Macrovipera lebetinus obtusa Venom and Its Fractions Affect Human Dermal Microvascular Endothelial and Fibrosarcoma Cells
Abstract
1. Introduction
2. Results
2.1. Fractionation of MLO Venom and Protein Identification
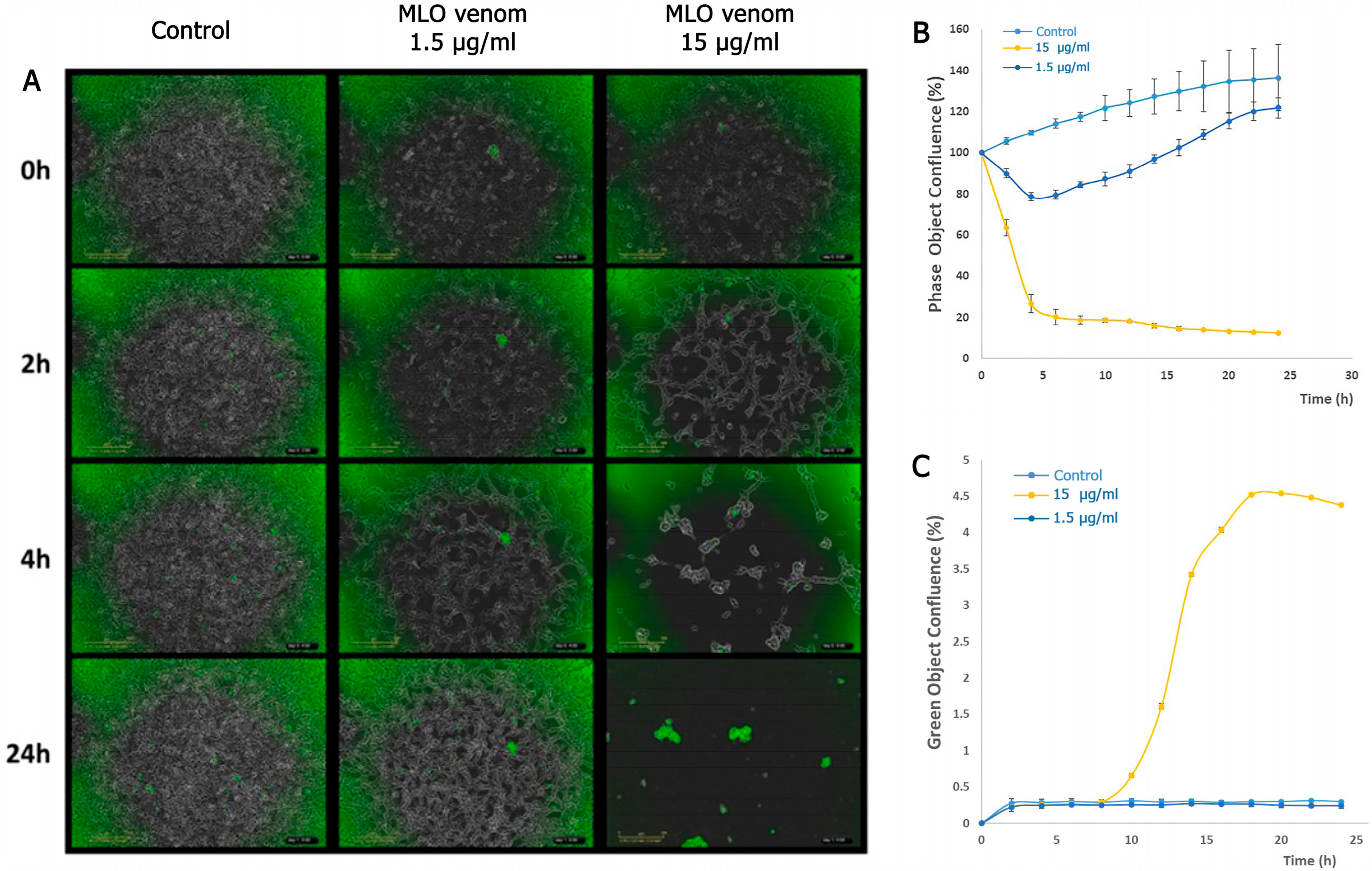
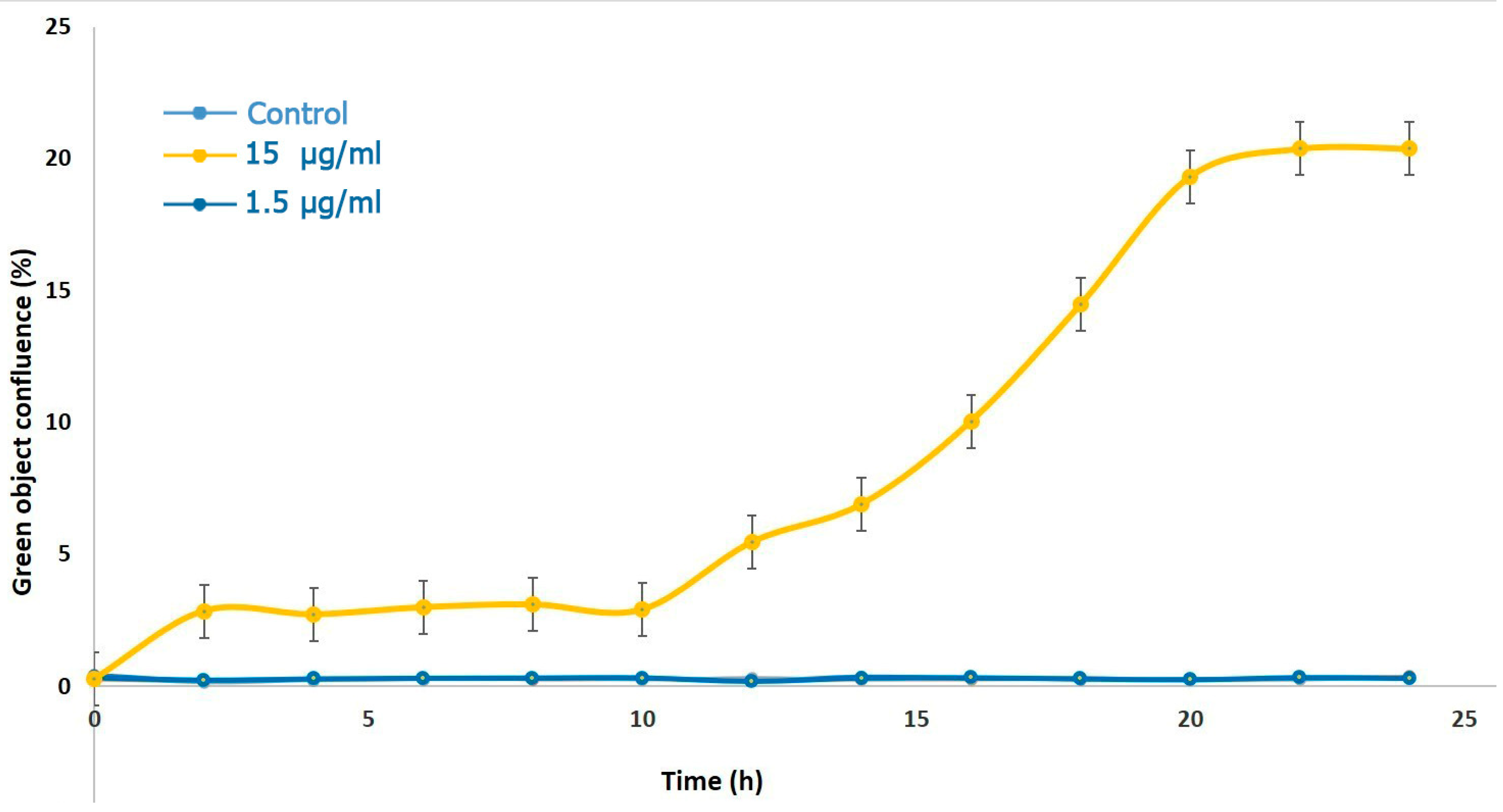
2.2. Detection of Cell Death by MLO Crude Venom
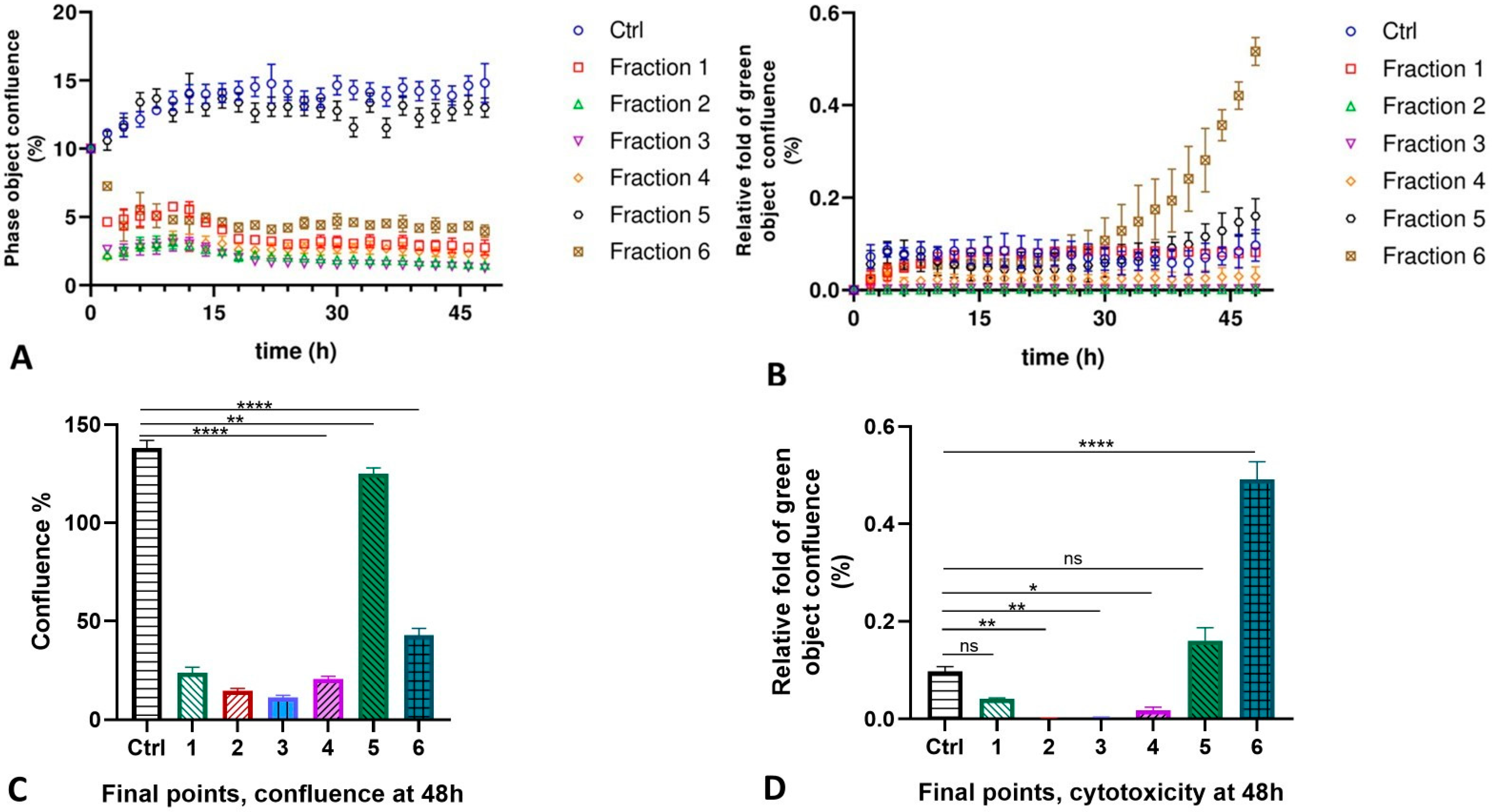
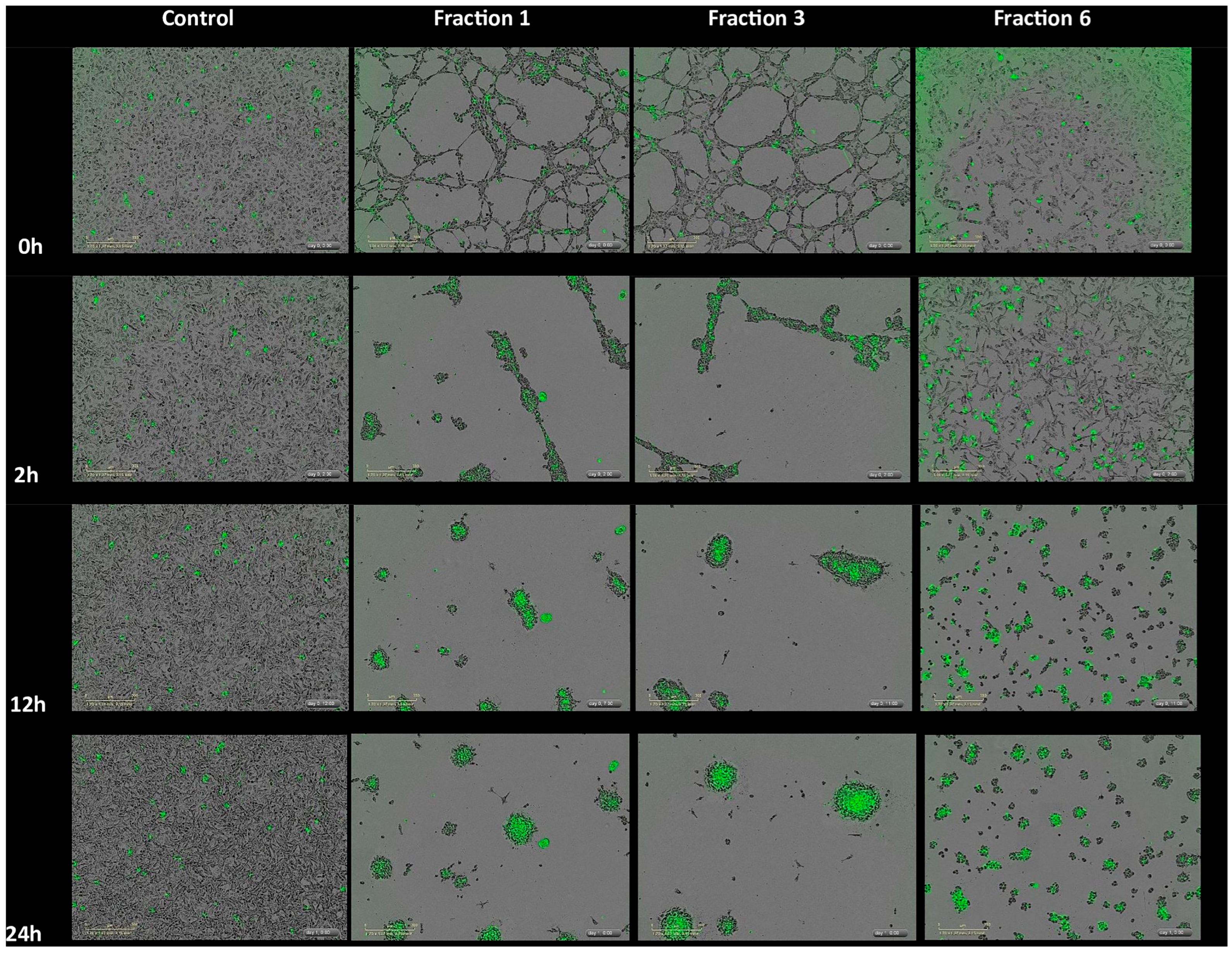
2.3. MLO Venom Fractions Effect on the HMVEC-D Cells
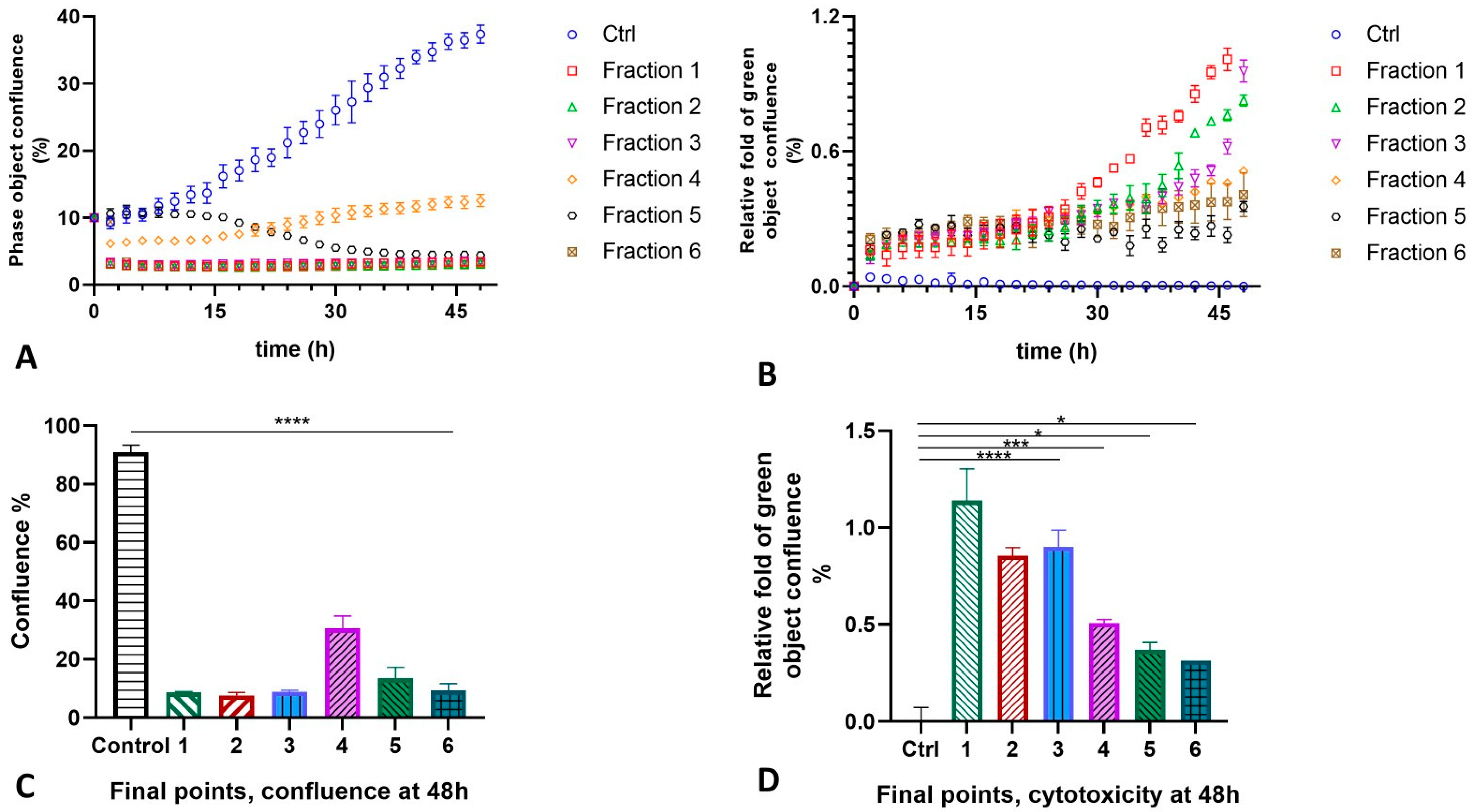
2.4. MLO Venom Fractions Effect on the HT-1080 Cells
3. Discussion
4. Materials and Methods
4.1. RP-HPLC for the Separation of MLO Venom Components
4.2. Gel Electrophoresis
4.3. Mass Spectrometric Protein Identification
4.4. IncuCyte Cytotox Green Detection of Cell Death
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLO | Macroipera lebetinus obtusa |
| HDEC/HMVEC-D | Human dermal microvascular endothelial cells |
| VEGF | Vascular endothelial growth factor |
| PLA2 | Phospholipase A2 |
| KTS/RGD | Lusine-threonine-serine/arginine-glycine-aspartic acid |
| CRISP | Cysteine-rich secretory protein |
References
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern venomics—Current insights, novel methods and future perspectives in biological and applied animal venom research. GigaScience 2022, 11, giac048. [Google Scholar] [CrossRef] [PubMed]
- Landová, E.; Bakhshaliyeva, N.; Janovcová, M.; Peléšková, Š.; Suleymanova, M.; Polák, J.; Guliev, A.; Frynta, D. Association Between Fear and Beauty Evaluation of Snakes: Cross-Cultural Findings. Front. Psychol. 2018, 9, 333. [Google Scholar] [CrossRef]
- Chowdhury, A.; Zdenek, C.N.; Dobson, J.S.; Bourke, L.A.; Soria, R.; Fry, B.G. Clinical implications of differential procoagulant toxicity of the palearctic viperid genus Macrovipera, and the relative neutralization efficacy of antivenoms and enzyme inhibitors. Toxicol. Lett. 2021, 340, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Stümpel, N.; Joger, U. Recent advances in phylogeny and taxonomy of near and Middle Eastern Vipers-an update. Zookeys 2008, 31, 179–191. [Google Scholar] [CrossRef]
- Damm, M.; Karış, M.; Petras, D.; Nalbantsoy, A.; Göçmen, B.; Süssmuth, R.D. Venomics and Peptidomics of Palearctic Vipers: A Clade-Wide Analysis of Seven Taxa of the Genera Vipera, Montivipera, Macrovipera, and Daboia across Türkiye. J. Proteome. Res. 2024, 23, 3524–3541. [Google Scholar] [CrossRef]
- Siigur, J.; Siigur, E. Biochemistry and toxicology of proteins and peptides purified from the venom of Vipera berus berus. Toxicon X 2022, 15, 100131. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Dufresnes, C.; Fritz, U.; Jablonski, D.; Lymberakis, P.; Martínez-Solano, I.; Razzetti, E.; Vamberger, M.; Vences, M.; et al. Species list of the European herpetofauna—2020 update by the Taxonomic Committee of the Societas Europaea Herpetologica. Amphibia-Reptilia 2020, 41, 139–189. [Google Scholar] [CrossRef]
- Sanz, L.; Ayvazyan, N.; Calvete, J.J. Snake venomics of the Armenian mountain vipers Macrovipera lebetina obtusa and Vipera raddei. J. Proteom. 2008, 71, 198–209. [Google Scholar] [CrossRef]
- Ghazaryan, N.; Movsisyan, N.; Macedo, J.C.; Vaz, S.; Ayvazyan, N.; Pardo, L.; Logarinho, E. The antitumor efficacy of monomeric disintegrin obtustatin in S-180 sarcoma mouse model. Investig. New Drugs. 2019, 193, 135–155. [Google Scholar] [CrossRef]
- Ghazaryan, N.; Ghulikyan, L.; Kishmiryan, A.; Kirakosyan, G.; Nazaryan, O.; Ghevondyan, T.; Zaqaryan, N.; Ayvazyan, N. Anti-tumor effect investigation of obtustatin and crude Macrovipera lebetina obtusa venom in S-180 sarcoma bearing mice. Eur. J. Pharmacol. 2015, 764, 340–345. [Google Scholar] [CrossRef]
- Brown, M.C.; Staniszewska, I.; Del Valle, L.; Tuszynski, G.P.; Marcinkiewicz, C. Angiostatic activity of obtustatin as alpha1beta1 integrin inhibitor in experimental melanoma growth. Int. J. Cancer 2008, 123, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Murciano, M.P.; Monleón, D.; Calvete, J.J.; Celda, B.; Marcinkiewicz, C. Amino acid sequence and homology modeling of obtustatin, a novel non-RGD-containing short disintegrin isolated from the venom of Vipera lebetina obtusa. Protein Sci. 2003, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, D.G.; Calvete, J.J.; Katzhendler, J.; Fertala, A.; Lazarovici, P.; Marcinkiewicz, C. Structural determinants of the selectivity of KTS-disintegrins for the alpha1beta1 integrin. FEBS. Lett. 2004, 577, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Marcinkiewicz, C.; Sanz, L. KTS and RTS-disintegrins: Anti-angiogenic viper venom peptides specifically targeting the alpha 1 beta 1 integrin. Curr. Pharm. Des. 2007, 13, 2853–2859. [Google Scholar] [CrossRef]
- Brown, M.C.; Eble, J.A.; Calvete, J.J.; Marcinkiewicz, C. Structural requirements of KTS-disintegrins for inhibition of alpha(1)beta(1) integrin. Biochem. J. 2009, 417, 95–101. [Google Scholar] [CrossRef]
- Watts, S.W.; Thompson, J.M.; Bhattacharya, S.; Panda, V.; Terrian, L.; Contreras, A.; Nault, R. Integrins play a role in stress relaxation of perivascular adipose tissue. Pharmacol. Res. 2024, 206, 107269. [Google Scholar] [CrossRef]
- Hunter, E.J.; Hamaia, S.W.; Gullberg, D.; Malcor, J.D.; Farndale, R.W. Selectivity of the collagen-binding integrin inhibitors, TC-I-15 and obtustatin. Toxicol. Appl. Pharmacol. 2021, 428, 115669. [Google Scholar] [CrossRef]
- Moraes, J.A.; Frony, A.C.; Dias, A.M.; Renovato-Martins, M.; Rodrigues, G.; Marcinkiewicz, C.; Assreuy, J.; Barja-Fidalgo, C. Alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Atherosclerosis 2015, 243, 477–485. [Google Scholar] [CrossRef]
- Horwacik, I.; Rokita, H. Modulation of interactions of neuroblastoma cell lines with extracellular matrix proteins affects their sensitivity to treatment with the anti-GD2 ganglioside antibody 14G2a. Int. J. Oncol. 2017, 50, 1899–1914. [Google Scholar] [CrossRef]
- Wang, J.Y.; Grabacka, M.; Marcinkiewicz, C.; Staniszewska, I.; Peruzzi, F.; Khalili, K.; Amini, S.; Reiss, K. Involvement of alpha1beta1 integrin in insulin-like growth factor-1-mediated protection of PC12 neuronal processes from tumor necrosis factor-alpha-induced injury. J. Neurosci. Res. 2006, 83, 7–18. [Google Scholar] [CrossRef]
- Guarin, J.R.; Fatherree, J.P.; Oudin, M.J. Chemotherapy treatment induces pro-invasive changes in liver ECM composition. Matrix. Biol. 2022, 112, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Savitri, C.; Ha, S.S.; Kwon, J.W.; Kim, S.H.; Kim, Y.M.; Park, H.M.; Kwon, H.; Ji, M.J.; Park, K. Human Fibroblast-Derived Matrix Hydrogel Accelerates Regenerative Wound Remodeling Through the Interactions with Macrophages. Adv. Sci. 2024, 11, e2305852. [Google Scholar] [CrossRef] [PubMed]
- Gawade, S.P. Therapeutic alternatives from venoms and toxins. Indian. J. Pharmacol. 2007, 39, 260–264. [Google Scholar] [CrossRef]
- Mackessy, S.P. Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kini, M.; Clemetson, K. Toxins and Hemostasis: From Bench to Bedside; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Fox, J.W.; Serrano, S.M.T. Snake toxins and hemostasis. Toxicon 2005, 45, 951–1181. [Google Scholar] [CrossRef]
- Ayvazyan, N.; Ghukasyan, G.; Ghulikyan, L.; Kirakosyan, G.; Sevoyan, G.; Voskanyan, A.; Karabekyan, Z. The Contribution of Phospholipase A2 and Metalloproteinases to the Synergistic Action of Viper Venom on the Bioenergetic Profile of Vero Cells. Toxins 2022, 14, 724. [Google Scholar] [CrossRef]
- Ghazaryan, N.A.; Ghulikyan, L.; Kishmiryan, A.; Andreeva, T.V.; Utkin, Y.N.; Tsetlin, V.I.; Lomonte, B.; Ayvazyan, N.M. Phospholipases a2 from Viperidae snakes: Differences in membranotropic activity between enzymatically active toxin and its inactive isoforms. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 463–468. [Google Scholar] [CrossRef]
- Kazaryan, N.A.; Gulikyan, L.; Lomonte, B.; Andreeva, T.V.; Tsetlin, V.I.; Utkin, Y.N.; Aivazyan, N.M. Comparative analysis of membranotropic properties of various phospholipases A2 from venom of snakes of the family viperidae. Dokl. Biochem. Biophys. 2014, 457, 125–127. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Wachtel, E.; Pereira, D.D.C.; Slagboom, J.; Casewell, N.R.; Jennings, P.; Kool, J.; Vonk, F.J. Development of a membrane-disruption assay using phospholipid vesicles as a proxy for the detection of cellular membrane degradation. Toxicon X 2024, 22, 100197. [Google Scholar] [CrossRef]
- Offor, B.C.; Piater, L.A. Snake venom toxins: Potential anticancer therapeutics. J. Appl. Toxicol. 2024, 44, 666–685. [Google Scholar] [CrossRef]
- Clissa, P.B.; Della-Casa, M.S.; Zychar, B.C.; Sanabani, S.S. The Role of Snake Venom Disintegrins in Angiogenesis. Toxins 2024, 16, 127. [Google Scholar] [CrossRef]
- Hboub, H.; Mrid, B.R.; Bouchmaa, N.; Oukkache, N.; El Fatimy, R. An in-depth exploration of snake venom-derived molecules for drug discovery in advancing antiviral therapeutics. Heliyon 2024, 10, e37321. [Google Scholar] [CrossRef] [PubMed]
- Daidone, I.; Aschi, M.; Patamia, M.; Bozzi, A.; Petruzzelli, R. Structural and dynamical properties of KTS-disintegrins: A comparison between Obtustatin and Lebestatin. Biopolymers 2013, 99, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Momic, T.; Katzehendler, J.; Benny, O.; Lahiani, A.; Cohen, G.; Noy, E.; Senderowitz, H.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vimocin and vidapin, cyclic KTS peptides, are dual antagonists of α1β1/α2β1 integrins with antiangiogenic activity. J. Pharmacol. Exp. Ther. 2014, 350, 506–519. [Google Scholar] [CrossRef]
- Tatulian, S.A. Toward Understanding Interfacial Activation of Secretory Phospholipase A2 (PLA2): Membrane Surface Properties and Membrane-Induced Structural Changes in the Enzyme Contribute Synergistically to PLA2 Activation. Biophys. J. 2001, 80, 789–800. [Google Scholar]
- Kini, R.M. Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism; John Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Ovsepian, S.V.; O’Leary, V.B.; Ayvazyan, N.M.; Al-Sabi, A.; Ntziachristos, V.; Dolly, J.O. Neurobiology and therapeutic applications of neurotoxins targeting transmitter release. Pharmacol. Ther. 2019, 193, 135–155. [Google Scholar] [CrossRef]
- McLane, M.A.; Marcinkiewicz, C.; Vijay-Kumar, S.; Wierzbicka-Patynowski, I.; Niewiarowski, S. Viper venom disintegrins and related molecules. Proc. Soc. Exp. Biol. Med. 1998, 219, 109–119. [Google Scholar] [CrossRef]
- Calvete, J.J. The continuing saga of snake venom disintegrins. Toxicon 2013, 62, 40–49. [Google Scholar] [CrossRef]
- Almeida, G.O.; de Oliveira, I.S.; Arantes, E.C.; Sampaio, S.V. Snake venom disintegrins update: Insights about new findings. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 18, e20230039. [Google Scholar] [CrossRef]
- Moreno-Murciano, P.; Monleón, D.; Marcinkiewicz, C.; Calvete, J.J.; Celda, B. NMR solution structure of the non-RGD disintegrin obtustatin. J. Mol. Biol. 2003, 329, 135–145. [Google Scholar] [CrossRef]
- Marcinkiewicz, C.; Weinreb, P.H.; Calvete, J.J.; Kisiel, D.G.; Mousa, S.A.; Tuszynski, G.P.; Lobb, R.R. Obtustatin: A potent selective inhibitor of alpha1beta1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003, 63, 2020–2023. [Google Scholar] [PubMed]
- Zakraoui, O.; Marcinkiewicz, C.; Aloui, Z.; Othman, H.; Grépin, R.; Haoues, M.; Essafi, M.; Srairi-Abid, N.; Gasmi, A.; Karoui, H.; et al. Lebein, a snake venom disintegrin, suppresses human colon cancer cells proliferation and tumor-induced angiogenesis through cell cycle arrest, apoptosis induction and inhibition of VEGF expression. Mol. Carcinog. 2017, 56, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Ben-Mabrouk, H.; Zouari-Kessentini, R.; Montassar, F.; Koubaa, Z.A.; Messaadi, E.; Guillonneau, X.; ElAyeb, M.; Srairi-Abid, N.; Luis, J.; Micheau, O.; et al. CC5 and CC8, two homologous disintegrins from Cerastes cerastes venom, inhibit in vitro and ex vivo angiogenesis. Cancer Res. J. Biol. Macromol. 2016, 86, 670–680. [Google Scholar] [CrossRef]
- Lucena, S.E.; Romo, K.; Suntravat, M.; Sánchez, E.E. Anti-angiogenic activities of two recombinant disintegrins derived from the Mohave and Prairie rattlesnakes. Toxicon 2014, 78, 10–17. [Google Scholar] [CrossRef]
- Saviola, A.J.; Burns, P.D.; Mukherjee, A.K.; Mackessy, S.P. The disintegrin tzabcanin inhibits adhesion and migration in melanoma and lung cancer cells. Int. J. Biol. Macromol. 2016, 88, 457–464. [Google Scholar] [CrossRef]
- Jahn, O.; Hesse, D.; Reinelt, M.; Kratzin, H.D. Technical innovations for the automated identification of gel-separated proteins by MALDI-TOF mass spectrometry. Anal. Bioanal. Chem. 2006, 386, 92–103. [Google Scholar] [CrossRef]
- Kusch, K.; Uecker, M.; Liepold, T.; Möbius, W.; Hoffmann, C.; Neumann, H.; Werner, H.B.; Jahn, O. Partial Immunoblotting of 2D-Gels: A Novel Method to Identify Post-Translationally Modified Proteins Exemplified for the Myelin Acetylome. Proteomes 2017, 5, 3. [Google Scholar] [CrossRef]
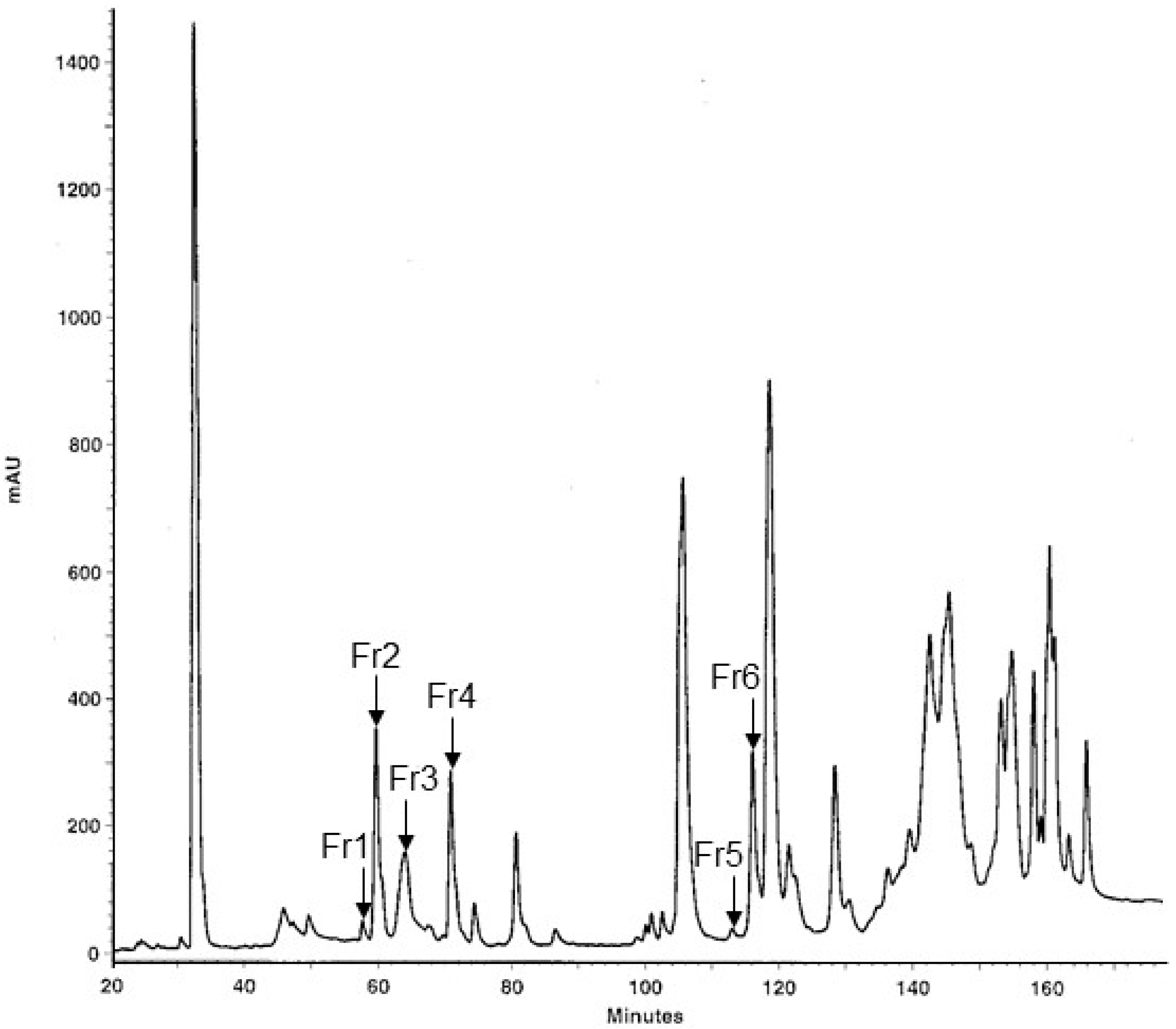
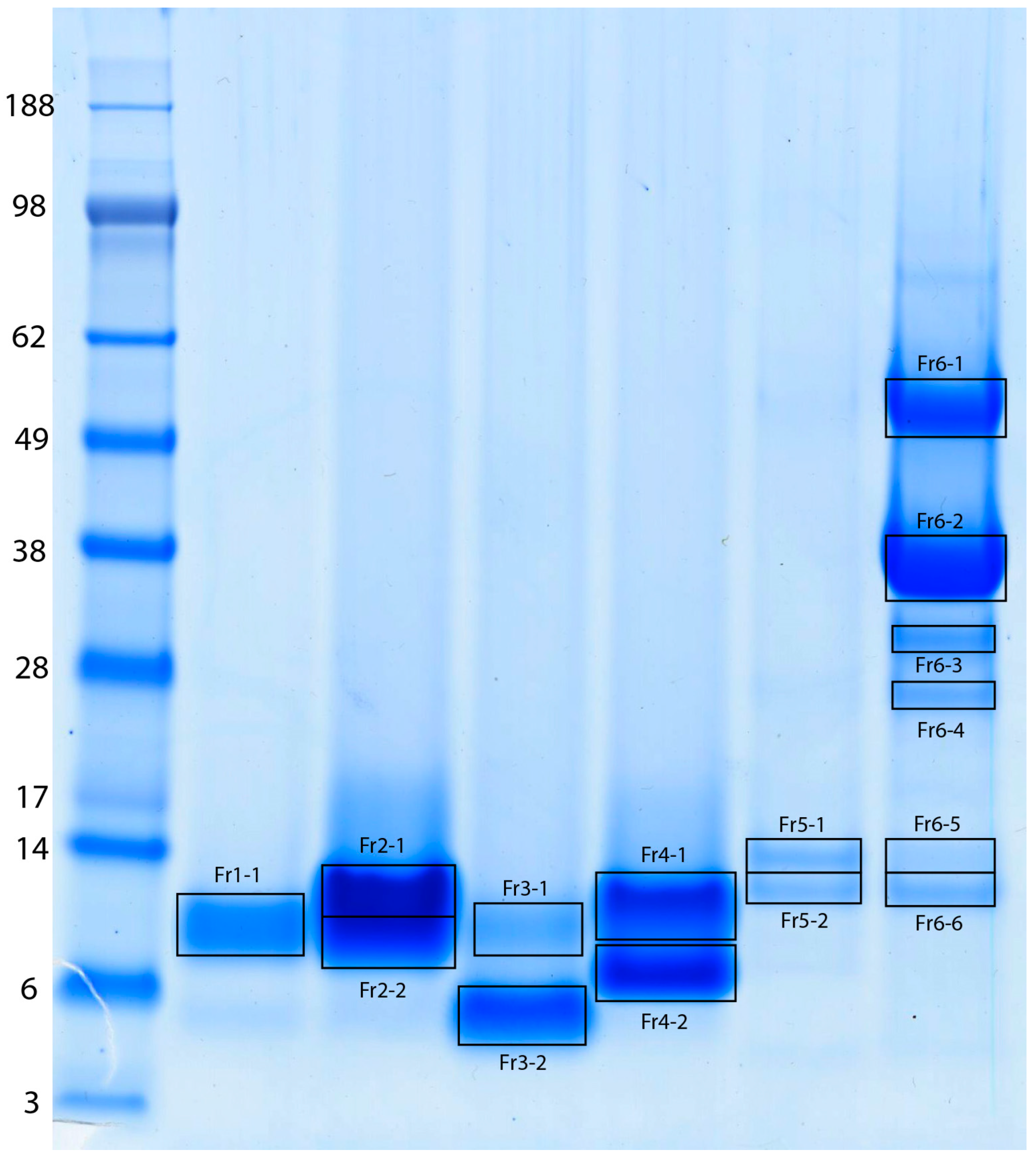
| Gel Band | Protein Name | UniProtKB Accession | UniProtKB ID | Species | MW [Da] | pI | PMF Score (a) | PMF % s.c. | Peptide Sequenced | MS/MS Ion Score (b) |
|---|---|---|---|---|---|---|---|---|---|---|
| Fr1-1 | Disintegrin lebein-1-alpha | P83253 | DID1A | MACLB | 12,718 | 7.42 | 46 | 39 | K.FLNAGTICNR.A R.RGEHCVSGPCCR.N R.GDDMNDYCTGISSDCPR.N | 71 62 141 |
| VGD-containing dimeric disintegrin subunit ML-G1 (Fragment) | Q1JRG9 | Q1JRG9 | MACLN | 7698 | 8.05 | 36 | 56 | R.RGEHCVSGPCCR.N R.AVGDDMDDYCTGISSDCPR.N | 62 146 | |
| Fr2-1 | Disintegrin lebein-1-alpha | P83253 | DID1A | MACLB | 12,718 | 7.42 | 57 | 39 | K.FLNAGTICNR.A R.RGEHCVSGPCCR.N R.GDDMNDYCTGISSDCPR.N | 70 60 144 |
| VGD-containing dimeric disintegrin subunit ML-G1 (Fragment) | Q1JRG9 | Q1JRG9 | MACLN | 7698 | 8.05 | 61 | 90 | R.RGEHCVSGPCCR.N R.AVGDDMDDYCTGISSDCPR.N | 60 151 | |
| Fr2-2 | Disintegrin lebein-1-alpha | P83253 | DID1A | MACLB | 12,718 | 7.42 | 67 | 39 | K.FLNAGTICNR.A R.GEHCVSGPCCR.N R.RGEHCVSGPCCR.N R.GDDMNDYCTGISSDCPR.N | 71 34 60 142 |
| Disintegrin VB7A | P0C6A6 | DID7A | VIPBB | 7574 | 8.10 | 76 | 68 | R.GEHCVSGPCCR.N R.RGEHCVSGPCCR.N -.NSGNPCCDPVTCKPR.R R.GDDMNDYCTGISSDCPR.N | 34 60 80 142 | |
| Disintegrin VLO4 | P0C6A8 | DID4 | MACLO | 7673 | 8.60 | 71 | 84 | R.GEHCVSGPCCR.N R.RGEHCVSGPCCR.N -.NSGNPCCDPVTCKPR.R -.MNSGNPCCDPVTCKPR.R | 34 60 80 77 | |
| VGD-containing dimeric disintegrin subunit ML-G1 (Fragment) | Q1JRG9 | Q1JRG9 | MACLN | 7698 | 8.05 | 51 | 70 | R.GEHCVSGPCCR.N R.RGEHCVSGPCCR.N R.AVGDDMDDYCTGISSDCPR.N | 34 60 110 | |
| Fr3-1 | Disintegrin lebein-1-alpha | P83253 | DID1A | MACLB | 12,718 | 7.42 | 49 | 56 | K.FLNAGTICNR.A R.GEHCVSGPCCR.N R.RGEHCVSGPCCR.N R.GDDMNDYCTGISSDCPR.N | 74 22 48 109 |
| Disintegrin VB7A | P0C6A6 | DID7A | VIPBB | 7574 | 8.10 | 57 | 68 | R.GEHCVSGPCCR.N R.RGEHCVSGPCCR.N -.NSGNPCCDPVTCKPR.R R.GDDMNDYCTGISSDCPR.N | 22 48 55 109 | |
| Fr3-2 | Disintegrin obtustatin | P83469 | DIS | MACLO | 4854 | 8.72 | 57 | 70 | -.CTTGPCCR.Q K.LKPAGTTCWK.T K.TSLTSHYCTGK.S K.LKPAGTTCWKTSLTSHYCTGK.S | 15 68 60 24 |
| Fr4-1 | Disintegrin VLO5A | P0C6A9 | VM25A | MACLO | 7570 | 8.11 | 80 | 87 | R.NCKFLR.A R.RGEHCVSGK.C -.NSGNPCCDPVTCQPR.R R.AVGDDMDDYCTGISSDCPR.N K.RAVGDDMDDYCTGISSDCPR.N | 24 31 36 151 58 |
| Disintegrin VB7B | P0C6A7 | VM27B | VIPBB | 7563 | 7.71 | 23 | 57 | -.ELLQNSGNPCCDPVTCKPR.E | 75 | |
| Fr4-2 | Disintegrin VLO5B | P0C6B0 | DID5B | MACLO | 8235 | 6.52 | 88 | 81 | K.FLNPGTICK.R K.FLNPGTICKR.T R.GEHCVSGPCCR.N M.NSANPCCDPITCKPR.R -.MNSANPCCDPITCKPR.R R.TMLDGLNDYCTGVTSDCPR.N | 53 30 25 54 83 155 |
| Disintegrin lebein-2-alpha | Q3BK13 | DID2A | MACLB | 14,638 | 7.45 | 43 | 32 | K.FLNPGTICK.R K.FLNPGTICKR.T K.GEHCVSGPCCR.N R.KGEHCVSGPCCR.N | 53 30 25 80 | |
| Fr5-1/2 | Acidic phospholipase A2 1 | C3W4R6 | PA2A1 | MACLB | 16,337 | 4.65 | 71 | 63 | K.YMLYSLFDCK.E K.YMLYSLFDCKEESEK.C R.CCFVHDCCYGSVNGCDPK.L K.LSTYSYSFQNGDIVCGDDDPCLR.A | 52 24 43 120 |
| Fr6-1 | Venom serine proteinase-like protein 2 | Q9PT40 | VSP2 | MACLB | 29,559 | 9.07 | 40 | 29 | R.FYCAGTLINQEWVLTAAR.C | 48 |
| Fr6-2 | Venom serine proteinase-like protein 2 | Q9PT40 | VSP2 | MACLB | 29,559 | 9.07 | 61 | 38 | R.WDKDIMLIR.L K.NVPNEDQQIR.V R.TLCAGILQGGIDSCK.V R.FYCAGTLINQEWVLTAAR.C K.VTYPDVPHCANINMFDYSVCR.K | 81 43 110 174 132 |
| Snake venom serine protease VaSP1 (Fragments) | P0DPS3 | VASP1 | VIPAA | 22,635 | 8.65 | 28 | 32 | R.WDKDIMLIR.L R.TLCAGILQGGIDSCK.G R.FYCAGTLINQEWVLTAAR.C -.VIGGDECNINEHPFLVALHTAR.X | 81 110 174 176 | |
| Fr6-3 | Factor V activator | Q9PT41 | VSPF5 | MACLB | 29,,261 | 8.81 | 68 | 46 | R.NEDEQIR.V K.FFCLNTK.F R.TLCAGILQGGK.D K.NSAHIAPISLPSSPSSPR.S K.ISTTEETYPDVPHCAK.I K.HAWCEALYPWVPADSR.T K.DTCEGDSGGPLICNGQIQGIVSGGSDPCGQR.L | 14 30 47 130 70 102 164 |
| Snake venom serine protease VaSP1 (Fragments) | P0DPS3 | VASP1 | VIPAA | 22,635 | 8.65 | 29 | 32 | R.FYCAGTLINQEWVLTAAR.C -.VIGGDECNINEHPFLVALHTAR.X | 76 129 | |
| Venom serine proteinase-like protein 2 | Q9PT40 | VSP2 | MACLB | 29,559 | 9.07 | 66 | 45 | R.FYCAGTLINQEWVLTAAR.C K.VTYPDVPHCANINMFDYSVCR.K | 76 43 | |
| Fr6-4 | Venom serine proteinase-like protein 2 | Q9PT40 | VSP2 | MACLB | 29,559 | 9.07 | 56 | 32 | K.FFCLSSK.T R.WDKDIMLIR.L R.IMGWGTITTTK.V K.NVPNEDQQIR.V R.FYCAGTLINQEWVLTAAR.C K.VTYPDVPHCANINMFDYSVCR.K | 26 81 41 29 115 97 |
| Snake venom serine protease VaSP1 (Fragments) | P0DPS3 | VASP1 | VIPAA | 22,635 | 8.65 | 22 | 24 | R.WDKDIMLIR.L R.FYCAGTLINQEWVLTAAR.C -.VIGGDECNINEHPFLVALHTAR.X | 81 115 139 | |
| Factor V activator | Q9PT41 | VSPF5 | MACLB | 29,261 | 8.81 | 20 | 22 | K.NSAHIAPISLPSSPSSPR.S | 108 | |
| Fr6-5 | L-amino-acid oxidase (Fragments) | P81375 | OXLA | MACLB | 12,541 | 4.52 | 23 | 31 | -.ADDKNPLEECFR.E | 47 |
| L-amino-acid oxidase | G8XQX1 | OXLA | DABRR | 57,251 | 8.55 | 41 | 21 | K.EGWYANLGPMR.V | 40 | |
| Fr6-6 | Acidic phospholipase A2 1 | C3W4R6 | PA2A1 | MACLB | 16,337 | 4.65 | 104 | 63 | K.YMLYSLFDCK.E R.VAAICFGENMNTYDK.K K.YMLYSLFDCKEESEK.C R.CCFVHDCCYGSVNGCDPK.L K.LSTYSYSFQNGDIVCGDDDPCLR.A | 56 87 44 31 98 |
| MACLB—Macrovipera lebetinus, MACLN—Macrovipera lebetinus transmediterranea, VIPBB—Vipera berus berus, MACLO—Macrovipera lebetinus obtusa, VIPAA—Vipera ammodytes ammodytes, DABRR—Daboia russelii | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazaryan, N.; Van Werven, L.; Liepold, T.; Jahn, O.; Pardo, L.A.; Ayvazyan, N. Macrovipera lebetinus obtusa Venom and Its Fractions Affect Human Dermal Microvascular Endothelial and Fibrosarcoma Cells. Int. J. Mol. Sci. 2025, 26, 3601. https://doi.org/10.3390/ijms26083601
Ghazaryan N, Van Werven L, Liepold T, Jahn O, Pardo LA, Ayvazyan N. Macrovipera lebetinus obtusa Venom and Its Fractions Affect Human Dermal Microvascular Endothelial and Fibrosarcoma Cells. International Journal of Molecular Sciences. 2025; 26(8):3601. https://doi.org/10.3390/ijms26083601
Chicago/Turabian StyleGhazaryan, Narine, Lars Van Werven, Thomas Liepold, Olaf Jahn, Luis A. Pardo, and Naira Ayvazyan. 2025. "Macrovipera lebetinus obtusa Venom and Its Fractions Affect Human Dermal Microvascular Endothelial and Fibrosarcoma Cells" International Journal of Molecular Sciences 26, no. 8: 3601. https://doi.org/10.3390/ijms26083601
APA StyleGhazaryan, N., Van Werven, L., Liepold, T., Jahn, O., Pardo, L. A., & Ayvazyan, N. (2025). Macrovipera lebetinus obtusa Venom and Its Fractions Affect Human Dermal Microvascular Endothelial and Fibrosarcoma Cells. International Journal of Molecular Sciences, 26(8), 3601. https://doi.org/10.3390/ijms26083601







