Detection of Human Circulating and Extracellular Vesicle-Derived miRNAs in Serum of Humanized Mice Transplanted with Human Breast Cancer (HER2+ and TNBC) Cells—A Proof of Principle Investigation
Abstract
1. Introduction
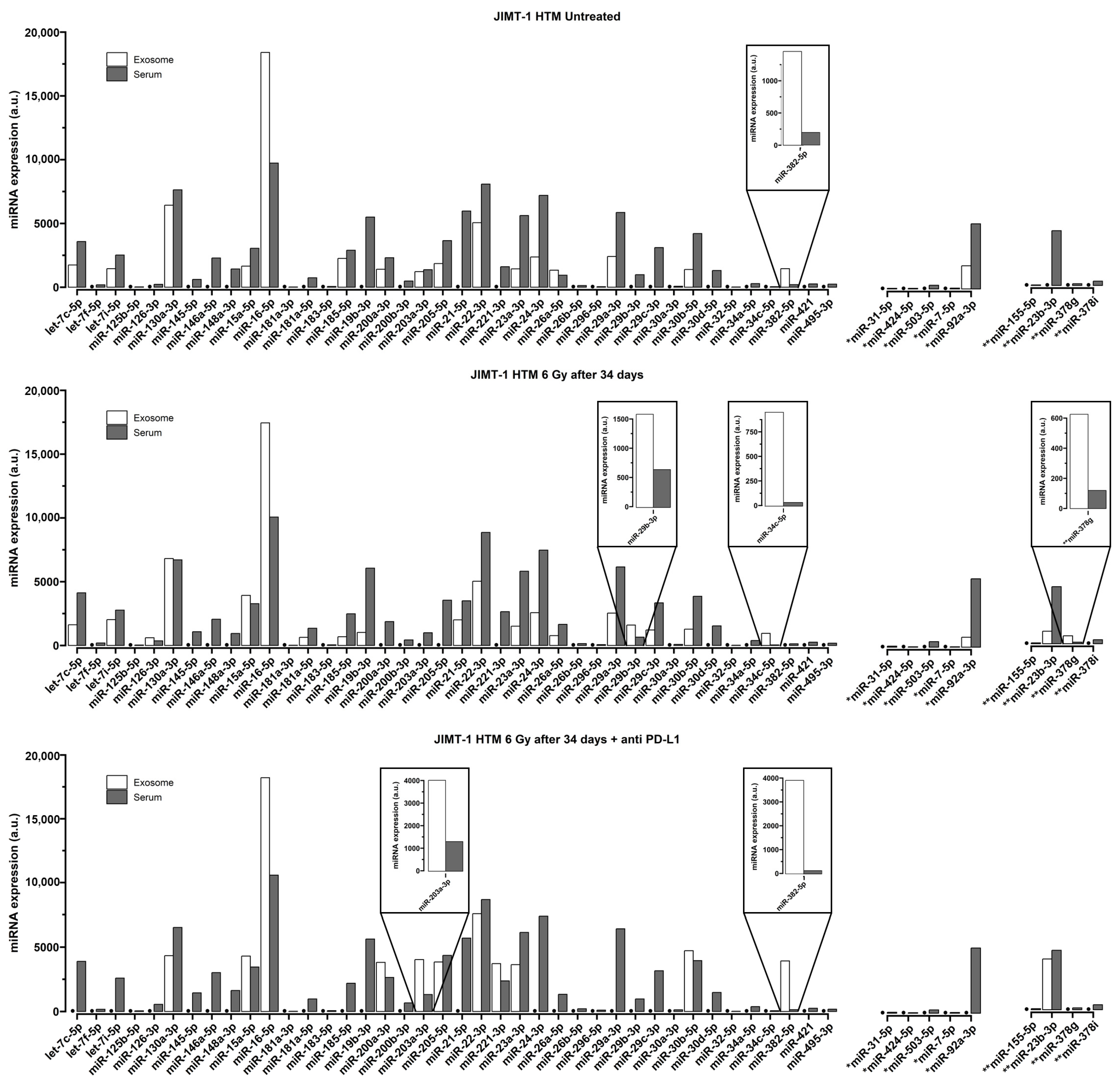
2. Results
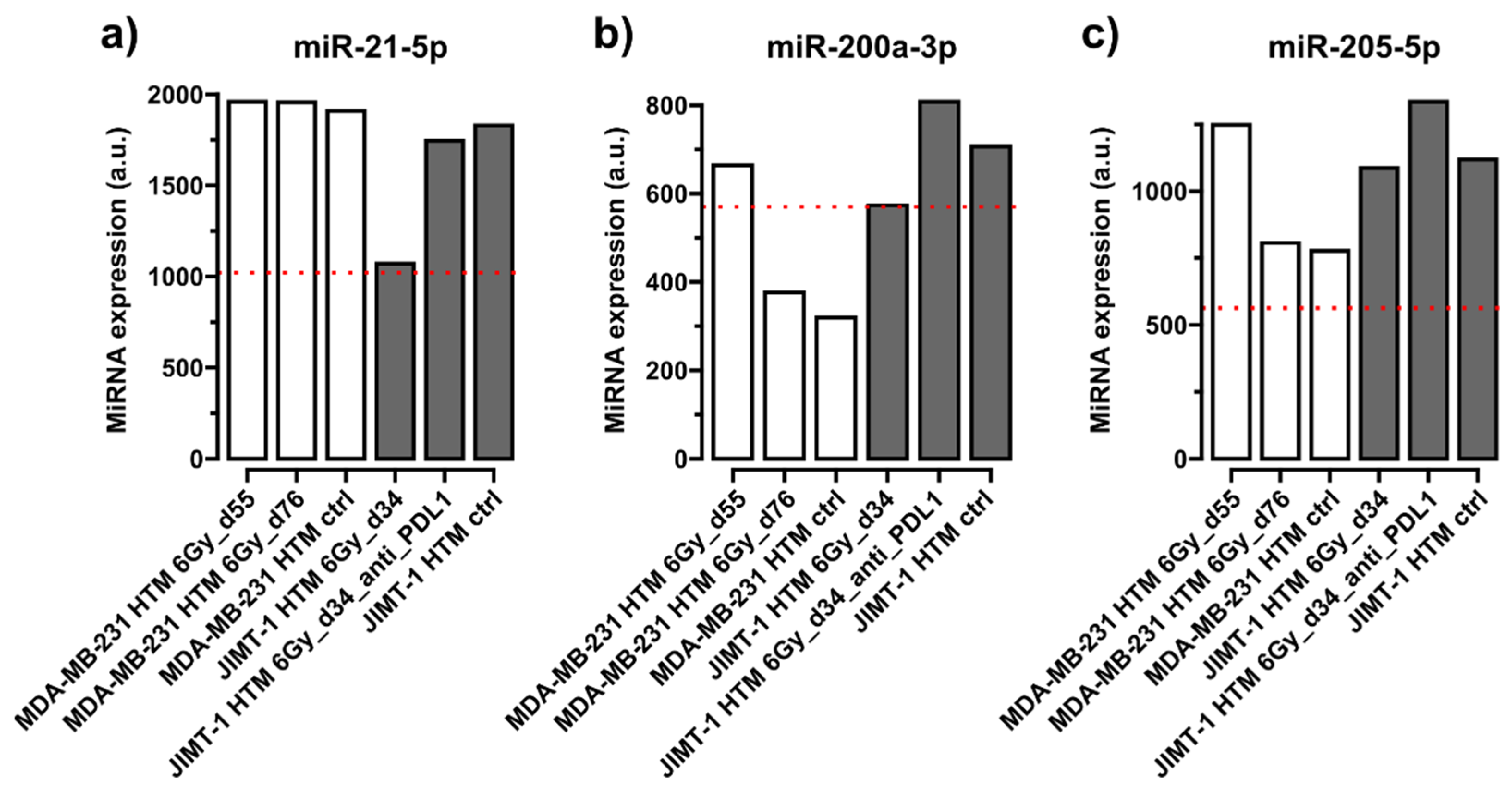
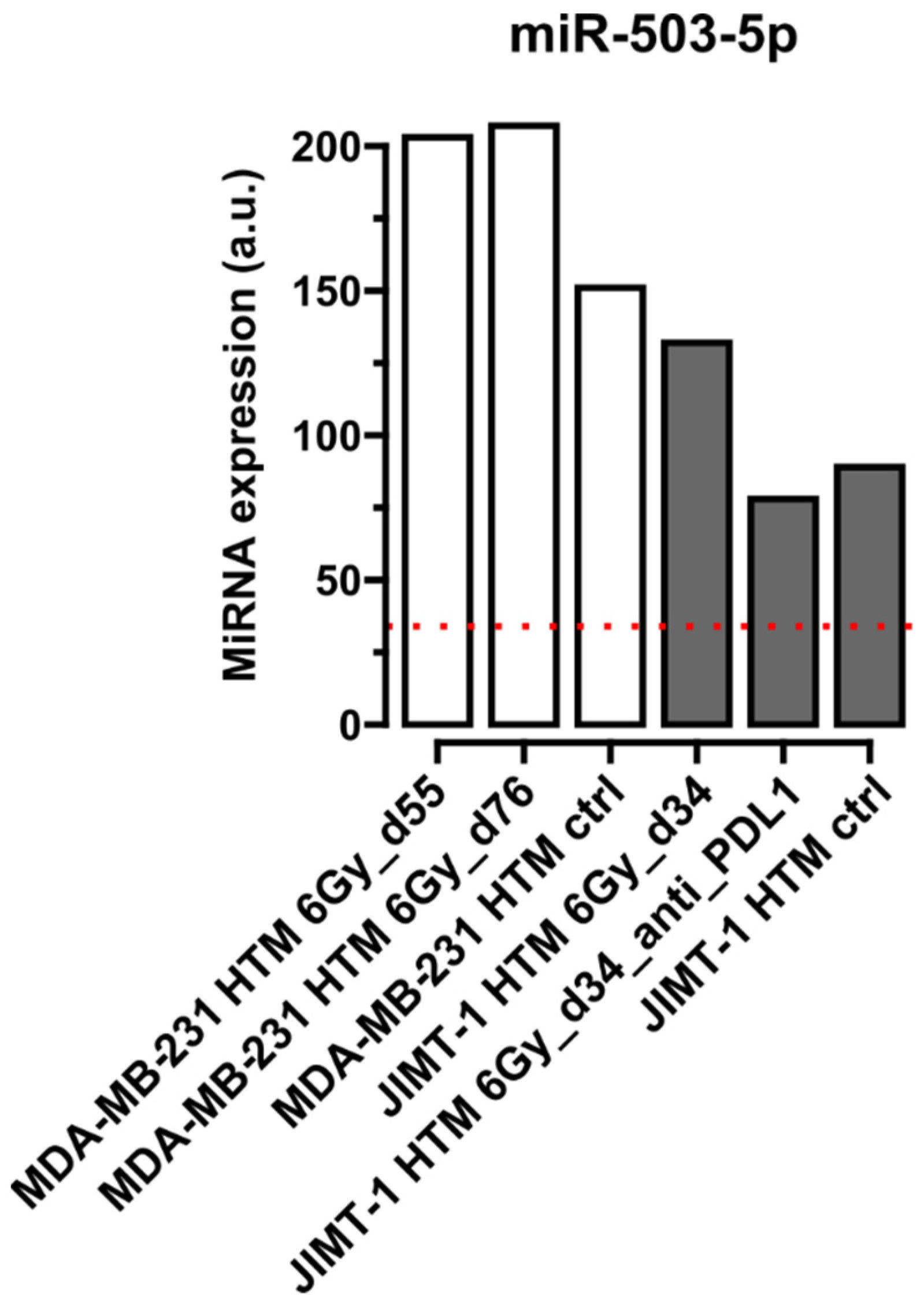
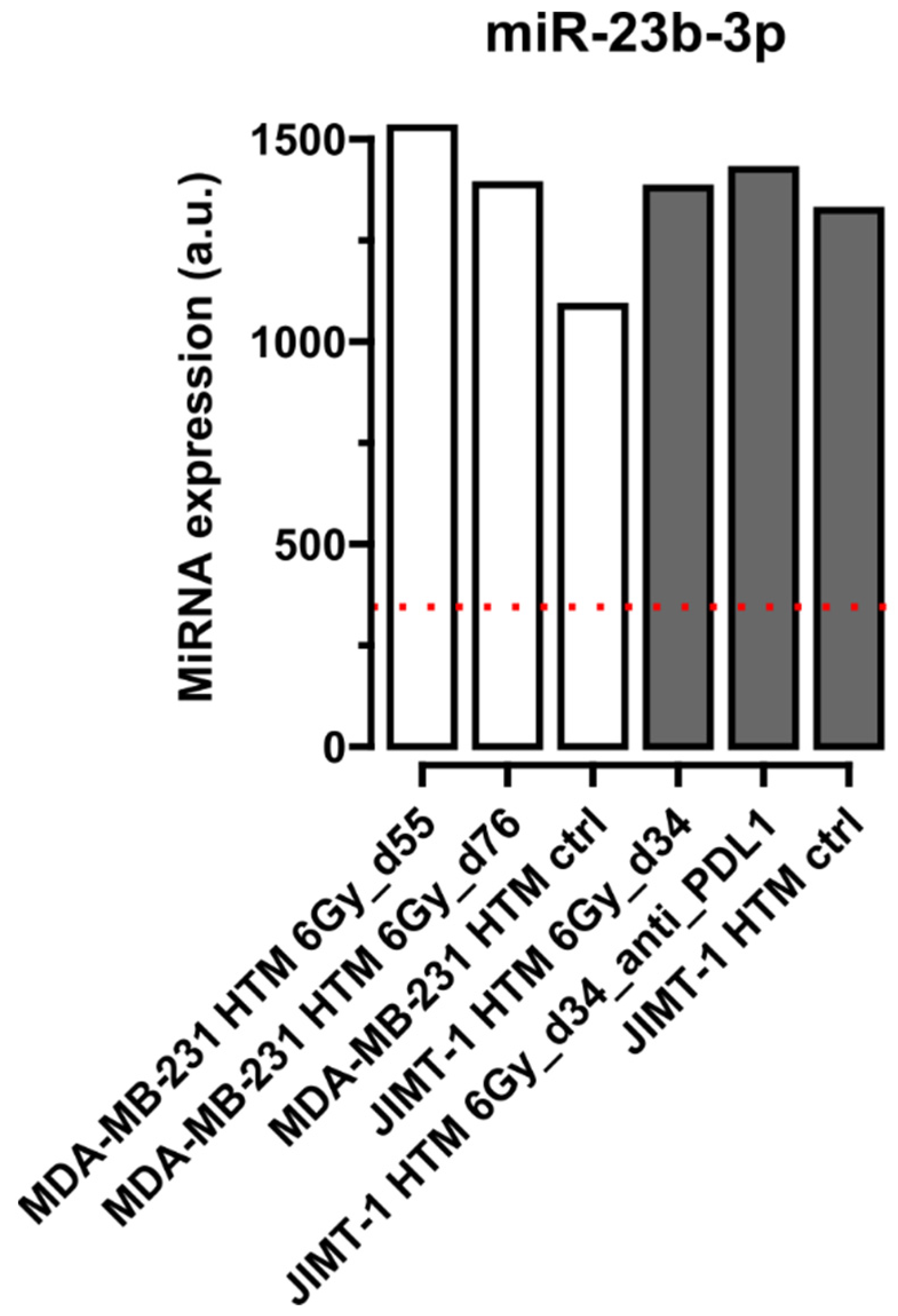
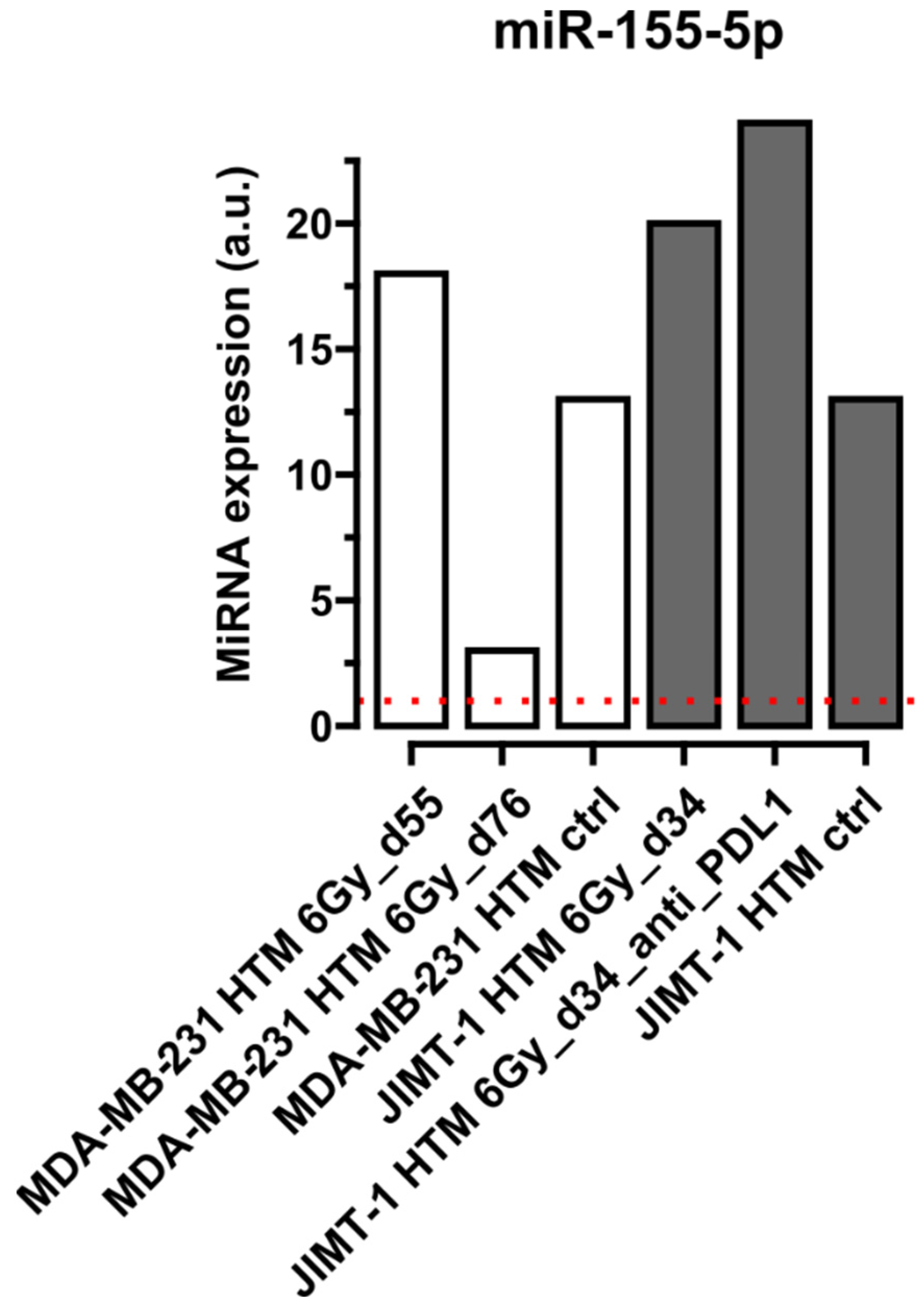
Quantification of miRNA in Serum of Treated and Control Mice
| No Mismatch—Conserved (Identical Nucleotide Sequence in Mice and Humans) | 1 Mismatch | 2 Mismatches | Prognostic, Predictive, Functional Role in HER2+/TNBC | |
|---|---|---|---|---|
| miR-21-5p | [41,61,62] | |||
| miR-26a-5p | [41] | |||
| miR-29a-3p | [41,43,44] | |||
| miR-30a-3p | [41] | |||
| miR-30b-5p | [41] | |||
| miR-34a-5p | [41,63] | |||
| miR-125b-5p | [36,41] | |||
| miR-145-5p | [41,44,63] | |||
| miR-148a-3p | [41,64] | |||
| miR-200a-3p | [41,44] | |||
| miR-200b-3p | [41,44] | |||
| miR-205-5p | [41,44,62,65] | |||
| miR-221-3p | [5,41,44] | |||
| miR-7-5p | [66,67] | |||
| miR-31-5p | [68] | |||
| miR-92a-3p | [5,44,69] | |||
| miR-424-5p | [70,71] | |||
| miR-503-5p | [5,72] | |||
| miR-23b-3p | [41] | |||
| miR-378g | [73] | |||
| miR-155-5p** | [41,74] |
3. Discussion
4. Materials and Methods
4.1. Humanized Mouse Model
4.1.1. Cell Lines Used to Induce BC in the Humanized Mouse Model
4.1.2. Irradiation and PD-L1 Treatment of Humanized Mouse
4.1.3. Blood Harvest, Serum (Plasma) Preparation of Treated (Section 4.1.2) Mice
4.2. miRNA Detection in the Humanized Mouse Model
4.2.1. miRNA Quantification in Serum of Humanized Mice
The FirePlex® Assay for miRNA Profiling in Serum and Exosome
miRNA Selection
4.2.2. Discrimination of Human and Mice miRNAs in a Humanized Mouse Model
4.3. sEV (Exosomes) and Their miRNA Cargo
4.3.1. Isolation of Exosomes from Serum of Humanized Mice
Size Exclusion Chromatography (SEC)
Tunable Resistive Pulse Sensing (TRPS) and Electron Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Fertal, S.A.; Poterala, J.E.; Ponik, S.M.; Wisinski, K.B. Stromal Characteristics and Impact on New Therapies for Metastatic Triple-Negative Breast Cancer. Cancers 2022, 14, 1238. [Google Scholar] [CrossRef] [PubMed]
- Fogazzi, V.; Kapahnke, M.; Cataldo, A.; Plantamura, I.; Tagliabue, E.; Di Cosimo, S.; Cosentino, G.; Iorio, M.V. The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective. Cancers 2022, 14, 5326. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Baselga, J. Phase I and II clinical trials of trastuzumab. Ann. Oncol. 2001, 12 (Suppl. 1), S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Vito, A.; Rathmann, S.; Mercanti, N.; El-Sayes, N.; Mossman, K.; Valliant, J. Combined Radionuclide Therapy and Immunotherapy for Treatment of Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 4843. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Carlino, F.; Diana, A.; Piccolo, A.; Ventriglia, A.; Bruno, V.; De Santo, I.; Letizia, O.; De Vita, F.; Daniele, B.; Ciardiello, F.; et al. Immune-Based Therapy in Triple-Negative Breast Cancer: From Molecular Biology to Clinical Practice. Cancers 2022, 14, 2102. [Google Scholar] [CrossRef] [PubMed]
- Rom-Jurek, E.-M.; Kirchhammer, N.; Ugocsai, P.; Ortmann, O.; Wege, A.K.; Brockhoff, G. Regulation of Programmed Death Ligand 1 (PD-L1) Expression in Breast Cancer Cell Lines In Vitro and in Immunodeficient and Humanized Tumor Mice. Int. J. Mol. Sci. 2018, 19, 563. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Hong, M.; Kim, J.W.; Kim, M.K.; Chung, B.W.; Ahn, S.K. Programmed cell death-ligand 1 expression in stromal immune cells is a marker of breast cancer outcome. J. Cancer 2020, 11, 7246–7252. [Google Scholar] [CrossRef]
- Nunez Abad, M.; Calabuig-Farinas, S.; Lobo de Mena, M.; Torres-Martinez, S.; Garcia Gonzalez, C.; Garcia Garcia, J.A.; Iranzo Gonzalez-Cruz, V.; Camps Herrero, C. Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer. Cancers 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Ghebeh, H.; Mohammed, S.; Al-Omair, A.; Qattant, A.; Lehe, C.; Al-Qudaihi, G.; Elkum, N.; Alshabanah, M.; Bin Amer, S.; Tulbah, A.; et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia 2006, 8, 190–198. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Cibulskis, K.; Rangel-Escareno, C.; Brown, K.K.; Carter, S.L.; Frederick, A.M.; Lawrence, M.S.; Sivachenko, A.Y.; Sougnez, C.; Zou, L.; et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012, 486, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.Y.; Tabrizi, S.; Dunn, S.A.; McArthur, H.L. Current advances in immune checkpoint inhibitor combinations with radiation therapy or cryotherapy for breast cancer. Breast Cancer Res. Treat. 2022, 191, 229–241. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Tan, J.; Siva, S.; Karroum, L.; Savas, P.; Loi, S. Combining Radiotherapy and Immunotherapy in Metastatic Breast Cancer: Current Status and Future Directions. Biomedicines 2022, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Debbi, K.; Grellier, N.; Loganadane, G.; Boukhobza, C.; Mahé, M.; Cherif, M.A.; Rida, H.; Gligorov, J.; Belkacemi, Y. Interaction between Radiation Therapy and Targeted Therapies in HER2-Positive Breast Cancer: Literature Review, Levels of Evidence for Safety and Recommendations for Optimal Treatment Sequence. Cancers 2023, 15, 2278. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012, 4, 143–159. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef]
- Zelli, V.; Compagnoni, C.; Capelli, R.; Cannita, K.; Sidoni, T.; Ficorella, C.; Capalbo, C.; Zazzeroni, F.; Tessitore, A.; Alesse, E. Circulating MicroRNAs as Prognostic and Therapeutic Biomarkers in Breast Cancer Molecular Subtypes. J. Pers. Med. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- El-Mogy, M.; Lam, B.; Haj-Ahmad, T.A.; McGowan, S.; Yu, D.; Nosal, L.; Rghei, N.; Roberts, P.; Haj-Ahmad, Y. Diversity and signature of small RNA in different bodily fluids using next generation sequencing. BMC Genom. 2018, 19, 408. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Lam, S.; Nagahara, M.; Hoon, D.S. Circulating microRNA Biomarkers as Liquid Biopsy for Cancer Patients: Pros and Cons of Current Assays. J. Clin. Med. 2015, 4, 1890–1907. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical utility of circulating non-coding RNAs—An update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, Z.; Talkhabi, M.; Taleahmad, S. Identification of potential microRNA diagnostic panels and uncovering regulatory mechanisms in breast cancer pathogenesis. Sci. Rep. 2022, 12, 20135. [Google Scholar] [CrossRef] [PubMed]
- Kleivi Sahlberg, K.; Bottai, G.; Naume, B.; Burwinkel, B.; Calin, G.A.; Borresen-Dale, A.L.; Santarpia, L. A serum microRNA signature predicts tumor relapse and survival in triple-negative breast cancer patients. Clin. Cancer Res. 2015, 21, 1207–1214. [Google Scholar] [CrossRef]
- Ritter, A.; Hirschfeld, M.; Berner, K.; Weiss, D.; Medl, M.; Gassner, S.; Asberger, J.; Erbes, T.; Rücker, G.; Jäger, M.; et al. Circulating noncoding RNAbiomarker potential in neoadjuvant chemotherapy of triple negative breast cancer? Int. J. Oncol. 2020, 56, 47–68. [Google Scholar] [PubMed]
- Qattan, A.; Al-Tweigeri, T.; Alkhayal, W.; Suleman, K.; Tulbah, A.; Amer, S. Clinical Identification of Dysregulated Circulating microRNAs and Their Implication in Drug Response in Triple Negative Breast Cancer (TNBC) by Target Gene Network and Meta-Analysis. Genes 2021, 12, 549. [Google Scholar] [CrossRef]
- Thomopoulou, K.; Papadaki, C.; Monastirioti, A.; Koronakis, G.; Mala, A.; Kalapanida, D.; Mavroudis, D.; Agelaki, S. MicroRNAs Regulating Tumor Immune Response in the Prediction of the Outcome in Patients With Breast Cancer. Front. Mol. Biosci. 2021, 8, 668534. [Google Scholar] [CrossRef]
- Di Agostino, S.; Vahabi, M.; Turco, C.; Fontemaggi, G. Secreted Non-Coding RNAs: Functional Impact on the Tumor Microenvironment and Clinical Relevance in Triple-Negative Breast Cancer. Noncoding RNA 2022, 8, 5. [Google Scholar] [CrossRef]
- Isca, C.; Piacentini, F.; Mastrolia, I.; Masciale, V.; Caggia, F.; Toss, A.; Piombino, C.; Moscetti, L.; Barbolini, M.; Maur, M.; et al. Circulating and Intracellular miRNAs as Prognostic and Predictive Factors in HER2-Positive Early Breast Cancer Treated with Neoadjuvant Chemotherapy: A Review of the Literature. Cancers 2021, 13, 4894. [Google Scholar] [CrossRef]
- Lowery, A.J.; Miller, N.; Devaney, A.; McNeill, R.E.; Davoren, P.A.; Lemetre, C.; Benes, V.; Schmidt, S.; Blake, J.; Ball, G.; et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009, 11, R27. [Google Scholar] [CrossRef]
- Bailey, S.T.; Westerling, T.; Brown, M. Loss of estrogen-regulated microRNA expression increases HER2 signaling and is prognostic of poor outcome in luminal breast cancer. Cancer Res. 2015, 75, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.P.; Cahill, H.F.; Marcato, P. Breast Cancer Subtype-Specific miRNAs: Networks, Impacts, and the Potential for Intervention. Biomedicines 2022, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, X.; Niu, W.; Wang, H.; Wen, Q.; Fan, S.; Zhao, R.; Li, Z.; Xiong, W.; Peng, S.; et al. Elevated microRNA-125b levels predict a worse prognosis in HER2-positive breast cancer patients. Oncol. Lett. 2017, 13, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The role of Exosomal miRNAs in cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Logozzi, M.; Campanella, C.; Bavisotto, C.C.; Marcilla, A.; Properzi, F.; Fais, S. Exosome levels in human body fluids: A tumor marker by themselves? Eur. J. Pharm. Sci. 2017, 96, 93–98. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Fais, S. Exosomes: A Source for New and Old Biomarkers in Cancer. Cancers 2020, 12, 2566. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Gutierrez-Vazquez, C.; Sanchez-Madrid, F.; Mittelbrunn, M. Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Barreca, M.M.; Zichittella, C.; Alessandro, R.; Conigliaro, A. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells 2021, 10, 3355. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Jia, J.; Yao, L.; Li, Z. Crosstalk of Exosomal Non-Coding RNAs in The Tumor Microenvironment: Novel Frontiers. Front. Immunol. 2022, 13, 900155. [Google Scholar] [CrossRef] [PubMed]
- Majood, M.; Rawat, S.; Mohanty, S. Delineating the role of extracellular vesicles in cancer metastasis: A comprehensive review. Front. Immunol. 2022, 13, 966661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Guo, S.; Li, F.; Sun, Q.; Liang, G. Exosomal PD-L1: New Insights Into Tumor Immune Escape Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 569219. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Yu, L.; Zhu, D.; Li, Y.; Xue, Z.; Hua, Z.; Luo, X.; Song, Z.; Lu, C.; et al. The Role of miRNA in Tumor Immune Escape and miRNA-Based Therapeutic Strategies. Front. Immunol. 2021, 12, 807895. [Google Scholar] [CrossRef]
- Samuels, M.; Cilibrasi, C.; Papanastasopoulos, P.; Giamas, G. Extracellular Vesicles as Mediators of Therapy Resistance in the Breast Cancer Microenvironment. Biomolecules 2022, 12, 132. [Google Scholar] [CrossRef]
- Aranza-Martinez, A.; Sanchez-Perez, J.; Brito-Elias, L.; Lopez-Camarillo, C.; Cantu de Leon, D.; Perez-Plasencia, C.; Lopez-Urrutia, E. Non-Coding RNAs Associated With Radioresistance in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 752270. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stahler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Roux, J.; Gonzalez-Porta, M.; Robinson-Rechavi, M. Comparative analysis of human and mouse expression data illuminates tissue-specific evolutionary patterns of miRNAs. Nucleic Acids Res. 2012, 40, 5890–5900. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Groger, L.; Tschernig, T.; Solomon, J.; Laham, O.; Schaum, N.; Wagner, V.; Kern, F.; Schmartz, G.P.; Li, Y.; et al. miRNATissueAtlas2: An update to the human miRNA tissue atlas. Nucleic Acids Res. 2022, 50, D211–D221. [Google Scholar] [CrossRef]
- Liu, B.; Su, F.; Lv, X.; Zhang, W.; Shang, X.; Zhang, Y.; Zhang, J. Serum microRNA-21 predicted treatment outcome and survival in HER2-positive breast cancer patients receiving neoadjuvant chemotherapy combined with trastuzumab. Cancer Chemother. Pharmacol. 2019, 84, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gautam, M.; Chaudhary, A.; Chaurasia, B. Impact of three miRNA signature as potential diagnostic marker for triple negative breast cancer patients. Sci. Rep. 2023, 13, 21643. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Tiberio, P.; Iorio, M.V.; Hilbers, F.; de Azambuja, E.; de la Peña, L.; Izquierdo, M.Á.; Huober, J.; et al. Plasma miRNA Levels for Predicting Therapeutic Response to Neoadjuvant Treatment in HER2-positive Breast Cancer: Results from the NeoALTTO Trial. Clin. Cancer Res. 2019, 25, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Silvestri, M.; Baselga, J.; Piccart, M.; Huober, J.; Izquierdo, M.; de la Pena, L.; Hilbers, F.S.; et al. Early Modulation of Circulating MicroRNAs Levels in HER2-Positive Breast Cancer Patients Treated with Trastuzumab-Based Neoadjuvant Therapy. Int. J. Mol. Sci. 2020, 21, 1386. [Google Scholar] [CrossRef]
- Cataldo, A.; Piovan, C.; Plantamura, I.; D’ippolito, E.; Camelliti, S.; Casalini, P.; Giussani, M.; Déas, O.; Cairo, S.; Judde, J.-G.; et al. MiR-205 as predictive biomarker and adjuvant therapeutic tool in combination with trastuzumab. Oncotarget 2018, 9, 27920–27928. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, Z.; Liu, L.; Gao, R.; Che, C.; Yang, G. Downregulation of exosomal miR-7-5p promotes breast cancer migration and invasion by targeting RYK and participating in the atypical WNT signalling pathway. Cell Mol. Biol. Lett. 2022, 27, 88. [Google Scholar] [CrossRef]
- Block, I.; Burton, M.; Sorensen, K.P.; Andersen, L.; Larsen, M.J.; Bak, M.; Cold, S.; Thomassen, M.; Tan, Q.; Kruse, T.A. Association of miR-548c-5p, miR-7-5p, miR-210-3p, miR-128-3p with recurrence in systemically untreated breast cancer. Oncotarget 2018, 9, 9030–9042. [Google Scholar] [CrossRef]
- Pizzamiglio, S.; Cosentino, G.; Ciniselli, C.M.; De Cecco, L.; Cataldo, A.; Plantamura, I.; Triulzi, T.; El-Abed, S.; Wang, Y.; Bajji, M.; et al. What if the future of HER2-positive breast cancer patients was written in miRNAs? An exploratory analysis from NeoALTTO study. Cancer Med. 2022, 11, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ling, Y.; Lin, Q.; Shi, Y.; Luo, Q.; Cen, Y.; Mehrpour, M.; Hamai, A.; Li, J.; Gong, C. MiR-92b-3p Inhibits Proliferation of HER2-Positive Breast Cancer Cell by Targeting circCDYL. Front. Cell Dev. Biol. 2021, 9, 707049. [Google Scholar] [CrossRef]
- Shadbad, M.A.; Safaei, S.; Brunetti, O.; Derakhshani, A.; Lotfinejad, P.; Mokhtarzadeh, A.; Hemmat, N.; Racanelli, V.; Solimando, A.G.; Argentiero, A.; et al. A Systematic Review on the Therapeutic Potentiality of PD-L1-Inhibiting MicroRNAs for Triple-Negative Breast Cancer: Toward Single-Cell Sequencing-Guided Biomimetic Delivery. Genes 2021, 12, 1206. [Google Scholar] [CrossRef]
- Dastmalchi, N.; Safaralizadeh, R.; Hosseinpourfeizi, M.A.; Baradaran, B.; Khojasteh, S.M.B. MicroRNA-424-5p enhances chemosensitivity of breast cancer cells to Taxol and regulates cell cycle, apoptosis, and proliferation. Mol. Biol. Rep. 2021, 48, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Gorbatenko, A.; Sokilde, R.; Sorensen, E.E.; Newie, I.; Persson, H.; Morancho, B.; Arribas, J.; Litman, T.; Rovira, C.; Pedersen, S.F. HER2 and p95HER2 differentially regulate miRNA expression in MCF-7 breast cancer cells and downregulate MYB proteins through miR-221/222 and miR-503. Sci. Rep. 2019, 9, 3352. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.J.; Muluhngwi, P.; Alizadeh-Rad, N.; Green, M.A.; Rouchka, E.C.; Waigel, S.J.; Klinge, C.M. Genome-wide miRNA response to anacardic acid in breast cancer cells. PLoS ONE 2017, 12, e0184471. [Google Scholar] [CrossRef]
- Guglielmi, L.; Nardella, M.; Musa, C.; Cifola, I.; Porru, M.; Cardinali, B.; Iannetti, I.; Di Pietro, C.; Bolasco, G.; Palmieri, V.; et al. Circulating miRNAs in Small Extracellular Vesicles Secreted by a Human Melanoma Xenograft in Mouse Brains. Cancers 2020, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Sebzari, A.; Kordi-Tamandani, D.M.; Dastjerdi, K. Involvement of the Dysregulation of miR-23b-3p, miR-195-5p, miR-656-5p, and miR-340-5p in Trastuzumab Resistance of HER2-Positive Breast Cancer Cells and System Biology Approach to Predict Their Targets Involved in Resistance. DNA Cell Biol. 2019, 38, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Basova, P.; Pesta, M.; Sochor, M.; Stopka, T. Prediction Potential of Serum miR-155 and miR-24 for Relapsing Early Breast Cancer. Int. J. Mol. Sci. 2017, 18, 2116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Yu, G.; Sun, Z.; Wang, T.; Tian, X.; Duan, X.; Zhang, C. Exosomal miR-1246 and miR-155 as predictive and prognostic biomarkers for trastuzumab-based therapy resistance in HER2-positive breast cancer. Cancer Chemother. Pharmacol. 2020, 86, 761–772. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O'Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Grassilli, S.; Bertagnolo, V.; Brugnoli, F. Mir-29b in Breast Cancer: A Promising Target for Therapeutic Approaches. Diagnostics 2022, 12, 2139. [Google Scholar] [CrossRef]
- Yan, S.; Teng, L.; Du, J.; Ji, L.; Xu, P.; Zhao, W.; Tao, W. Long non-coding RNA DANCR aggravates breast cancer through the miR-34c/E2F1 feedback loop. Mol. Med. Rep. 2024, 29, 93. [Google Scholar] [CrossRef]
- Yang, X.; Fan, L.; Huang, J.; Li, Y. Plasma Exosome miR-203a-3p is a Potential Liquid Biopsy Marker for Assessing Tumor Progression in Breast Cancer Patients. Breast Cancer 2024, 16, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Y.; Hsu, R.J.; Liu, J.M.; Chen, S.C.; Liao, G.S.; Gao, H.W.; Yu, C.P. MicroRNA-382-5p aggravates breast cancer progression by regulating the RERG/Ras/ERK signaling axis. Oncotarget 2017, 8, 22443–22459. [Google Scholar] [CrossRef]
- Luo, W.; Zhou, Y.; Wang, J.; Wang, K.; Lin, Q.; Li, Y.; Xie, Y.; Li, M.; Xiong, L. YTHDF1’s Regulatory Involvement in Breast Cancer Prognosis, Immunity, and the ceRNA Network. Int. J. Mol. Sci. 2024, 25, 1879. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhou, F.; Zhou, H.; Pan, X.; Sun, Z.; Peng, G. MicroRNA-378g enhanced radiosensitivity of NPC cells partially by targeting protein tyrosine phosphatase SHP-1. Int. J. Radiat. Biol. 2015, 91, 859–866. [Google Scholar] [CrossRef]
- Wege, A.K.; Ernst, W.; Eckl, J.; Frankenberger, B.; Vollmann-Zwerenz, A.; Mannel, D.N.; Ortmann, O.; Kroemer, A.; Brockhoff, G. Humanized tumor mice--a new model to study and manipulate the immune response in advanced cancer therapy. Int. J. Cancer 2011, 129, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Wege, A.K.; Weber, F.; Kroemer, A.; Ortmann, O.; Nimmerjahn, F.; Brockhoff, G. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM). Oncotarget 2017, 8, 2731–2744. [Google Scholar] [CrossRef]
- Bender, M.; Chen, I.P.; Henning, S.; Degenhardt, S.; Mhamdi-Ghodbani, M.; Starzonek, C.; Volkmer, B.; Greinert, R. Knockdown of Simulated-Solar-Radiation-Sensitive miR-205-5p Does Not Induce Progression of Cutaneous Squamous Cell Carcinoma In Vitro. Int. J. Mol. Sci. 2023, 24, 16428. [Google Scholar] [CrossRef]


| Mice | Cord Blood Donor | Transplanted Cells | Tumor Volume ~ 50 mm3 (Days Post Tumor Transplant) | Treatment of Mice (Days Post Tumor Transplant) | Isolation of |
|---|---|---|---|---|---|
| Her2+ HTM | CB donor 7 | JIMT-1 | 30 | 6 Gy day 34 | Serum miRNA, Exosomal miRNA |
| Her2+ HTM | CB donor 7 | JIMT-1 | 34 | 6 Gy day 34 + anti-PD-L1 | Serum miRNA, Exosomal miRNA |
| Her2+ HTM | CB donor 7 | JIMT-1 | 37 | No treatment | Serum miRNA, Exosomal miRNA |
| TNBC HTM | CB donor 10 | MDA-MB-231 | 50 | 6 Gy day 55 | Serum miRNA |
| TNBC HTM | CB donor 10 | MDA-MB-231 | 71 | 6 Gy day 55 | Serum miRNA |
| TNBC HTM | CB donor 10 | MDA-MB-231 | 66 | No treatment | Serum miRNA |
| NSG (control) | No | No treatment | Serum miRNA |
| SERUM | IRRADIATED * | ICI * | PARTICLES/ML | MEAN DIAMETER [NM] |
|---|---|---|---|---|
| HER2+ HTM, JIMT-1 | + | − | 1.3 × 1010 | 80 |
| HER2+ HTM, JIMT-1 | + | + | 8.6 × 1010 | 78 |
| HER2+ HTM, JIMT-1 | − | − | 3 × 1010 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.-P.; Henning, S.; Bender, M.; Degenhardt, S.; Mhamdi Ghodbani, M.; Bergmann, A.K.; Volkmer, B.; Brockhoff, G.; Wege, A.K.; Greinert, R. Detection of Human Circulating and Extracellular Vesicle-Derived miRNAs in Serum of Humanized Mice Transplanted with Human Breast Cancer (HER2+ and TNBC) Cells—A Proof of Principle Investigation. Int. J. Mol. Sci. 2025, 26, 3629. https://doi.org/10.3390/ijms26083629
Chen I-P, Henning S, Bender M, Degenhardt S, Mhamdi Ghodbani M, Bergmann AK, Volkmer B, Brockhoff G, Wege AK, Greinert R. Detection of Human Circulating and Extracellular Vesicle-Derived miRNAs in Serum of Humanized Mice Transplanted with Human Breast Cancer (HER2+ and TNBC) Cells—A Proof of Principle Investigation. International Journal of Molecular Sciences. 2025; 26(8):3629. https://doi.org/10.3390/ijms26083629
Chicago/Turabian StyleChen, I-Peng, Stefan Henning, Marc Bender, Sarah Degenhardt, Mouna Mhamdi Ghodbani, Ann Kathrin Bergmann, Beate Volkmer, Gero Brockhoff, Anja K. Wege, and Rüdiger Greinert. 2025. "Detection of Human Circulating and Extracellular Vesicle-Derived miRNAs in Serum of Humanized Mice Transplanted with Human Breast Cancer (HER2+ and TNBC) Cells—A Proof of Principle Investigation" International Journal of Molecular Sciences 26, no. 8: 3629. https://doi.org/10.3390/ijms26083629
APA StyleChen, I.-P., Henning, S., Bender, M., Degenhardt, S., Mhamdi Ghodbani, M., Bergmann, A. K., Volkmer, B., Brockhoff, G., Wege, A. K., & Greinert, R. (2025). Detection of Human Circulating and Extracellular Vesicle-Derived miRNAs in Serum of Humanized Mice Transplanted with Human Breast Cancer (HER2+ and TNBC) Cells—A Proof of Principle Investigation. International Journal of Molecular Sciences, 26(8), 3629. https://doi.org/10.3390/ijms26083629







