.; Yao, L.; Lv, J.; Wang, L.; Shi, M.; Zhang, W.; et al. Effects of Prevotella copri on insulin, gut microbiota and bile acids. Gut Microbes 2024, 16, 2340487. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 69, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Nii, T.; Maeda, Y.; Motooka, D.; Naito, M.; Matsumoto, Y.; Ogawa, T.; Oguro-Igashira, E.; Kishikawa, T.; Yamashita, M.; Koizumi, S.; et al. Genomic repertoires linked with pathogenic potency of arthritogenic Prevotella copri isolated from the gut of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2023, 82, 621–629. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, B.; Xia, X.; Chen, S.; Deng, Y.; Wang, Y.; Wu, L.; Tian, Y.; Zhao, B.; Xu, H.; et al. Prevotella copri is associated with carboplatin-induced gut toxicity. Cell Death Dis. 2019, 10, 714. [Google Scholar] [CrossRef]
- Nevermann, J.; Silva, A.; Otero, C.; Oyarzún, D.P.; Barrera, B.; Gil, F.; Calderón, I.L.; Fuentes, J.A. Identification of Genes Involved in Biogenesis of Outer Membrane Vesicles (OMVs) in Salmonella enterica Serovar Typhi. Front. Microbiol. 2019, 10, 104. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Lam, R.S.; O’Brien-Simpson, N.M.; Holden, J.A.; Lenzo, J.C.; Fong, S.B.; Reynolds, E.C. Unprimed, M1 and M2 Macrophages Differentially Interact with Porphyromonas gingivalis. PLoS ONE 2016, 11, e0158629. [Google Scholar] [CrossRef]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell. Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef]
- Pollak, C.N.; Delpino, M.V.; Fossati, C.A.; Baldi, P.C. Outer Membrane Vesicles from Brucella abortus Promote Bacterial Internalization by Human Monocytes and Modulate Their Innate Immune Response. PLoS ONE 2012, 7, e50214. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Yamamoto, S.; Hashimoto, H.; Nishii, M.; Miyaura, M.; Tomada, K.; Nakase, I.; Takeda-Morishita, M. Optimization of the method for analyzing endocytosis of fluorescently tagged molecules: Impact of incubation in the cell culture medium and cell surface wash with glycine-hydrochloric acid buffer. J. Control. Release 2019, 310, 127–140. [Google Scholar] [CrossRef]
- Hansen, S.H.; Sandvig, K.; van Deurs, B. Clathrin and HA2 adaptors: Effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 1993, 121, 61–72. [Google Scholar] [CrossRef]
- Cecil, J.D.; O’brien-Simpson, N.M.; Lenzo, J.C.; Holden, J.A.; Singleton, W.; Perez-Gonzalez, A.; Mansell, A.; Reynolds, E.C. Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo. Front. Immunol. 2017, 8, 1017. [Google Scholar] [CrossRef]
- Durant, L.; Stentz, R.; Noble, A.; Brooks, J.; Gicheva, N.; Reddi, D.; O’connor, M.J.; Hoyles, L.; McCartney, A.L.; Man, R.; et al. Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease. Microbiome 2020, 8, 88. [Google Scholar] [CrossRef]
- Mofrad, L.Z.; Fateh, A.; Sotoodehnejadnematalahi, F.; Asbi, D.N.S.; Siadat, S.D. The Effect of Akkermansia muciniphila and Its Outer Membrane Vesicles on MicroRNAs Expression of Inflammatory and Anti-inflammatory Pathways in Human Dendritic Cells. Probiotics Antimicrob. Proteins 2023, 16, 367–382. [Google Scholar] [CrossRef]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]
- Chen, S.; Lei, Q.; Zou, X.; Ma, D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front. Immunol. 2023, 14, 1157813. [Google Scholar] [CrossRef]
- Catalan, E.A.; Seguel-Fuentes, E.; Fuentes, B.; Aranguiz-Varela, F.; Castillo-Godoy, D.P.; Rivera-Asin, E.; Bocaz, E.; Fuentes, J.A.; Bravo, D.; Schinnerling, K.; et al. Oral Pathobiont-Derived Outer Membrane Vesicles in the Oral–Gut Axis. Int. J. Mol. Sci. 2024, 25, 11141. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, W.J.; Johnston, E.L.; Bitto, N.J.; Zavan, L.; O’Brien-Simpson, N.; Hill, A.F.; Kaparakis-Liaskos, M. Bacteroides fragilis outer membrane vesicles preferentially activate innate immune receptors compared to their parent bacteria. Front. Immunol. 2022, 13, 970725. [Google Scholar] [CrossRef]
- Krsek, D.; Yara, D.A.; Hrbáčková, H.; Daniel, O.; Mančíková, A.; Schüller, S.; Bielaszewska, M. Translocation of outer membrane vesicles from enterohemorrhagic Escherichia coli O157 across the intestinal epithelial barrier. Front. Microbiol. 2023, 14, 1198945. [Google Scholar] [CrossRef]
- Bhar, S.; Zhao, G.; Bartel, J.D.; Sterchele, H.; Del Mazo, A.; Emerson, L.E.; Edelmann, M.J.; Jones, M.K. Bacterial extracellular vesicles control murine norovirus infection through modulation of antiviral immune responses. Front. Immunol. 2022, 13, 909949. [Google Scholar] [CrossRef]
- Jiao, C.-Y.; Delaroche, D.; Burlina, F.; Alves, I.D.; Chassaing, G.; Sagan, S. Translocation and Endocytosis for Cell-penetrating Peptide Internalization. J. Biol. Chem. 2009, 284, 33957–33965. [Google Scholar] [CrossRef]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori Outer Membrane Vesicles by Gastric Epithelial Cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef]
- Jarzab, M.; Posselt, G.; Meisner-Kober, N.; Wessler, S. Helicobacter pylori-Derived Outer Membrane Vesicles (OMVs): Role in Bacterial Pathogenesis? Microorganisms 2020, 8, 1328. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Soderblom, T.; Oxhamre, C.; Wai, S.N.; Uhlen, P.; Aperia, A.; Uhlin, B.E.; Richter-Dahlfors, A. Effects of the Escherichia coli toxin cytolysin A on mucosal immunostimulation via epithelial Ca2+ signalling and Toll-like receptor 4. Cell Microbiol. 2005, 7, 779–788. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef]
- Boisvert, H.; Duncan, M.J. Clathrin-dependent entry of a gingipain adhesin peptide and Porphyromonas gingivalis into host cells. Cell Microbiol. 2008, 10, 2538–2552. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.-Y.; Yan, J.; Wei, J.-T.; Zeng, Y.-H.; Feng, L.-Y.; Que, D.-D.; Li, S.-C.; Guo, J.-B.; Fan, Y.; Ding, Y.-F.; et al. Prevotella copri promotes vascular calcification via lipopolysaccharide through activation of NF-κB signaling pathway. Gut Microbes 2024, 16, 2351532. [Google Scholar] [CrossRef]
- Geng, J.; Shi, Y.; Zhang, J.; Yang, B.; Wang, P.; Yuan, W.; Zhao, H.; Li, J.; Qin, F.; Hong, L.; et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat. Commun. 2021, 12, 3519. [Google Scholar] [CrossRef] [PubMed]
- Skjesol, A.; Yurchenko, M.; Bösl, K.; Gravastrand, C.; Nilsen, K.E.; Grøvdal, L.M.; Agliano, F.; Patane, F.; Lentini, G.; Kim, H.; et al. The TLR4 adaptor TRAM controls the phagocytosis of Gram-negative bacteria by interacting with the Rab11-family interacting protein 2. PLoS Pathog. 2019, 15, e1007684. [Google Scholar] [CrossRef] [PubMed]
- Veith, P.D.; Chen, Y.-Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyromonas gingivalis Outer Membrane Vesicles Exclusively Contain Outer Membrane and Periplasmic Proteins and Carry a Cargo Enriched with Virulence Factors. J. Proteome Res. 2014, 13, 2420–2432. [Google Scholar] [CrossRef]
- Liu, J.; Hsieh, C.-L.; Gelincik, O.; Devolder, B.; Sei, S.; Zhang, S.; Lipkin, S.M.; Chang, Y.-F. Proteomic characterization of outer membrane vesicles from gut mucosa-derived fusobacterium nucleatum. J. Proteom. 2019, 195, 125–137. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Cai, Z.; Zhou, K.; Chang, L.; Bai, Y.; Ma, Y. Prevotella Induces the Production of Th17 Cells in the Colon of Mice. J. Immunol. Res. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Tardito, S.; Martinelli, G.; Soldano, S.; Paolino, S.; Pacini, G.; Patane, M.; Alessandri, E.; Smith, V.; Cutolo, M. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 2019, 18, 102397. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, A.J.; Lee, M.K.; Singleton, W.; Achuthan, A.; Lee, M.-C.; O’Brien-Simpson, N.M.; Cook, A.D.; Murphy, A.J.; Dashper, S.G.; Reynolds, E.C.; et al. Metabolic Remodeling, Inflammasome Activation, and Pyroptosis in Macrophages Stimulated by Porphyromonas gingivalis and Its Outer Membrane Vesicles. Front. Cell Infect. Microbiol. 2017, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage Polarization in Bacterial Infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.J.; Booth, C.; Fonseca, S.; Parker, A.; Cross, K.; Miquel-Clopés, A.; Hautefort, I.; Mayer, U.; Wileman, T.; Stentz, R.; et al. The Uptake, Trafficking, and Biodistribution of Bacteroides thetaiotaomicron Generated Outer Membrane Vesicles. Front. Microbiol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Palacios, E.; Lobos-González, L.; Guerrero, S.; Kogan, M.J.; Shao, B.; Heinecke, J.W.; Quest, A.F.G.; Leyton, L.; Valenzuela-Valderrama, M. Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa B activation and cause neuronal damage in vivo in a murine model. J. Neuroinflamm. 2023, 20, 66. [Google Scholar] [CrossRef]
- Schaack, B.; Mercier, C.; Katby, M.; Hannani, D.; Vollaire, J.; Robert, J.S.; Caffaratti, C.; Blanquet, F.; Nicoud, O.; Josserand, V.; et al. Rapid Biodistribution of Fluorescent Outer-Membrane Vesicles from the Intestine to Distant Organs via the Blood in Mice. Int. J. Mol. Sci. 2024, 25, 1821. [Google Scholar] [CrossRef]
- Schinnerling, K.; Aguillón, J.C.; Catalán, D.; Soto, L. The role of interleukin-6 signalling and its therapeutic blockage in skewing the T cell balance in rheumatoid arthritis. Clin. Exp. Immunol. 2017, 189, 12–20. [Google Scholar] [CrossRef]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.; Baron, B. The role of TNF-α in rheumatoid arthritis: A focus on regulatory T cells. J. Clin. Transl. Res. 2016, 2, 84–90. [Google Scholar] [CrossRef]
- Yoshida, Y.; Tanaka, T. Interleukin 6 and Rheumatoid Arthritis. BioMed Res. Int. 2014, 2014, 698313. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-Hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2013, 20, 62–68. [Google Scholar] [CrossRef]
- Hou, X.; Tian, F. STAT3-mediated osteogenesis and osteoclastogenesis in osteoporosis. Cell Commun. Signal. 2022, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2018, 15, 9–17. [Google Scholar] [CrossRef]
- Brzustewicz, E.; Bryl, E. The role of cytokines in the pathogenesis of rheumatoid arthritis—Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 2015, 76, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial tissue macrophages in joint homeostasis, rheumatoid arthritis and disease remission. Nat. Rev. Rheumatol. 2022, 18, 384–397. [Google Scholar] [CrossRef]
- Johnston, E.L.; Heras, B.; Kufer, T.A.; Kaparakis-Liaskos, M. Detection of Bacterial Membrane Vesicles by NOD-Like Receptors. Int. J. Mol. Sci. 2021, 22, 1005. [Google Scholar] [CrossRef]
- Ajam-Hosseini, M.; Akhoondi, F.; Parvini, F.; Fahimi, H. Gram-negative bacterial sRNAs encapsulated in OMVs: An emerging class of therapeutic targets in diseases. Front. Cell Infect. Microbiol. 2024, 13, 1305510. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda-Pontigo, A.; Chávez-Villacreses, K.; Madrid-Muñoz, C.; Conejeros-Lillo, S.; Parra, F.; Melo-González, F.; Regaldiz, A.; González, V.P.I.; Méndez-Pérez, I.; Castillo-Godoy, D.P.; et al. Segatella copri Outer-Membrane Vesicles Are Internalized by Human Macrophages and Promote a Pro-Inflammatory Profile. Int. J. Mol. Sci. 2025, 26, 3630. https://doi.org/10.3390/ijms26083630
Sepúlveda-Pontigo A, Chávez-Villacreses K, Madrid-Muñoz C, Conejeros-Lillo S, Parra F, Melo-González F, Regaldiz A, González VPI, Méndez-Pérez I, Castillo-Godoy DP, et al. Segatella copri Outer-Membrane Vesicles Are Internalized by Human Macrophages and Promote a Pro-Inflammatory Profile. International Journal of Molecular Sciences. 2025; 26(8):3630. https://doi.org/10.3390/ijms26083630
Chicago/Turabian StyleSepúlveda-Pontigo, Alison, Karissa Chávez-Villacreses, Cristóbal Madrid-Muñoz, Sabrina Conejeros-Lillo, Francisco Parra, Felipe Melo-González, Alejandro Regaldiz, Valentina P. I. González, Isabel Méndez-Pérez, Daniela P. Castillo-Godoy, and et al. 2025. "Segatella copri Outer-Membrane Vesicles Are Internalized by Human Macrophages and Promote a Pro-Inflammatory Profile" International Journal of Molecular Sciences 26, no. 8: 3630. https://doi.org/10.3390/ijms26083630
APA StyleSepúlveda-Pontigo, A., Chávez-Villacreses, K., Madrid-Muñoz, C., Conejeros-Lillo, S., Parra, F., Melo-González, F., Regaldiz, A., González, V. P. I., Méndez-Pérez, I., Castillo-Godoy, D. P., Soto, J. A., Fuentes, J. A., & Schinnerling, K. (2025). Segatella copri Outer-Membrane Vesicles Are Internalized by Human Macrophages and Promote a Pro-Inflammatory Profile. International Journal of Molecular Sciences, 26(8), 3630. https://doi.org/10.3390/ijms26083630











