The In Vitro Antioxidant and Immunomodulatory Effects of the Irish Monofloral Ivy and Heather Honey Varieties †
Abstract
1. Introduction
2. Results
2.1. Antioxidant Capacity of Irish Heather and Ivy Honey Varieties
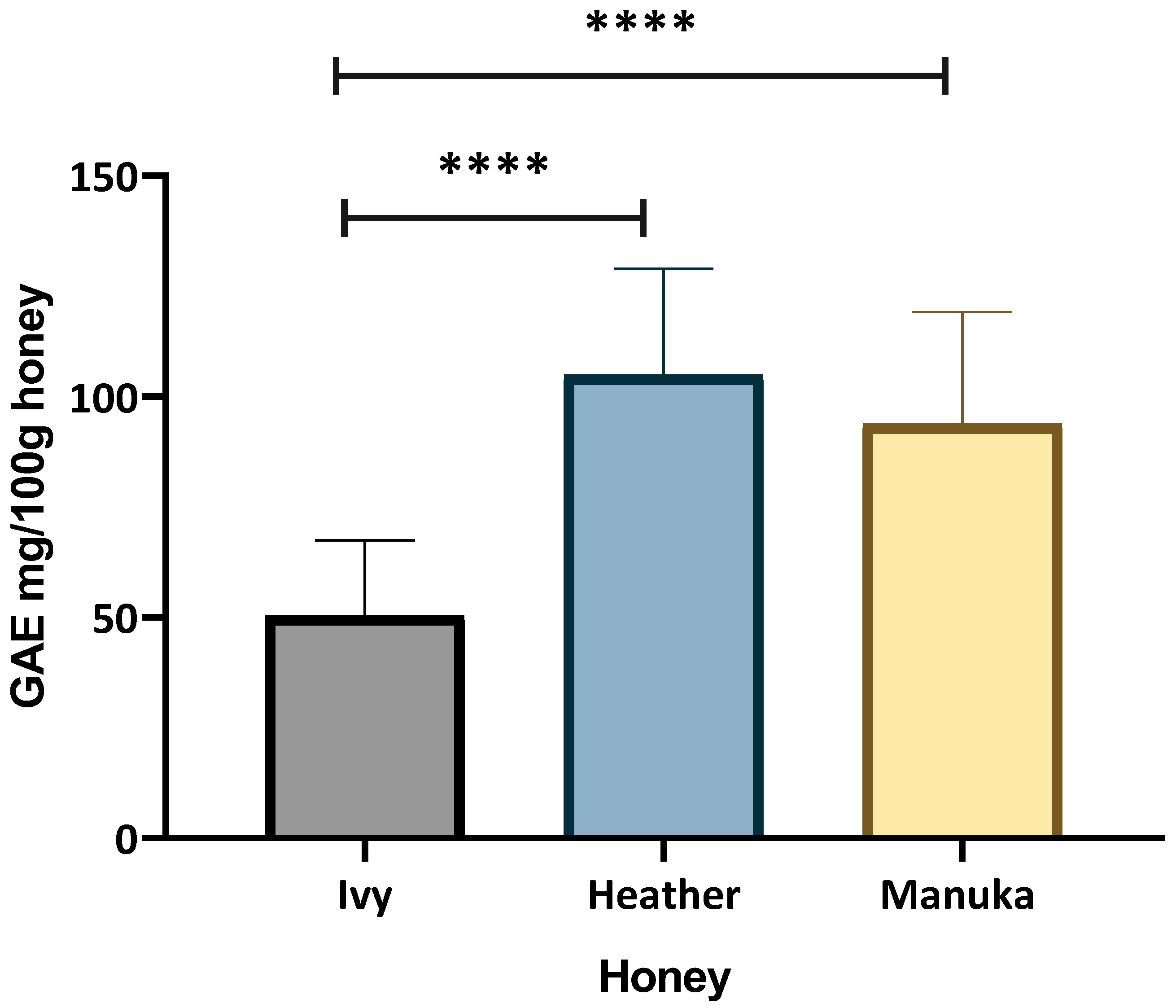
2.1.1. Total Phenolic Content
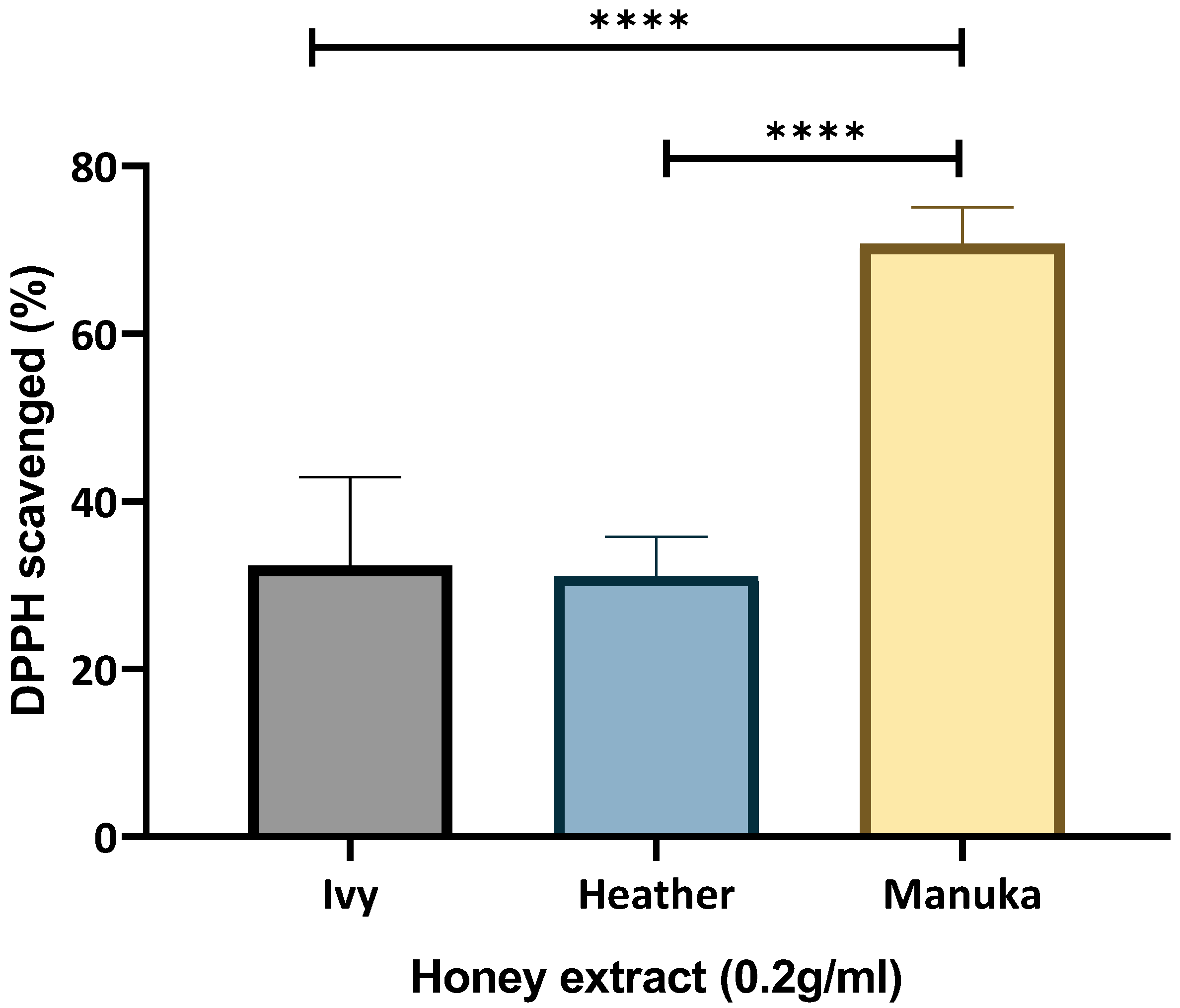
2.1.2. Free Radical Scavenging
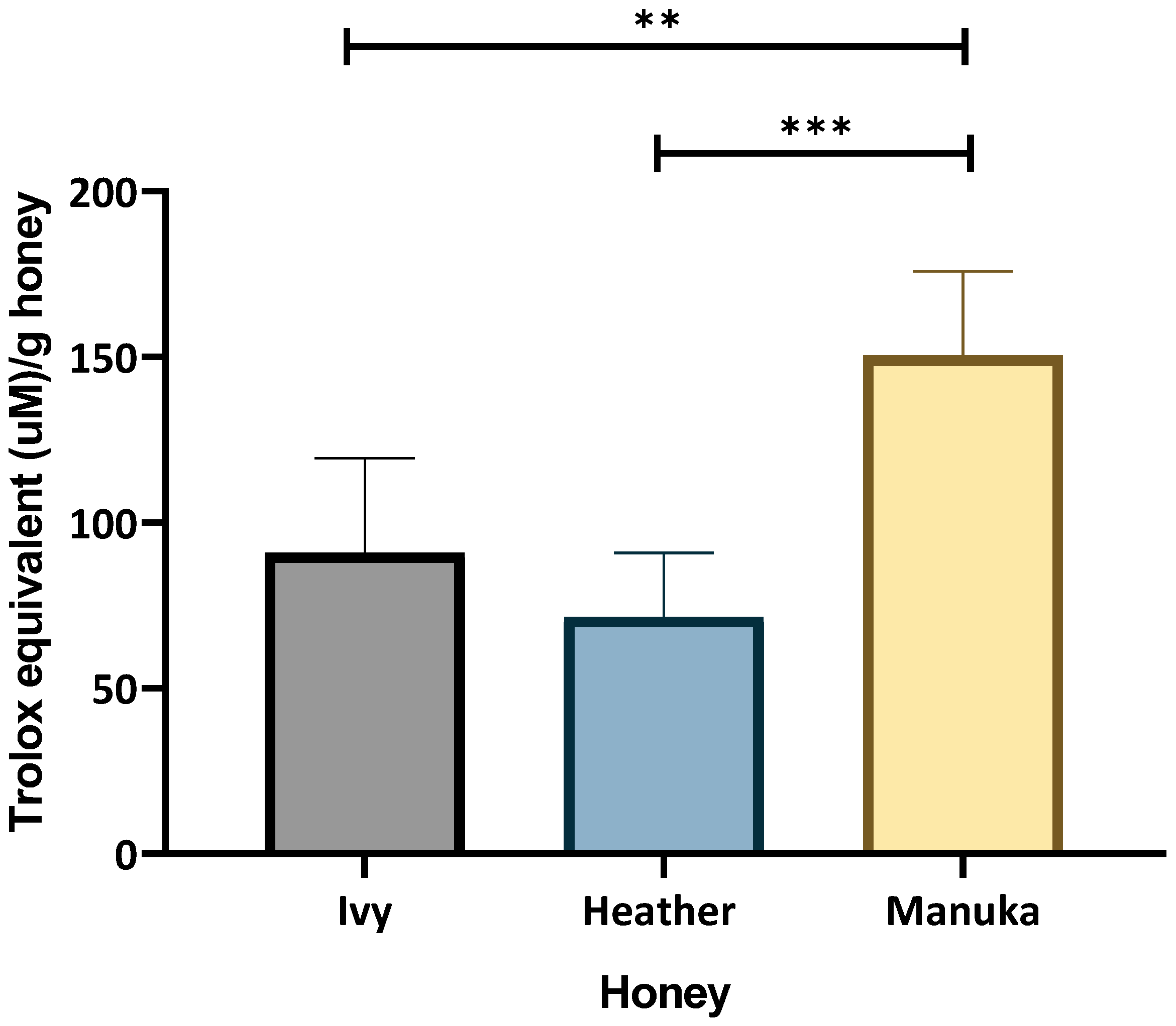
2.1.3. Peroxyl Radical Neutralising (ORAC) Assay
2.2. The Immunomodulatory and Antioxidant Effects of Irish Honey Samples
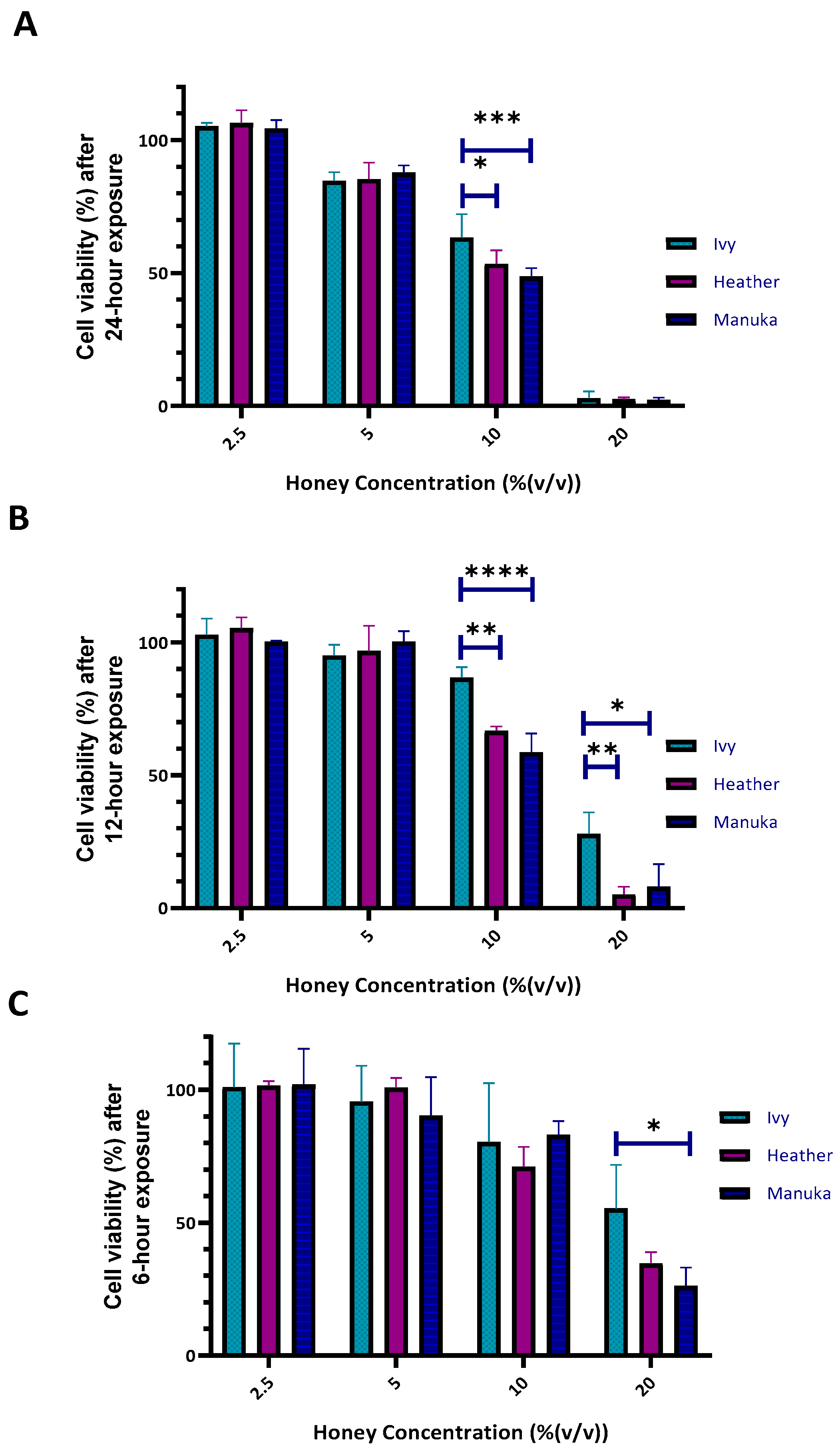
2.2.1. Cytotoxicity of Honey on Differentiated Macrophages (Resazurin Assay)
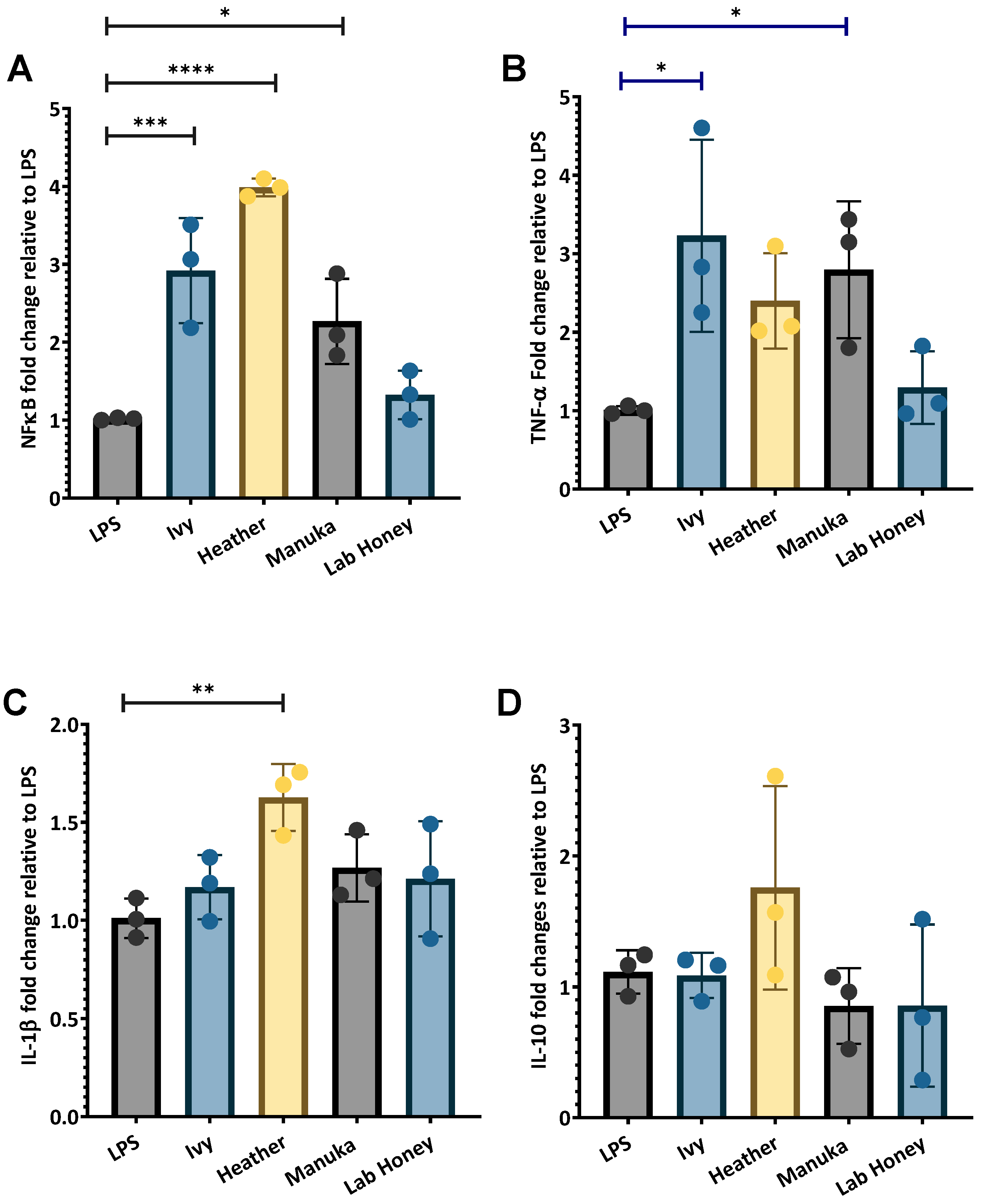
2.2.2. The Immunomodulatory Effects of Irish Ivy and Heather Honey Samples on Inflammatory Marker Gene Expression
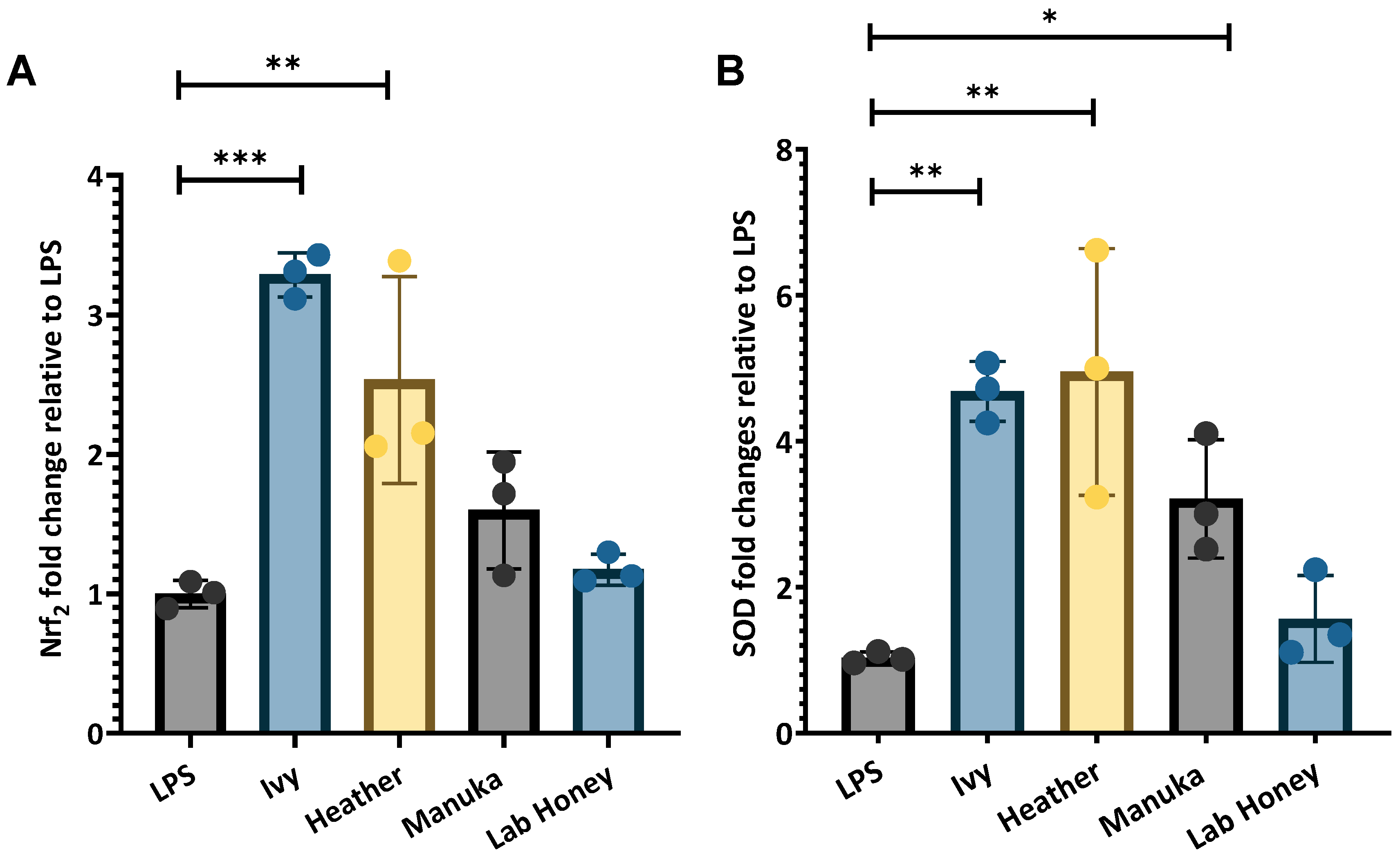
2.2.3. Response of Nrf2 Antioxidant Transcriptional Regulator and Downstream SOD to Honey Exposure
3. Discussion
3.1. Radical Scavenging Potential
3.2. Changes in Inflammatory and Antioxidant Marker Gene Expression by Irish Honey Varieties
3.2.1. Resazurin-Based Cytotoxicity Evaluation of Irish Honey
3.2.2. The Effects of Irish Honey Varieties on Pro- and Anti-Inflammatory Marker Gene Expression
3.2.3. Effects of Irish Honey Varieties on Antioxidant Transcription Factor and Associated Enzyme Expression
4. Materials and Methods
4.1. Materials
4.2. Honey Samples
4.3. Antioxidant Assays
4.3.1. Total Phenolic Content
4.3.2. Preparation of Honey Extracts for DPPH and ORAC Assays
4.3.3. DPPH Assay
4.3.4. The ORAC Assay
4.4. Immunomodulatory and Antioxidant Effects on Differentiated THP-1 Cells
4.4.1. Culture of THP-1 Cells
4.4.2. Honey Cytotoxicity
4.4.3. Gene Expression Analysis of M1 THP-1 Macrophages Exposed to Honey
| Gene | Sequence (5′-3′) | Reference |
|---|---|---|
| GAPDH | F-GGTGGTCTCCTCTGACTTCAACA | [93] |
| R-GTTGCTG TAGCCAAATTCGTTGC | ||
| RPL37A | F-ATTGAAATCAGCCAGCACGC | [92] |
| R-AGGAACCACAGTGCCAGATCC | ||
| Β-actin | F-ATTGCCGACAGGATGCAGAA | [92] |
| R-GCTGATCCACATCTGCTGGAA | ||
| NF-κB | F-TGAGTCCTGCTCCTTCCA | [94] |
| R-GCTTCGGTGTAGCCCATT | ||
| TNF-α | F-CCTCTCTCTAATCAGCCCTCTG | [95] |
| R-GAGGACCTGGGAGTAGATGAG | ||
| IL-1β | F-ATGATGGCTTATTACAGTGGCAA | [93] |
| R-GTCGGAGATTCGTAGCTGGA | ||
| IL-10 | F-GACTTTAAGGGTTACCTGGGTTG | [93] |
| R-TCACATGCGCCTTGATGTCT | ||
| Nrf2 | F-CTTGGCCTCAGTGATTCTGAAGTG | [96] |
| R-CCTGAGATGGTGACAAGGGTTGTA | ||
| Mn-SOD | F-GGGAGCACGCTTACTACCTTC | [97] |
| R-TCTTGCTGGGATCATTAGGGTAT |
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-Azobis(2-amidinopropane) dihydrochloride |
| AGE | Advanced glycation end-product |
| AMPK | AMP-activated Protein Kinase |
| AUC | Area under the curve |
| CD14 | Cluster of Differentiation 14 |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| DPPH RSA | DPPH radical scavenging activity |
| EC50 | Half-Maximal Effective Concentration |
| MAPK | Mitogen-Activated Protein Kinase |
| MGO | Methylglyoxal |
| MMP | Matrix Metalloproteinase |
| MRJP | Major royal jelly protein |
| NO | Nitric Oxide |
| ORAC | Oxygen Radical Absorbance Capacity |
| ROS | Reactive Oxygen Species |
| TLR | Toll-like Receptor |
| TPC | Total phenolic content |
| TE | Trolox equivalent |
| GAE | Gallic Acid Equivalent |
References
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-Bacterial, Anti-Biofilm and Anti-Quorum Sensing Activities of Honey: A Review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef] [PubMed]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef]
- Wilczyńska, A.; Żak, N. Polyphenols as the Main Compounds Influencing the Antioxidant Effect of Honey—A Review. Int. J. Mol. Sci. 2024, 25, 10606. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, E.Y.; Serin, E.K. Use of Honey in Diabetic Foot Ulcer: Systematic Review and Meta-Analysis. J. Tissue Viability 2023, 32, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Yaghoobi, R.; Kazerouni, A.; Kazerouni, O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-Bacterial, Anti-Inflammatory Anti-Oxidant and Anti-Viral Agent: A Review. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 100–104. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical Properties and Phenolic Content of Honey from Different Floral Origins and from Rural versus Urban Landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]
- Angioi, R.; Morrin, A.; White, B. Advantages of a Multifaceted Characterization of Honey, Illustrated with Irish Honey Marketed as Heather Honey. ACS Food Sci. Technol. 2024, 4, 606–616. [Google Scholar] [CrossRef]
- Farkas, Á.; Balázs, V.L.; Kõszegi, T.; Csepregi, R.; Kerekes, E.; Horváth, G.; Szabó, P.; Gaál, K.; Kocsis, M. Antibacterial and Biofilm Degradation Effects of Hungarian Honeys Linked with Botanical Origin, Antioxidant Capacity and Mineral Content. Front. Nutr. 2022, 9, 953470. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Dong, A.Z.; Levina, A.; Cokcetin, N.N.; Brooks, P.; Carter, D.A. Long-Term Stability and the Physical and Chemical Factors Predictive for Antimicrobial Activity in Australian Honey. PLoS ONE 2024, 19, e0303095. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Badrulhisham, N.S.R.; Ab Hamid, S.N.P.; Ismail, M.A.H.; Yong, Y.K.; Zakuan, N.M.; Harith, H.H.; Saidi, H.I.; Nurdin, A. Harvested Locations Influence the Total Phenolic Content, Antioxidant Levels, Cytotoxic, and Anti-Inflammatory Activities of Stingless Bee Honey. J. Asia-Pac. Entomol. 2020, 23, 950–956. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Seraglio, S.K.T.; Bergamo, G.; Rocha, G.d.O.; Valese, A.C.; Daguer, H.; Miotto, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Physicochemical Properties and Biological Activities of Bracatinga Honeydew Honey from Different Geographical Locations. J. Food Sci. Technol. 2021, 58, 3417–3429. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional Analysis of Scottish Honeys with Antimicrobial Activity against Antibiotic-Resistant Bacteria Reveals Novel Antimicrobial Components. LWT Food Sci. Technol. 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Starowicz, M.; Ostaszyk, A.; Zieliński, H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021, 10, 967. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Karpińska, E.; Moskwa, J.; Socha, K. Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants 2022, 11, 1312. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Mărghitaş, L.A.; Fiţ, N.; Chirilă, F.; Gherman, B.; Mărgăoan, R.; Aurori, A.; Bobiş, O. Antibacterial Effect of Heather Honey (Calluna vulgaris) against Different Microorganisms of Clinical Importance. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2015, 72, 72–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakač, M.; Jovanov, P.; Marić, A.; Četojević-Simin, D.; Novaković, A.; Plavšić, D.; Škrobot, D.; Kovač, R. Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans. Antioxidants 2022, 11, 1120. [Google Scholar] [CrossRef]
- Carnwath, R.; Graham, E.M.; Reynolds, K.; Pollock, P.J. The Antimicrobial Activity of Honey against Common Equine Wound Bacterial Isolates. Vet. J. 2014, 199, 110–114. [Google Scholar] [CrossRef]
- Rodrigues, F.; Moreira, T.; Pinto, D.; Pimentel, F.B.; Costa, A.S.G.; Nunes, M.A.; Albuquerque, T.G.; Costaa, H.S.; Palmeira-de-Oliveira, A.; Oliveira, A.I.; et al. The Phytochemical and Bioactivity Profiles of Wild Calluna vulgaris L. Flowers. Food Res. Int. 2018, 111, 724–731. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.R.; Chisté, R.C.; Fernandes, E. Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris. Separations 2021, 8, 177. [Google Scholar] [CrossRef]
- Michalkiewicz, A.; Biesaga, M.; Pyrzynska, K. Solid-Phase Extraction Procedure for Determination of Phenolic Acids and Some Flavonols in Honey. J. Chromatogr. A 2008, 1187, 18–24. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; D’Acierno, A.; Caputo, L.; Amato, G.; De Feo, V.; Coppola, R.; Nazzaro, F. Polyphenols Content and in vitro α-Glycosidase Activity of Different Italian Monofloral Honeys, and Their Effect on Selected Pathogenic and Probiotic Bacteria. Microorganisms 2021, 9, 1694. [Google Scholar] [CrossRef]
- Kolaylı, S.; Baltas, N.; Sahin, H.; Karaoglu, S. Evaluation of Anti-Helicobacter Pylori Activity and Urease Inhibition by Some Turkish Authentic Honeys. J. Food Sci. Eng. 2017, 7, 67–73. [Google Scholar] [CrossRef]
- Tavakoli, M.; Tarkesh Esfahani, M.; Soltani, S.; Karamian, R.; Aliarabi, H. Effects of Ecological Factors on Phenolic Compounds in Salvia multicaulis Vahl (Lamiaceae). Biochem. Syst. Ecol. 2022, 104, 104484. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An Overview of Plant Phenolics and Their Involvement in Abiotic Stress Tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Navaei-Alipour, N.; Mastali, M.; Ferns, G.; Saberi-Karimian, M.; Ghayour-Mobarhan, M. The Effects of Honey on Pro- and Anti-Inflammatory Cytokines: A Narrative Review. Phytother. Res. PTR 2021, 35, 3690–3701. [Google Scholar] [CrossRef] [PubMed]
- Panezai, J.; Van Dyke, T.E. Resolution of Inflammation: Intervention Strategies and Future Applications. Toxicol. Appl. Pharmacol. 2022, 449, 116089. [Google Scholar] [CrossRef]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-Derived Antibacterial Peptide, Defensin-1, Promotes Wound Re-Epithelialisation in vitro and in Vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef]
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of Honey and Its Major Royal Jelly Protein 1 on Cytokine and MMP-9 mRNA Transcripts in Human Keratinocytes. Exp. Dermatol. 2010, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Majtán, J.; Kováčová, E.; Bíliková, K.; Šimúth, J. The Immunostimulatory Effect of the Recombinant Apalbumin 1-Major Honeybee Royal Jelly Protein-on TNFα Release. Int. Immunopharmacol. 2006, 6, 269–278. [Google Scholar] [CrossRef]
- Gannabathula, S.; Skinner, M.A.; Rosendale, D.; Greenwood, J.M.; Mutukumira, A.N.; Steinhorn, G.; Stephens, J.; Krissansen, G.W.; Schlothauer, R.C. Arabinogalactan Proteins Contribute to the Immunostimulatory Properties of New Zealand Honeys. Immunopharmacol. Immunotoxicol. 2012, 34, 598–607. [Google Scholar] [CrossRef]
- Gasparrini, M.; Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective Effects of Manuka Honey on LPS-Treated RAW 264.7 Macrophages. Part 2: Control of Oxidative Stress Induced Damage, Increase of Antioxidant Enzyme Activities and Attenuation of Inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef]
- Browne, E.; Kavanagh, S.; Devery, S. The Cytotoxicity of Phorbol 12-Myristate 13-Acetate and Lipopolysaccharide on THP-1 Cells and an Optimized Differentiation Protocol. Med. Sci. Forum 2022, 11, 5. [Google Scholar] [CrossRef]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical Properties, Antibacterial and Cellular Antioxidant Activities of Buckwheat Honey in Comparison to Manuka Honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef]
- Ndungu, N.N.; Kegode, T.M.; Kurgat, J.K.; Baleba, S.B.S.; Cheseto, X.; Turner, S.; Tasse Taboue, G.C.; Kasina, J.M.; Subramanian, S.; Nganso, B.T. Bio-Functional Properties and Phytochemical Composition of Selected Apis Mellifera Honey from Africa. Heliyon 2024, 10, e30839. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef] [PubMed]
- Jasicka-Misiak, I.; Gruyaert, S.; Poliwoda, A.; Kafarski, P. Chemical Profiling of Polyfloral Belgian Honey: Ellagic Acid and Pinocembrin as Antioxidants and Chemical Markers. J. Chem. 2017, 2017, 5393158. [Google Scholar] [CrossRef]
- Broznić, D.; Ratkaj, I.; Staver, M.M.; Pavelić, S.K.; Žurga, P.; Bubalo, D.; Gobin, I. Evaluation of the Antioxidant Capacity, Antimicrobial and Antiproliferative Potential of Fir (Abies Alba Mill.) Honeydew Honey Collected from Gorski Kotar (Croatia). Food Technol. Biotechnol. 2018, 56, 533–545. [Google Scholar] [CrossRef]
- Polak-Śliwińska, M.; Tańska, M. Conventional and Organic Honeys as a Source of Water- and Ethanol-Soluble Molecules with Nutritional and Antioxidant Characteristics. Molecules 2021, 26, 3746. [Google Scholar] [CrossRef] [PubMed]
- Biluca, F.C.; de Gois, J.S.; Schulz, M.; Braghini, F.; Gonzaga, L.V.; Maltez, H.F.; Rodrigues, E.; Vitali, L.; Micke, G.A.; Borges, D.L.G.; et al. Phenolic Compounds, Antioxidant Capacity and Bioaccessibility of Minerals of Stingless Bee Honey (Meliponinae). J. Food Compos. Anal. 2017, 63, 89–97. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules 2021, 26, 2825. [Google Scholar] [CrossRef]
- Kocsis, M.; Bodó, A.; Kőszegi, T.; Csepregi, R.; Filep, R.; Hoffmann, G.; Farkas, Á. Quality Assessment of Goldenrod, Milkweed and Multifloral Honeys Based on Botanical Origin, Antioxidant Capacity and Mineral Content. Int. J. Mol. Sci. 2022, 23, 769. [Google Scholar] [CrossRef]
- Majtan, J. In vitro Testing of Honey Quality and Biological Functionality: Underestimated Elements in the Clinical Testing of Honey. Front. Nutr. 2024, 11, 1433786. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-Mediated Production of Hydrogen Peroxide Is Crucial for High Antibacterial Activity of Honeydew Honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Marshall, S.M.; Schneider, K.R.; Cisneros, K.V.; Gu, L. Determination of Antioxidant Capacities, α-Dicarbonyls, and Phenolic Phytochemicals in Florida Varietal Honeys Using HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2014, 62, 8623–8631. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Ki, S.H.; Shin, S.M. Methylglyoxal Induces Mitochondrial Dysfunction and Cell Death in Liver. Toxicol. Res. 2014, 30, 193–198. [Google Scholar] [CrossRef]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The Role of Methylglyoxal and the Glyoxalase System in Diabetes and Other Age-Related Diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Prestes, A.d.S.; dos Santos, M.M.; Kamdem, J.P.; Mancini, G.; da Silva, L.C.S.; de Bem, A.F.; Barbosa, N.V. Methylglyoxal Disrupts the Functionality of Rat Liver Mitochondria. Chem. Biol. Interact. 2022, 351, 109677. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.T.; Gonzalez, E.D.J.L.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Battino, M.; Giampieri, F. Protective Effects of Manuka Honey on LPS-Treated RAW 264.7 Macrophages. Part 1: Enhancement of Cellular Viability, Regulation of Cellular Apoptosis and Improvement of Mitochondrial Functionality. Food Chem. Toxicol. 2018, 121, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.C.; Yaacob, M.; Rajab, N.F.; Shahar, S.; Sharif, R. The Stingless Bee Honey Protects against Hydrogen Peroxide-Induced Oxidative Damage and Lipopolysaccharide-Induced Inflammation in vitro. Saudi J. Biol. Sci. 2021, 28, 2987–2994. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-kappaB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Raynaud, A.; Ghezali, L.; Gloaguen, V.; Liagre, B.; Quero, F.; Petit, J.M. Honey-Induced Macrophage Stimulation: AP-1 and NF-κB Activation and Cytokine Production Are Unrelated to LPS Content of Honey. Int. Immunopharmacol. 2013, 17, 874–879. [Google Scholar] [CrossRef]
- Webster, J.D.; Vucic, D. The Balance of TNF Mediated Pathways Regulates Inflammatory Cell Death Signaling in Healthy and Diseased Tissues. Front. Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Tonks, A.J.; Dudley, E.; Porter, N.G.; Parton, J.; Brazier, J.; Smith, E.L.; Tonks, A. A 5.8-kDa Component of Manuka Honey Stimulates Immune Cells via TLR4. J. Leukoc. Biol. 2007, 82, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.B.W.; Basu, S.; Vivekanand, P. Honey Gold Nanoparticles Attenuate the Secretion of IL-6 by LPS-Activated Macrophages. PLoS ONE 2023, 18, e0291076. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 Signalling by Manuka Honey Protects Human Dermal Fibroblasts against Oxidative Damage by Improving Antioxidant Response and Mitochondrial Function Promoting Wound Healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey Stimulates Inflammatory Cytokine Production from Monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Wusiman, A.; He, J.; Zhu, T.; Liu, Z.; Gu, P.; Hu, Y.; Liu, J.; Wang, D. Macrophage Immunomodulatory Activity of the Cationic Polymer Modified PLGA Nanoparticles Encapsulating Alhagi Honey Polysaccharide. Int. J. Biol. Macromol. 2019, 134, 730–739. [Google Scholar] [CrossRef]
- Zhang, H.; Kuchroo, V. Epigenetic and Transcriptional Mechanisms for the Regulation of IL-10. Semin. Immunol. 2019, 44, 101324. [Google Scholar] [CrossRef]
- Harakeh, S.; Saber, S.H.; Akefe, I.O.; Shaker, S.; Barkaat Hussain, M.; Almasaudi, A.S.; Saleh, S.M.M.; Almasaudi, S. Saudi Honey Alleviates Indomethacin-Induced Gastric Ulcer via Improving Antioxidant and Anti-Inflammatory Responses in Male Albino Rats. Saudi J. Biol. Sci. 2022, 29, 3040–3050. [Google Scholar] [CrossRef]
- Masad, R.J.; Nasser, R.A.; Bashir, G.; Mohamed, Y.A.; Al-Sbiei, A.; Al-Saafeen, B.H.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Characterization of Immunomodulatory Responses Induced by Manuka Honey. Front. Immunol. 2022, 13, 1020574. [Google Scholar] [CrossRef]
- Romário-Silva, D.; Lazarini, J.G.; Franchin, M.; de Alencar, S.M.; Rosalen, P.L. Brazilian Organic Honey from Atlantic Rainforest Decreases Inflammatory Process in Mice. Vet. Sci. 2022, 9, 268. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Alvarez-Suarez, J.M.; Ansary, J.; Quinzi, D.; Amici, A.; Navarro-Hortal, M.D.; Esteban-Muñoz, A.; Quiles, J.L.; Battino, M.; et al. Anti-Inflammatory Activities of Italian Chestnut and Eucalyptus Honeys on Murine RAW 264.7 Macrophages. J. Funct. Foods 2021, 87, 104752. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Ranneh, Y.; Mahmoud, A.M.; Fadel, A.; Albujja, M.; Akim, A.M.; Hamid, H.A.; Khazaai, H. Acute Inflammation and Oxidative Stress Induced by Lipopolysaccharide and the Ameliorative Effect of Stingless Bee Honey. Comb. Chem. High Throughput Screen. 2020, 24, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Arigela, C.S.; Nelli, G.; Gan, S.H.; Sirajudeen, K.N.S.; Krishnan, K.; Rahman, N.A.; Pasupuleti, V.R. Bitter Gourd Honey Ameliorates Hepatic and Renal Diabetic Complications on Type 2 Diabetes Rat Models by Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms. Foods 2021, 10, 2872. [Google Scholar] [CrossRef]
- Borrelli, A.; Schiattarella, A.; Bonelli, P.; Tuccillo, F.M.; Buonaguro, F.M.; Mancini, A. The Functional Role of MnSOD as a Biomarker of Human Diseases and Therapeutic Potential of a New Isoform of a Human Recombinant MnSOD. BioMed Res. Int. 2014, 2014, 476789. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, X.; Cao, M.; Zheng, S.; Ma, Y.; Huang, Q. NF-κB and AMPK-Nrf2 Pathways Support the Protective Effect of Polysaccharides from Polygonatum Cyrtonema Hua in Lipopolysaccharide-Induced Acute Lung Injury. J. Ethnopharmacol. 2022, 291, 115153. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, X.; Bo, J.; Lv, Z.; Li, G.; Chen, Y.; Liang, J.; Zhang, C.; Jin, X.; Liu, C.; et al. A Polysaccharide from Alhagi Honey Protects the Intestinal Barrier and Regulates the Nrf2/HO-1-TLR4/MAPK Signaling Pathway to Treat Alcoholic Liver Disease in Mice. J. Ethnopharmacol. 2024, 321, 117552. [Google Scholar] [CrossRef] [PubMed]
- Malkoç, M.; Yaman, S.Ö.; Imamoğlu, Y.; İnce, İ.; Kural, B.V.; Mungan, S.; Livaoglu, M.; Yıldız, O.; Kolaylı, S.; Orem, A. Anti-Inflammatory, Antioxidant and Wound-Healing Effects of Mad Honey in Streptozotocin-Induced Diabetic Rats. J. Apic. Res. 2020, 59, 426–436. [Google Scholar] [CrossRef]
- Abouzed, T.K.; Eldomany, E.B.; Khatab, S.A.; Aldhahrani, A.; Gouda, W.M.; Elgazzar, A.M.; Soliman, M.M.; Kassab, M.A.; El-Shazly, S.A.; Althobaiti, F.; et al. The Modulatory Effect of Bee Honey against Diethyl Nitrosamine and Carbon Tetrachloride Instigated Hepatocellular Carcinoma in Wistar Rats. Toxicol. Res. 2021, 10, 1092–1103. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Martin, A.C.; Harvey, W.J. The Global Pollen Project: A New Tool for Pollen Identification and the Dissemination of Physical Reference Collections. Methods Ecol. Evol. 2017, 8, 892–897. [Google Scholar] [CrossRef]
- Faegri, K.; Iversen, J. Textbook of Pollen Analysis; Blackwell: Hoboken, NJ, USA, 1964. [Google Scholar]
- Proaño, A.; Coello, D.; Villacrés-Granda, I.; Ballesteros, I.; Debut, A.; Vizuete, K.; Brenciani, A.; Álvarez-Suarez, J.M. The Osmotic Action of Sugar Combined with Hydrogen Peroxide and Bee-Derived Antibacterial Peptide Defensin-1 Is Crucial for the Antibiofilm Activity of Eucalyptus Honey. LWT 2021, 136, 110379. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. An Updated Review of Functional Ingredients of Manuka Honey and Their Value-Added Innovations. Food Chem. 2024, 440, 138060. [Google Scholar] [CrossRef] [PubMed]
- Nayik, G.A.; Suhag, Y.; Majid, I.; Nanda, V. Discrimination of High Altitude Indian Honey by Chemometric Approach According to Their Antioxidant Properties and Macro Minerals. J. Saudi Soc. Agric. Sci. 2018, 17, 200–207. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ali, A.; Chong, C.H.; Mah, S.H.; Abdullah, L.C.; Choong, T.S.Y.; Chua, B.L. Impact of Storage Conditions on the Stability of Predominant Phenolic Constituents and Antioxidant Activity of Dried Piper Betle Extracts. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 484. [Google Scholar] [CrossRef]
- Yasin, Z.N.M.; Idrus, F.N.M.; Hoe, C.H.; Yvonne-Tee, G.B. Macrophage Polarization in THP-1 Cell Line and Primary Monocytes: A Systematic Review. Differentiation 2022, 128, 67–82. [Google Scholar] [CrossRef]
- Nascimento, C.R.; Rodrigues Fernandes, N.A.; Gonzalez Maldonado, L.A.; Rossa Junior, C. Comparison of Monocytic Cell Lines U937 and THP-1 as Macrophage Models for in vitro Studies. Biochem. Biophys. Rep. 2022, 32, 101383. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Abel, S.D.A.; Baird, S.K. Honey Is Cytotoxic towards Prostate Cancer Cells but Interacts with the MTT Reagent: Considerations for the Choice of Cell Viability Assay. Food Chem. 2018, 241, 70–78. [Google Scholar] [CrossRef]
- Maeß, M.B.; Sendelbach, S.; Lorkowski, S. Selection of Reliable Reference Genes during THP-1 Monocyte Differentiation into Macrophages. BMC Mol. Biol. 2010, 11, 90. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, Y.; Sun, J.; Li, H.; Huang, M.; Sun, X.; Zhao, M. Elucidation of The Anti-Inflammatory Effect of Vanillin in Lps-Activated THP-1 Cells. J. Food Sci. 2019, 84, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.; Savelkoul, H.F.J.; Wichers, H.J. Transcription Profiles of LPS-Stimulated THP-1 Monocytes and Macrophages: A Tool to Study Inflammation Modulating Effects of Food-Derived Compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, J.; Zhao, M.; Sun, W.; Sun, J.; Li, H.; Huang, M. Immunomodulatory Activity of a Novel Polysaccharide Extracted from Huangshui on THP-1 Cells through NO Production and Increased IL-6 and TNF-α Expression. Food Chem. 2020, 330, 127257. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Jiang, J.; Zhang, J.; Zhang, L.; Wang, T. Curcumin Protects Human Trophoblast HTR8/SVneo Cells from H2O2-Induced Oxidative Stress by Activating Nrf2 Signaling Pathway. Antioxidants 2020, 9, 121. [Google Scholar] [CrossRef]
- Linares, C.; Ferrín, G.; Aguilar-Melero, P.; González Rubio, S.; Rodríguez-Perálvarez, M.; Sanchez Aragó, M.; Chicano-Galvez, E.; Cuezva, J.; Montero-Álvarez, J.; Muntané, J.; et al. Sensitivity to Anti-Fas Is Independent of Increased Cathepsin D Activity and Adrenodoxin Reductase Expression Occurring in NOS-3 Overexpressing HepG2 Cells. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2015, 1853, 1182–1194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browne, E.; Kavanagh, S.; Devery, S. The In Vitro Antioxidant and Immunomodulatory Effects of the Irish Monofloral Ivy and Heather Honey Varieties. Int. J. Mol. Sci. 2025, 26, 3625. https://doi.org/10.3390/ijms26083625
Browne E, Kavanagh S, Devery S. The In Vitro Antioxidant and Immunomodulatory Effects of the Irish Monofloral Ivy and Heather Honey Varieties. International Journal of Molecular Sciences. 2025; 26(8):3625. https://doi.org/10.3390/ijms26083625
Chicago/Turabian StyleBrowne, Emma, Siobhán Kavanagh, and Sinead Devery. 2025. "The In Vitro Antioxidant and Immunomodulatory Effects of the Irish Monofloral Ivy and Heather Honey Varieties" International Journal of Molecular Sciences 26, no. 8: 3625. https://doi.org/10.3390/ijms26083625
APA StyleBrowne, E., Kavanagh, S., & Devery, S. (2025). The In Vitro Antioxidant and Immunomodulatory Effects of the Irish Monofloral Ivy and Heather Honey Varieties. International Journal of Molecular Sciences, 26(8), 3625. https://doi.org/10.3390/ijms26083625








