Connecting the Dots: How MicroRNAs Link Asthma and Atherosclerosis
Abstract
1. Introduction
2. MicroRNAs at the Crossroads of Human Health and Disease
3. Key MiRNAs Associated with Atherosclerosis
3.1. MiRNAs Involved in the Regulation of Endothelial Cell Function
3.2. MiRNAs Involved in the Regulation of Vascular Smooth Muscle Cells
3.3. MiRNAs Involved in the Regulation of Macrophages
3.4. MiRNAs Involved in the Regulation of Inflammation
3.5. MiRNAs Involved in the Regulation of Cholesterol Homeostasis
4. MicroRNAs as Modulators of Atherosclerosis Pathogenesis
4.1. MicroRNAs as Drivers of Atherosclerosis: Pro-Atherogenic Effects and Mechanisms
4.2. MicroRNAs as Guardians Against Atherosclerosis: Anti-Atherogenic Effects and Mechanisms
5. Dysregulated MicroRNAs in Asthma: Pathogenic Insights and Implications
5.1. Inflammatory Signaling Pathways Regulated by MicroRNAs in Asthma
5.2. Role in Regulating Asthma-Related Bronchoconstriction
5.3. Impact on Airway Epithelial Barrier Dysfunction
5.4. Allergic Sensitization and T Cell Polarization in Asthma Pathogenesis
5.5. Epithelial–Mesenchymal Crosstalk
5.6. The Interplay Between Oxidative Imbalance and Antioxidant Protection
5.7. Environmental Triggers and MicroRNA-Mediated Responses
6. MicroRNAs as Modulators of Asthma Pathogenesis
6.1. Pro-Asthmatic Pathways: Drivers of Airway Hyper-Responsiveness and Remodeling
6.2. Anti-Asthmatic Effects: Exploring Molecular Pathways and Functional Outcomes
7. Overlapping MiRNAs in Asthma and Atherosclerosis
8. Agomir and Antagomir Therapy: From Bench to Bedside—Unlocking the Potential of miRNA Modulation for Disease Targeting
9. Conclusion and Future Perspectives: Bridging Asthma and Atherosclerosis Through MiRNA Signaling
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-Binding Cassette Subfamily A Member 1 |
| ABCG1 | ATP-Binding Cassette Subfamily G Member 1 |
| ADAM17 | ADAM Metallopeptidase Domain 17 |
| ADAMTS-7 | A disintegrin and metalloproteinase with thrombospondin motifs 7 |
| AKT | Protein Kinase B |
| AMPK | AMP-Activated Protein Kinase |
| Angptl3 | Angiopoietin-like 3 |
| ATG6 | Autophagy-Related 6 (also known as Beclin-1) |
| BCL-6 | B-cell lymphoma 6 |
| Bim1 | BCL2 Interacting Mediator of Cell Death |
| c-Maf | c-Maf Transcription Factor |
| CCR3 | C-C Motif Chemokine Receptor 3 |
| CD | Cluster of Differentiation |
| CEBPβ | CCAAT/enhancer-binding protein beta |
| CLCA1 | Chloride Channel Accessory 1 |
| CNTN4 | Contactin 4 |
| COX-2 | Cyclooxygenase-2 |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| CTNND2 | Catenin Delta 2 |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 (also known as SDF-1) |
| DHCR24 | 24-Dehydrocholesterol Reductase |
| DOCK4 | Dedicator of Cytokinesis 4 |
| E2F1 | E2F1 Transcription Factor |
| EC | Endothelial Cells |
| ELK1 | ETS-Like Protein 1 |
| ELN | Elastin |
| eNOS | Endothelial Nitric Oxide Synthase |
| ErbB | Erb-B Receptor Tyrosine Kinase Family |
| Ets-1 | Ets-1 Transcription Factor |
| FGFR1 | Fibroblast Growth Factor Receptor 1 |
| FOXP3 | Forkhead Box P3 |
| Gpam | Glycerol-3-phosphate acyltransferase |
| GSK-3β | Glycogen Synthase Kinase 3 Beta |
| HAS-2 | Hyaluronan Synthase 2 |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl-Coenzyme A |
| HMGA2 | High Mobility Group AT-Hook 2 |
| HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| IGF2 | Insulin-like Growth Factor 2 |
| IKKα | IκB Kinase Alpha |
| IL | Interleukin |
| IRAK1 | Interleukin-1 Receptor-Associated Kinase 1 |
| IκBα | Inhibitor of kappa B Alpha |
| JAK2 | Janus Kinase 2 |
| KIF3A | Kinesin Family Member 3A |
| KLF2 | Kruppel-Like Factor 2 |
| KLF4 | Krüppel-like factor 4 |
| LOX-1 | Lectin-like Oxidized LDL Receptor-1 |
| LPP | LIM Domain-Containing Protein |
| MAP2K3 | Mitogen-Activated Protein Kinase Kinase 3 |
| MAP3K10 | Mitogen-Activated Protein Kinase Kinase Kinase 10 |
| MAP3K3 | Mitogen-Activated Protein Kinase Kinase Kinase 3 |
| MAP3K7 | Mitogen-Activated Protein Kinase Kinase Kinase 7 |
| MAPK | Mitogen-Activated Protein Kinase |
| MMP | Matrix Metalloproteinases |
| NEMO | NF-κB Essential Modulator |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NOX4 | NADPH oxidase 4 |
| OBF 1 | Oct binding factor 1 |
| ORMDL3 | ORM1-like 3 |
| P27KIP1 | Cyclin-Dependent Kinase Inhibitor 1B |
| PAK1 | p21-Activated Kinase 1 |
| PDCD4 | Programmed cell death protein 4 |
| PDGFR-β | Platelet-Derived Growth Factor Receptor Beta |
| PDK1 | 3-Phosphoinositide-Dependent Protein Kinase-1 |
| PP2A | Protein phosphatase 2A regulatory subunit B |
| Pparg | Peroxisome Proliferator-Activated Receptor Gamma |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PPM1A | Protein Phosphatase, Magnesium-Dependent 1A |
| pSTAT3 | Phosphorylated Signal Transducer and Activator of Transcription 3 |
| PTEN | Phosphatase and Tensin Homolog |
| Pu.1 | Purine Rich Box-1 Transcription Factor |
| RAS | Rat Sarcoma |
| RASA1 | RAS p21 Protein Activator 1 |
| Rb | Retinoblastoma Protein |
| RUNX3 | Runt-related transcription factor 3 |
| SC5D | Sterol-C5-Desaturase |
| SCARB1 | Scavenger Receptor Class B Type 1 |
| SFRP4 | Secreted Frizzled-Related Protein 4 |
| SIRT1 | Sirtuin 1 |
| SIX1 | SIX Homeobox 1 |
| SLC26A2 | Solute Carrier Family 26 Member 2 |
| SMAD3 | SMAD Family Member 3 |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| SOCS3 | Suppressor of Cytokine Signaling 3 |
| SOCS5 | Suppressor of Cytokine Signaling 5 |
| SOD2 | Superoxide Dismutase 2 |
| SOX6 | SRY-Box Transcription Factor 6 |
| SP-7 | Osterix |
| SPRY1 | Sprouty Homolog 1 |
| SREBP2 | Sterol Regulatory Element-Binding Protein 2 |
| STATs | Signal Transducers and Activators of Transcription |
| TAB2 | TAK1 Binding Protein 2 |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TIMP3 | Tissue Inhibitor of Metalloproteinases 3 |
| TLR4 | Toll-Like Receptor 4 |
| TPM4 | Tropomyosin 4 |
| TRAF6 | TNF Receptor-Associated Factor 6 |
| Vav3 | Vav3 Guanine Nucleotide Exchange Factor |
| VCAM1 | Vascular Cell Adhesion Molecule 1 |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| vSMC | Vascular smooth muscle cell |
| βTRC | Beta-Transducin Repeat-Containing |
References
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global Burden of Cardiovascular Diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, zwae281. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the Life Cycle of the Atherosclerotic Plaque. Cardiovasc. Res. 2021, 117, cvab303. [Google Scholar] [CrossRef]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and Unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Gans, M.D.; Gavrilova, T. Understanding the Immunology of Asthma: Pathophysiology, Biomarkers, and Treatments for Asthma Endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef]
- Vartak, T.; Kumaresan, S.; Brennan, E. Decoding microRNA Drivers in Atherosclerosis. Biosci. Rep. 2022, 42, BSR20212355. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting Key Proximal Drivers of Type 2 Inflammation in Disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Sahin, K.O. Pleiotropic Effects of Statins: New Evidences. Turk. Kardiyol. Dernegi Arsivi/Arch. Turk. Soc. Cardiol. 2021, 49, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F. Inhibition of the ANGPTL3/8 Complex for the Prevention and Treatment of Atherosclerotic Cardiovascular Disease. Curr. Atheroscler. Rep. 2024, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Maidman, S.D.; Hegele, R.A.; Rosenson, R.S. The Emerging Potential of Apolipoprotein C-III Inhibition for ASCVD Prevention: A State-of-the-Art Review. Curr. Atheroscler. Rep. 2024, 27, 3. [Google Scholar] [CrossRef]
- Szarek, M.; Bittner, V.A.; Aylward, P.; Baccara-Dinet, M.; Bhatt, D.L.; Diaz, R.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Harrington, R.A.; et al. Lipoprotein(a) Lowering by Alirocumab Reduces the Total Burden of Cardiovascular Events Independent of Low-Density Lipoprotein Cholesterol Lowering: ODYSSEY OUTCOMES Trial. Eur. Heart J. 2020, 41, 4245–4255. [Google Scholar] [CrossRef]
- Libby, P.; Everett, B.M. Novel Antiatherosclerotic Therapies. Arter. Thromb. Vasc. Biol. 2019, 39, 538–545. [Google Scholar] [CrossRef]
- Wohlford Th, G.F.; Van Tassell, B.W.; Ravindra, K.; Abbate, A. COLCOT and CANTOS: Piecing Together the Puzzle of Inflammation and Cardiovascular Events. Minerva Cardioangiol. 2020, 68, 5–8. [Google Scholar] [CrossRef]
- Kraler, S.; Wenzl, F.A.; Lüscher, T.F. Repurposing Colchicine to Combat Residual Cardiovascular Risk: The LoDoCo2 Trial. Eur. J. Clin. Investig. 2020, 50, e13424. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Suguro, S.; Suguro, R. MicroRNAs Regulate Function in Atherosclerosis and Clinical Implications. Oxidative Med. Cell Longev. 2023, 2023, 2561509. [Google Scholar] [CrossRef]
- Javadifar, A.; Rastgoo, S.; Banach, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Foam Cells as Therapeutic Targets in Atherosclerosis with a Focus on the Regulatory Roles of Non-Coding RNAs. Int. J. Mol. Sci. 2021, 22, 2529. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, A.; Rani, S. miRNA Biogenesis and Regulation of Diseases: An Updated Overview. In MicroRNA Profiling; Rani, S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2023; Volume 2595, ISBN 978-1-07-162822-5. [Google Scholar]
- AL-Noshokaty, T.M.; Fathi, D.; Abulsoud, A.I.; Moustafa, Y.M.; Abdel Mageed, S.S.; Mohammed, O.A.; Abdel-Reheim, M.A.; Abdelmaksoud, N.M.; Doghish, A.S. Harnessing the Power of miRNAs: The Molecular Architects of Asthma Pathogenesis and Potential Targets for Therapeutic Innovation. Pathol. Res. Pract. 2024, 253, 155054. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Panaitescu, C.; Haidar, L.; Buzan, M.R.; Grijincu, M.; Spanu, D.E.; Cojanu, C.; Laculiceanu, A.; Bumbacea, R.; Agache, I. Precision Medicine in the Allergy Clinic: The Application of Component Resolved Diagnosis. Expert Rev. Clin. Immunol. 2022, 18, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, R.; Dumitru, C.S.; Laza, R.; Besliu, R.S.; Gug, M.; Zara, F.; Laitin, S.M.D. The Role of Age and Comorbidity Interactions in COVID-19 Mortality: Insights from Cardiac and Pulmonary Conditions. J. Clin. Med. 2024, 13, 7510. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Sehmi, R.; Ambrose, C.S.; Griffiths, J.M. Thymic Stromal Lymphopoietin: Its Role and Potential as a Therapeutic Target in Asthma. Expert Opin. Ther. Targets 2020, 24, 777–792. [Google Scholar] [CrossRef]
- Carr, T.F.; Zeki, A.A.; Kraft, M. Eosinophilic and Noneosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 22–37. [Google Scholar] [CrossRef]
- Tliba, O.; Panettieri, R.A. Paucigranulocytic Asthma: Uncoupling of Airway Obstruction from Inflammation. J. Allergy Clin. Immunol. 2019, 143, 1287–1294. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Agache, I.; Bavbek, S.; Bakirtas, A.; Blanca, M.; Bochenek, G.; Bonini, M.; Heffler, E.; Klimek, L.; Laidlaw, T.M.; et al. Diagnosis and Management of NSAID-Exacerbated Respiratory Disease (N-ERD)—A EAACI Position Paper. Allergy 2019, 74, 28–39. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision Medicine and Phenotypes, Endotypes, Genotypes, Regiotypes, and Theratypes of Allergic Diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- Pelaia, C.; Pelaia, G.; Longhini, F.; Crimi, C.; Calabrese, C.; Gallelli, L.; Sciacqua, A.; Vatrella, A. Monoclonal Antibodies Targeting Alarmins: A New Perspective for Biological Therapies of Severe Asthma. Biomedicines 2021, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Zimbru, R.-I.; Zimbru, E.-L.; Ordodi, V.-L.; Bojin, F.-M.; Crîsnic, D.; Grijincu, M.; Mirica, S.-N.; Tănasie, G.; Georgescu, M.; Huțu, I.; et al. The Impact of High-Fructose Diet and Co-Sensitization to House Dust Mites and Ragweed Pollen on the Modulation of Airway Reactivity and Serum Biomarkers in Rats. Int. J. Mol. Sci. 2024, 25, 8868. [Google Scholar] [CrossRef]
- Sharma, R.; Tiwari, A.; McGeachie, M.J. Recent miRNA Research in Asthma. Curr. Allergy Asthma Rep. 2022, 22, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Boateng, E.; Kovacevic, D.; Oldenburg, V.; Rådinger, M.; Krauss-Etschmann, S. Role of Airway Epithelial Cell miRNAs in Asthma. Front. Allergy 2022, 3, 962693. [Google Scholar] [CrossRef] [PubMed]
- Maneechotesuwan, K. Role of microRNA in Severe Asthma. Respir. Investig. 2019, 57, 9–19. [Google Scholar] [CrossRef]
- Weidner, J.; Bartel, S.; Kılıç, A.; Zissler, U.M.; Renz, H.; Schwarze, J.; Schmidt-Weber, C.B.; Maes, T.; Rebane, A.; Krauss-Etschmann, S.; et al. Spotlight on microRNAs in Allergy and Asthma. Allergy 2021, 76, 1661–1678. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Nadeem, A.; Al-Harbi, M.M.; Imam, F.; Al-Shabanah, O.A.; Ahmad, S.F.; Sayed-Ahmed, M.M.; Bahashwan, S.A. Oxidative Airway Inflammation Leads to Systemic and Vascular Oxidative Stress in a Murine Model of Allergic Asthma. Int. Immunopharmacol. 2015, 26, 237–245. [Google Scholar] [CrossRef]
- Zimbru, E.-L.; Zimbru, R.-I.; Ordodi, V.-L.; Bojin, F.-M.; Crîsnic, D.; Andor, M.; Mirica, S.-N.; Huțu, I.; Tănasie, G.; Haidar, L.; et al. Rosuvastatin Attenuates Vascular Dysfunction Induced by High-Fructose Diets and Allergic Asthma in Rats. Nutrients 2024, 16, 4104. [Google Scholar] [CrossRef]
- Hirata, T. Asthma as Risk for Incident Cardiovascular Disease and Its Subtypes. Hypertens. Res. 2023, 46, 2056–2058. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tung, K.Y.; Tsai, C.H.; Su, M.W.; Wang, P.C.; Chen, C.H.; Lee, Y.L. Lipid Profiles in Children with and without Asthma: Interaction of Asthma and Obesity on Hyperlipidemia. Diabetes Metab. Syndr. Clin. Res. Rev. 2013, 7, 20–25. [Google Scholar] [CrossRef]
- Cazzola, M.; Hanania, N.A.; Rogliani, P.; Matera, M.G. Cardiovascular Disease in Asthma Patients: From Mechanisms to Therapeutic Implications. Kardiol. Pol. 2023, 81, 232–241. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, W.; Zheng, L. Genetic Liability to Asthma and Risk of Cardiovascular Diseases: A Mendelian Randomization Study. Front. Genet. 2022, 13, 879468. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Hernández, C.A.; Del Greco, M.F.; Sundaram, V.; Portas, L.; Minelli, C.; Bloom, C.I. Asthma and Incident Coronary Heart Disease: An Observational and Mendelian Randomisation Study. Eur. Respir. J. 2023, 62, 2301788. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Bansal, V.; Mahmood, R.; Kanagala, S.G.; Jain, R. Asthma and Cardiovascular Diseases: Uncovering Common Ground in Risk Factors and Pathogenesis. Cardiol. Rev. 2023, 33, 219–226. [Google Scholar] [CrossRef]
- Cîrnaţu, D.; Szentesi, S.G.; Cuc, L.D.; Ciurariu, E.; Bran, L.R.; Bâtcă-Dumitru, G.-C.; Joldes, C.S.R.; Pantea, M.F.; Pârvu, S. Investigation and Modeling of the Variables of the Decision to Vaccinate as the Foundation of an Algorithm for Reducing Vaccination Reluctance. Systems 2023, 11, 220. [Google Scholar] [CrossRef]
- Hosen, M.R.; Goody, P.R.; Zietzer, A.; Nickenig, G.; Jansen, F. MicroRNAs as Master Regulators of Atherosclerosis: From Pathogenesis to Novel Therapeutic Options. Antioxid. Redox Signal. 2020, 33, 621–644. [Google Scholar] [CrossRef]
- Plotnikova, O.; Baranova, A.; Skoblov, M. Comprehensive Analysis of Human microRNA–mRNA Interactome. Front. Genet. 2019, 10, 933. [Google Scholar] [CrossRef]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.-P.; et al. An Estimate of the Total Number of True Human miRNAs. Nucleic Acids Res. 2019, 47, 3353–3364. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007. [Google Scholar] [CrossRef] [PubMed]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. The miRNA–Target Interactions: An Underestimated Intricacy. Nucleic Acids Res. 2024, 52, 1544–1557. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The Nuclear RNase III Drosha Initiates microRNA Processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Görlich, D. Exportin 5 Is a RanGTP-Dependent dsRNA-Binding Protein That Mediates Nuclear Export of Pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hur, I.; Park, S.-Y.; Kim, Y.-K.; Suh, M.R.; Kim, V.N. The Role of PACT in the RNA Silencing Pathway. EMBO J. 2006, 25, 522–532. [Google Scholar] [CrossRef]
- Ho, S.-M. Environmental Epigenetics of Asthma: An Update. J. Allergy Clin. Immunol. 2010, 126, 453–465. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Cañas, J.A.; Rodrigo-Muñoz, J.M.; Sastre, B.; Gil-Martinez, M.; Redondo, N.; Del Pozo, V. MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2021, 11, 608666. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic Advances of miRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ntontsi, P.; Photiades, A.; Zervas, E.; Xanthou, G.; Samitas, K. Genetics and Epigenetics in Asthma. Int. J. Mol. Sci. 2021, 22, 2412. [Google Scholar] [CrossRef] [PubMed]
- Skuratovskaia, D.; Vulf, M.; Komar, A.; Kirienkova, E.; Litvinova, L. Promising Directions in Atherosclerosis Treatment Based on Epigenetic Regulation Using MicroRNAs and Long Noncoding RNAs. Biomolecules 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and Challenges in Translating the Biology of Atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Tian, Y.; Li, X.; Bai, C.; Yang, Z.; Zhang, L.; Luo, J. MiR-17-5p Promotes the Endothelialization of Endothelial Progenitor Cells to Facilitate the Vascular Repair of Aneurysm by Regulating PTEN-Mediated PI3K/AKT/VEGFA Pathway. Cell Cycle 2020, 19, 3608–3621. [Google Scholar] [CrossRef]
- Liu, D.; Sun, X.; Ye, P. miR-31 Overexpression Exacerbates Atherosclerosis by Targeting NOX4 in apoE-/- Mice. Clin. Lab. 2015, 61, 1617–1624. [Google Scholar] [CrossRef]
- Zeng, P.; Yang, J.; Liu, L.; Yang, X.; Yao, Z.; Ma, C.; Zhu, H.; Su, J.; Zhao, Q.; Feng, K.; et al. ERK1/2 Inhibition Reduces Vascular Calcification by Activating miR-126-3p-DKK1/LRP6 Pathway. Theranostics 2021, 11, 1129–1146. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Wu, Y.; Zuo, L.; Zhang, S.; Zhou, Q.; Wei, W.; Wang, Y.; Zhu, H. MicroRNA-126 Attenuates Palmitate-Induced Apoptosis by Targeting TRAF7 in HUVECs. Mol. Cell Biochem. 2015, 399, 123–130. [Google Scholar] [CrossRef]
- Sabzevari Rad, R.; Shirvani, H.; Mahmoodzadeh Hosseini, H.; Shamsoddini, A.; Samadi, M. Micro RNA-126 Promoting Angiogenesis in Diabetic Heart by VEGF/Spred-1/Raf-1 Pathway: Effects of High-Intensity Interval Training. J. Diabetes Metab. Disord. 2020, 19, 1089–1096. [Google Scholar] [CrossRef]
- Bassand, K.; Metzinger, L.; Naïm, M.; Mouhoubi, N.; Haddad, O.; Assoun, V.; Zaïdi, N.; Sainte-Catherine, O.; Butt, A.; Guyot, E.; et al. miR-126-3p Is Essential for CXCL12-induced Angiogenesis. J. Cell Mol. Med. 2021, 25, 6032–6045. [Google Scholar] [CrossRef]
- Daniel, J.-M.; Penzkofer, D.; Teske, R.; Dutzmann, J.; Koch, A.; Bielenberg, W.; Bonauer, A.; Boon, R.A.; Fischer, A.; Bauersachs, J.; et al. Inhibition of miR-92a Improves Re-Endothelialization and Prevents Neointima Formation Following Vascular Injury. Cardiovasc. Res. 2014, 103, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Samak, M.; Kaltenborn, D.; Kues, A.; Le Noble, F.; Hinkel, R.; Germena, G. Micro-RNA 92a as a Therapeutic Target for Cardiac Microvascular Dysfunction in Diabetes. Biomedicines 2021, 10, 58. [Google Scholar] [CrossRef]
- Linna-Kuosmanen, S.; Tomas Bosch, V.; Moreau, P.R.; Bouvy-Liivrand, M.; Niskanen, H.; Kansanen, E.; Kivelä, A.; Hartikainen, J.; Hippeläinen, M.; Kokki, H.; et al. NRF2 Is a Key Regulator of Endothelial microRNA Expression under Proatherogenic Stimuli. Cardiovasc. Res. 2021, 117, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Bonafè, M.; Spazzafumo, L.; Gobbi, M.; Prattichizzo, F.; Recchioni, R.; Marcheselli, F.; Sala, L.L.; Galeazzi, R.; Rippo, M.R.; et al. Age- and Glycemia-Related miR-126-3p Levels in Plasma and Endothelial Cells. Aging 2014, 6, 771–786. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Ortega, A.; Flores-Chova, A.; Sanchez-Garcia, B.; Garcia-Garcia, A.B.; Chaves, F.J.; Martin-Escudero, J.C.; Forner, M.J.; Redon, J.; Cortes, R. High miR-126-3p Levels Associated with Cardiovascular Events in a General Population. Eur. J. Intern. Med. 2023, 113, 49–56. [Google Scholar] [CrossRef]
- Marchegiani, F.; Recchioni, R.; Di Rosa, M.; Piacenza, F.; Marcheselli, F.; Bonfigli, A.R.; Galeazzi, R.; Matacchione, G.; Cardelli, M.; Procopio, A.D.; et al. Low Circulating Levels of miR-17 and miR-126-3p Are Associated with Increased Mortality Risk in Geriatric Hospitalized Patients Affected by Cardiovascular Multimorbidity. GeroScience 2023, 46, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Wang, Y. miR-126 Overexpression Attenuates Oxygen-glucose Deprivation/Reperfusion Injury by Inhibiting Oxidative Stress and Inflammatory Response via the Activation of SIRT1/Nrf2 Signaling Pathway in Human Umbilical Vein Endothelial Cells. Mol. Med. Rep. 2020, 23, 165. [Google Scholar] [CrossRef]
- Gao, H.; Yu, Z.; Li, Y.; Wang, X. miR-100-5p in Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Mediates Eosinophilic Inflammation to Alleviate Atherosclerosis via the FZD5/Wnt/β-Catenin Pathway. Acta Biochim. Biophys. Sin. 2021, 53, 1166–1176. [Google Scholar] [CrossRef]
- Shoeibi, S. Diagnostic and Theranostic microRNAs in the Pathogenesis of Atherosclerosis. Acta Physiol. 2020, 228, e13353. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zhao, Y.; Feng, Z. MicroRNA Control of P53. J. Cell Biochem. 2017, 118, 7–14. [Google Scholar] [CrossRef]
- Xu, S.; Wu, W.; Huang, H.; Huang, R.; Xie, L.; Su, A.; Liu, S.; Zheng, R.; Yuan, Y.; Zheng, H.; et al. The P53/miRNAs/Ccna2 Pathway Serves as a Novel Regulator of Cellular Senescence: Complement of the Canonical P53/P21 Pathway. Aging Cell 2019, 18, e12918. [Google Scholar] [CrossRef] [PubMed]
- Stelmaszyk, A.; Mikołajczak, P.; Dworacka, M. Sirtuin 1 as the Mechanism of Action of Agents Used in the Diabetes Mellitus Pharmacotherapy. Eur. J. Pharmacol. 2021, 907, 174289. [Google Scholar] [CrossRef]
- Dhahri, W.; Dussault, S.; Légaré, É.; Rivard, F.; Desjarlais, M.; Mathieu, R.; Rivard, A. Reduced Expression of microRNA-130a Promotes Endothelial Cell Senescence and Age-Dependent Impairment of Neovascularization. Aging 2020, 12, 10180–10193. [Google Scholar] [CrossRef]
- Vasa-Nicotera, M.; Chen, H.; Tucci, P.; Yang, A.L.; Saintigny, G.; Menghini, R.; Mahè, C.; Agostini, M.; Knight, R.A.; Melino, G.; et al. miR-146a Is Modulated in Human Endothelial Cell with Aging. Atherosclerosis 2011, 217, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wufuer, D.; Ding, J.; Wang, J. MicroRNA miR-146a-5p Inhibits the Inflammatory Response and Injury of Airway Epithelial Cells via Targeting TNF Receptor-Associated Factor 6. Bioengineered 2021, 12, 1916–1926. [Google Scholar] [CrossRef]
- Sun, D.; Xiang, G.; Wang, J.; Li, Y.; Mei, S.; Ding, H.; Yan, J. miRNA 146b-5p Protects Against Atherosclerosis by Inhibiting Vascular Smooth Muscle Cell Proliferation and Migration. Epigenomics 2020, 12, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a Inhibits Oxidized Low-Density Lipoprotein-Induced Lipid Accumulation and Inflammatory Response via Targeting Toll-like Receptor 4. FEBS Lett. 2011, 585, 854–860. [Google Scholar] [CrossRef]
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative Stress-Induced miR-200c Disrupts the Regulatory Loop Among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 2017, 27, 328–344. [Google Scholar] [CrossRef]
- Sapp, R.M.; Chesney, C.A.; Springer, C.B.; Laskowski, M.R.; Singer, D.B.; Eagan, L.E.; Mascone, S.E.; Evans, W.S.; Prior, S.J.; Hagberg, J.M.; et al. Race-Specific Changes in Endothelial Inflammation and microRNA in Response to an Acute Inflammatory Stimulus. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2371–H2384. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y.V. Human miR-221/222 in Physiological and Atherosclerotic Vascular Remodeling. BioMed Res. Int. 2015, 2015, 354517. [Google Scholar] [CrossRef]
- De Yébenes, V.G.; Briones, A.M.; Martos-Folgado, I.; Mur, S.M.; Oller, J.; Bilal, F.; González-Amor, M.; Méndez-Barbero, N.; Silla-Castro, J.C.; Were, F.; et al. Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arter. Thromb. Vasc. Biol. 2020, 40, 2408–2424. [Google Scholar] [CrossRef]
- Giuliani, A.; Londin, E.; Ferracin, M.; Mensà, E.; Prattichizzo, F.; Ramini, D.; Marcheselli, F.; Recchioni, R.; Rippo, M.R.; Bonafè, M.; et al. Long-Term Exposure of Human Endothelial Cells to Metformin Modulates miRNAs and isomiRs. Sci. Rep. 2020, 10, 21782. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, R.; Xiao, X.; Deng, Z.-L.; Jian, D.; Xie, H.-F.; Li, J. Microrna-217 Modulates Human Skin Fibroblast Senescence by Directly Targeting DNA Methyltransferase 1. Oncotarget 2017, 8, 33475–33486. [Google Scholar] [CrossRef]
- Soufi-zomorrod, M.; Hajifathali, A.; Kouhkan, F.; Mehdizadeh, M.; Rad, S.M.A.H.; Soleimani, M. MicroRNAs Modulating Angiogenesis: miR-129-1 and miR-133 Act as Angio-miR in HUVECs. Tumor Biol. 2016, 37, 9527–9534. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Jahantigh, M.; Wei, Y.; Schober, A. The Role of microRNAs in Arterial Remodelling. Thromb. Haemost. 2012, 107, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Ardinal, A.P.; Wiyono, A.V.; Estiko, R.I. Unveiling the Therapeutic Potential of miR-146a: Targeting Innate Inflammation in Atherosclerosis. J. Cell Mol. Med. 2024, 28, e70121. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sit, A.; Feinberg, M.W. Role of miR-181 Family in Regulating Vascular Inflammation and Immunity. Trends Cardiovasc. Med. 2014, 24, 105–112. [Google Scholar] [CrossRef]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Vera, M.P.; Blackwell, T.S.; Baron, R.M.; et al. MicroRNA-181b Regulates NF-κB–Mediated Vascular Inflammation. J. Clin. Investig. 2012, 122, JCI61495. [Google Scholar] [CrossRef]
- Heath, J.M.; Fernandez Esmerats, J.; Khambouneheuang, L.; Kumar, S.; Simmons, R.; Jo, H. Mechanosensitive microRNA-181b Regulates Aortic Valve Endothelial Matrix Degradation by Targeting TIMP3. Cardiovasc. Eng. Technol. 2018, 9, 141–150. [Google Scholar] [CrossRef]
- Jun-Hao, E.T.; Gupta, R.R.; Shyh-Chang, N. Lin28 and Let-7 in the Metabolic Physiology of Aging. Trends Endocrinol. Metab. 2016, 27, 132–141. [Google Scholar] [CrossRef]
- Chen, K.-C.; Hsieh, I.-C.; Hsi, E.; Wang, Y.-S.; Dai, C.-Y.; Chou, W.-W.; Juo, S.-H.H. Negative Feedback Regulation between microRNA Let-7g and the oxLDL Receptor LOX-1. J. Cell Sci. 2011, 124, 4115–4124. [Google Scholar] [CrossRef]
- Bao, M.-H.; Feng, X.; Zhang, Y.-W.; Lou, X.-Y.; Cheng, Y.; Zhou, H.-H. Let-7 in Cardiovascular Diseases, Heart Development and Cardiovascular Differentiation from Stem Cells. Int. J. Mol. Sci. 2013, 14, 23086–23102. [Google Scholar] [CrossRef] [PubMed]
- Neth, P.; Nazari-Jahantigh, M.; Schober, A.; Weber, C. MicroRNAs in Flow-Dependent Vascular Remodelling. Cardiovasc. Res. 2013, 99, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Telkoparan-Akillilar, P.; Cevik, D. Identification of miR-17, miR-21, miR-27a, miR-106b and miR-222 as Endoplasmic Reticulum Stress-Related Potential Biomarkers in Circulation of Patients with Atherosclerosis. Mol. Biol. Rep. 2021, 48, 3503–3513. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, H.; Wu, J.; Zhao, Y. miR-125a Suppresses TrxR1 Expression and Is Involved in H2O2-Induced Oxidative Stress in Endothelial Cells. J. Immunol. Res. 2018, 2018, 61402320. [Google Scholar] [CrossRef]
- Sun, B.; Liu, S.; Hao, R.; Dong, X.; Fu, L.; Han, B. RGD-PEG-PLA Delivers MiR-133 to Infarct Lesions of Acute Myocardial Infarction Model Rats for Cardiac Protection. Pharmaceutics 2020, 12, 575. [Google Scholar] [CrossRef]
- Kurdi, M.; Booz, G.W. Carvedilol Protects the Infarcted Heart by Upregulating miR-133: First Evidence That Disease State Affects β-Adrenergic Arrestin-Biased Signaling? J. Mol. Cell Cardiol. 2014, 76, 12–14. [Google Scholar] [CrossRef]
- Xu, C.; Hu, Y.; Hou, L.; Ju, J.; Li, X.; Du, N.; Guan, X.; Liu, Z.; Zhang, T.; Qin, W.; et al. β-Blocker Carvedilol Protects Cardiomyocytes against Oxidative Stress-Induced Apoptosis by up-Regulating miR-133 Expression. J. Mol. Cell Cardiol. 2014, 75, 111–121. [Google Scholar] [CrossRef]
- Anastasio, C.; Donisi, I.; Colloca, A.; D’Onofrio, N.; Balestrieri, M.L. MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction. Int. J. Mol. Sci. 2024, 25, 5087. [Google Scholar] [CrossRef]
- Wang, K.; Huang, X.-T.; Miao, Y.-P.; Bai, X.-L.; Jin, F. MiR-148a-3p Attenuates Apoptosis and Inflammation by Targeting CNTN4 in Atherosclerosis. Ann. Transl. Med. 2022, 10, 1201. [Google Scholar] [CrossRef]
- Holliday-Ankeny, C.J.; Ankeny, R.F.; Ferdous, Z.; Nerem, R.M.; Jo, H. Shear- and Side-Dependent microRNAs and Messenger RNAs in Aortic Valvular Endothelium. In Proceedings of the 5th Biennial Conference on Heart Valve Biology and Tissue Engineering, Mykonos Island, Greece, 18–20 May 2012; Hamad bin Khalifa University Press (HBKU Press): Mykonos Island, Greece, 2012. [Google Scholar]
- Girona, J.; Rosales, R.; Saavedra, P.; Masana, L.; Vallvé, J.-C. Palmitate Decreases Migration and Proliferation and Increases Oxidative Stress and Inflammation in Smooth Muscle Cells: Role of the Nrf2 Signaling Pathway. Am. J. Physiol. Cell Physiol. 2019, 316, C888–C897. [Google Scholar] [CrossRef] [PubMed]
- Citrin, K.M.; Fernández-Hernando, C.; Suárez, Y. MicroRNA Regulation of Cholesterol Metabolism. Ann. N. Y. Acad. Sci. 2021, 1495, 55–77. [Google Scholar] [CrossRef]
- Hergenreider, E.; Heydt, S.; Tréguer, K.; Boettger, T.; Horrevoets, A.J.G.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective Communication between Endothelial Cells and Smooth Muscle Cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Ahmed, S.; Warren, D.T. Vascular Smooth Muscle Cell Contractile Function and Mechanotransduction. Vessel. Plus 2018, 2, 36. [Google Scholar] [CrossRef]
- Song, Z.; Li, G. Role of Specific MicroRNAs in Regulation of Vascular Smooth Muscle Cell Differentiation and the Response to Injury. J Cardiovasc. Transl. Res. 2010, 3, 246–250. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, B.; Sun, L.; Sun, B.; Li, Y. Association of miR-192-5p with Atherosclerosis and Its Effect on Proliferation and Migration of Vascular Smooth Muscle Cells. Mol. Biotechnol. 2021, 63, 1244–1251. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Dong, X.; Ding, J.; Ma, H.; Han, W. Circ_GRN Promotes the Proliferation, Migration, and Inflammation of Vascular Smooth Muscle Cells in Atherosclerosis Through miR-214-3p/FOXO1 Axis. J. Cardiovasc. Pharmacol. 2021, 77, 470–479. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, S.-P.; Zhao, Y.-H. MicroRNA-143/-145 in Cardiovascular Diseases. BioMed Res. Int. 2015, 2015, 531740. [Google Scholar] [CrossRef] [PubMed]
- Vacante, F.; Denby, L.; Sluimer, J.C.; Baker, A.H. The Function of miR-143, miR-145 and the MiR-143 Host Gene in Cardiovascular Development and Disease. Vascul. Pharmacol. 2019, 112, 24–30. [Google Scholar] [CrossRef]
- Torella, D.; Iaconetti, C.; Catalucci, D.; Ellison, G.M.; Leone, A.; Waring, C.D.; Bochicchio, A.; Vicinanza, C.; Aquila, I.; Curcio, A.; et al. MicroRNA-133 Controls Vascular Smooth Muscle Cell Phenotypic Switch In Vitro and Vascular Remodeling In Vivo. Circ. Res. 2011, 109, 880–893. [Google Scholar] [CrossRef]
- Letonja, J.; Petrovič, D. A Review of MicroRNAs and lncRNAs in Atherosclerosis as Well as Some Major Inflammatory Conditions Affecting Atherosclerosis. Biomedicines 2024, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hu, X.; Zhang, Q.; Wang, J.; Li, J.; Liu, B.; Shao, Y.; Li, X.; Zhang, J.; Xin, S. Upregulation of Let-7a Inhibits Vascular Smooth Muscle Cell Proliferation in Vitro and in Vein Graft Intimal Hyperplasia in Rats. J. Surg. Res. 2014, 192, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.M.; Hall, I.F.; Serio, S.; Zani, S.; Climent, M.; Salvarani, N.; Carullo, P.; Civilini, E.; Condorelli, G.; Elia, L.; et al. miR-128-3p Is a Novel Regulator of Vascular Smooth Muscle Cell Phenotypic Switch and Vascular Diseases. Circ. Res. 2020, 126, E120–E135. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Sheu, J.-J.; Sun, C.-K.; Huang, T.-H.; Lin, Y.-P.; Yip, H.-K. MicroRNA-214 Modulates the Senescence of Vascular Smooth Muscle Cells in Carotid Artery Stenosis. Mol. Med. 2020, 26, 46. [Google Scholar] [CrossRef]
- Wang, H.; He, F.; Liang, B.; Jing, Y.; Zhang, P.; Liu, W.; Zhao, H. P53-Dependent LincRNA-P21 Protects Against Proliferation and Anti-Apoptosis of Vascular Smooth Muscle Cells in Atherosclerosis by Upregulating SIRT7 via MicroRNA-17-5p. J. Cardiovasc. Transl. Res. 2021, 14, 426–440. [Google Scholar] [CrossRef] [PubMed]
- An, J.-H.; Chen, Z.-Y.; Ma, Q.-L.; Li, Y.-B.; Shi, F.-W. Liraglutide Improves Atherosclerosis by Regulating Long Non-Coding RNA RMRP/miR-128-1-5P/Gadd45g Axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2725–2737. [Google Scholar] [CrossRef]
- Chong, H.; Wei, Z.; Na, M.; Sun, G.; Zheng, S.; Zhu, X.; Xue, Y.; Zhou, Q.; Guo, S.; Xu, J.; et al. The PGC-1α/NRF1/miR-378a Axis Protects Vascular Smooth Muscle Cells from FFA-Induced Proliferation, Migration and Inflammation in Atherosclerosis. Atherosclerosis 2020, 297, 136–145. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. A Necessary Role of miR-221 and miR-222 in Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia. Circ. Res. 2009, 104, 476–487. [Google Scholar] [CrossRef]
- Barreto, J.; Karathanasis, S.K.; Remaley, A.; Sposito, A.C. Role of LOX-1 (Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1) as a Cardiovascular Risk Predictor: Mechanistic Insight and Potential Clinical Use. Arter. Thromb. Vasc. Biol. 2021, 41, 153–166. [Google Scholar] [CrossRef]
- Nguyen, M.-A.; Hoang, H.-D.; Rasheed, A.; Duchez, A.-C.; Wyatt, H.; Cottee, M.L.; Graber, T.E.; Susser, L.; Robichaud, S.; Berber, İ.; et al. miR-223 Exerts Translational Control of Proatherogenic Genes in Macrophages. Circ. Res. 2022, 131, 42–58. [Google Scholar] [CrossRef]
- Li, B.-R.; Xia, L.-Q.; Liu, J.; Liao, L.-L.; Zhang, Y.; Deng, M.; Zhong, H.-J.; Feng, T.-T.; He, P.-P.; Ouyang, X.-P. miR-758-5p Regulates Cholesterol Uptake via Targeting the CD36 3′UTR. Biochem. Biophys. Res. Commun. 2017, 494, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Lightbody, R.J.; Taylor, J.M.W.; Dempsie, Y.; Graham, A. MicroRNA Sequences Modulating Inflammation and Lipid Accumulation in Macrophage “Foam” Cells: Implications for Atherosclerosis. World J. Cardiol. 2020, 12, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Monk, C.E.; Hutvagner, G.; Arthur, J.S.C. Regulation of miRNA Transcription in Macrophages in Response to Candida Albicans. PLoS ONE 2010, 5, e13669. [Google Scholar] [CrossRef]
- Curtale, G.; Rubino, M.; Locati, M. MicroRNAs as Molecular Switches in Macrophage Activation. Front. Immunol. 2019, 10, 799. [Google Scholar] [CrossRef]
- Rayner, K.J.; Moore, K.J. MicroRNA Control of High-Density Lipoprotein Metabolism and Function. Circ. Res. 2014, 114, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-B.; Zhu, D.; Dai, F.; Huang, Y.-Q.; Zheng, J.-X.; Tang, Y.-P.; Dong, Z.-R.; Liao, X.; Qing, Y.-F. MicroRNA-223 Suppresses IL-1β and TNF-α Production in Gouty Inflammation by Targeting the NLRP3 Inflammasome. Front. Pharmacol. 2021, 12, 637415. [Google Scholar] [CrossRef]
- Deng, W.; Chen, K.; Liu, S.; Wang, Y. Silencing Circular ANRIL Protects HK-2 Cells from Lipopolysaccharide-Induced Inflammatory Injury through up-Regulating microRNA-9. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3478–3484. [Google Scholar] [CrossRef]
- Lai, T.-C.; Lee, T.-L.; Chang, Y.-C.; Chen, Y.-C.; Lin, S.-R.; Lin, S.-W.; Pu, C.-M.; Tsai, J.-S.; Chen, Y.-L. MicroRNA-221/222 Mediates ADSC-Exosome-Induced Cardioprotection Against Ischemia/Reperfusion by Targeting PUMA and ETS-1. Front. Cell Dev. Biol. 2020, 8, 569150. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Shi, H.; Xu, J.; Zhang, D.; Wu, Y.; Zhou, S.; Sun, X. MiR-21 Modulates Human Airway Smooth Muscle Cell Proliferation and Migration in Asthma through Regulation of PTEN Expression. Exp. Lung Res. 2015, 41, 535–545. [Google Scholar] [CrossRef]
- Price, N.L.; Rotllan, N.; Zhang, X.; Canfrán-Duque, A.; Nottoli, T.; Suarez, Y.; Fernández-Hernando, C. Specific Disruption of Abca1 Targeting Largely Mimics the Effects of miR-33 Knockout on Macrophage Cholesterol Efflux and Atherosclerotic Plaque Development. Circ. Res. 2019, 124, 874–880. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, X.; Du, G.; Zhang, Z.; Zhai, Y.; Xiong, X.; Luo, X. MicroRNA-122-5p Inhibition Improves Inflammation and Oxidative Stress Damage in Dietary-Induced Non-Alcoholic Fatty Liver Disease Through Targeting FOXO3. Front. Physiol. 2022, 13, 803445. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Peng, J.; Guo, Y.; Li, F. MicroRNA-33-5p Inhibits Cholesterol Efflux in Vascular Endothelial Cells by Regulating Citrate Synthase and ATP-Binding Cassette Transporter A1. BMC Cardiovasc. Disord. 2021, 21, 433. [Google Scholar] [CrossRef]
- Yerlikaya, F.H.; Öz, M. Aberrant Expression of miRNA Profiles in High-Fat and High-Sucrose Fed Rats. Clin. Nutr. Exp. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Qiao, X.-R.; Wang, L.; Liu, M.; Tian, Y.; Chen, T. MiR-210-3p Attenuates Lipid Accumulation and Inflammation in Atherosclerosis by Repressing IGF2. Biosci. Biotechnol. Biochem. 2020, 84, 321–329. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Zhu, Y.; Sun, H.; Juguilon, C.; Li, F.; Fan, D.; Yin, L.; Zhang, Y. Macrophage miR-34a Is a Key Regulator of Cholesterol Efflux and Atherosclerosis. Mol. Ther. 2020, 28, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.; Enrick, M.; Diaz, A.; Yin, L. Is miR-21 A Therapeutic Target in Cardiovascular Disease? Int. J. Drug Discov. Pharmacol. 2023, 2, 26–36. [Google Scholar] [CrossRef]
- Ouimet, M.; Ediriweera, H.; Afonso, M.S.; Ramkhelawon, B.; Singaravelu, R.; Liao, X.; Bandler, R.C.; Rahman, K.; Fisher, E.A.; Rayner, K.J.; et al. microRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2017, 37, 1058–1067. [Google Scholar] [CrossRef]
- Frutos, M.F.; Pardo-Marqués, V.; Torrecilla-Parra, M.; Rada, P.; Pérez-García, A.; Martín-Martín, Y.; De La Peña, G.; Gómez, A.; Toledano-Zaragoza, A.; Gómez-Coronado, D.; et al. “MiR-7 Controls Cholesterol Biosynthesis through Posttranscriptional Regulation of DHCR24 Expression”. Biochim. Biophys. Acta Gene Regul. Mech. 2023, 1866, 194938. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Q.; Wang, W.; Ling, Y.; Yan, Y.; Xia, P. Hepatocyte-Derived Extracellular Vesicles Promote Endothelial Inflammation and Atherogenesis via microRNA-1. J. Hepatol. 2020, 72, 156–166. [Google Scholar] [CrossRef]
- Lv, Y.-C.; Tang, Y.-Y.; Peng, J.; Zhao, G.-J.; Yang, J.; Yao, F.; Ouyang, X.-P.; He, P.-P.; Xie, W.; Tan, Y.-L.; et al. MicroRNA-19b Promotes Macrophage Cholesterol Accumulation and Aortic Atherosclerosis by Targeting ATP-Binding Cassette Transporter A1. Atherosclerosis 2014, 236, 215–226. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Li, P.; Zhang, B.; Zhang, J. HDAC3 Protects against Atherosclerosis through Inhibition of Inflammation via the microRNA-19b/PPARγ/NF-κB Axis. Atherosclerosis 2021, 323, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Baker, M.B.; Moore, J.P.; Searles, C.D. MiR-21 Is Induced in Endothelial Cells by Shear Stress and Modulates Apoptosis and eNOS Activity. Biochem. Biophys. Res. Commun. 2010, 393, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Davoudi, M.; Alitotonchi, Z.; Ahmadi, E.S.; Amraee, F.; Alemi, A.; Afrisham, R. Managing Cardiovascular Events, Hyperglycemia, and Obesity in Type 2 Diabetes through microRNA Regulation Linked to Glucagon-like Peptide-1 Receptor Agonists. Diabetol. Metab. Syndr. 2025, 17, 13. [Google Scholar] [CrossRef]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. Microrna-27b Is a Regulatory Hub in Lipid Metabolism and Is Altered in Dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, C.; Liu, Z.; Wang, L.; Liu, B.; He, F.; Zhang, T.; Wang, Y.; Wang, X.; Xu, M.; et al. Upregulation of a Disintegrin and Metalloproteinase with Thrombospondin Motifs-7 by miR-29 Repression Mediates Vascular Smooth Muscle Calcification. Arter. Thromb. Vasc. Biol. 2012, 32, 2580–2588. [Google Scholar] [CrossRef]
- Price, N.L.; Goedeke, L.; Suárez, Y.; Fernández-Hernando, C. miR-33 in Cardiometabolic Diseases: Lessons Learned from Novel Animal Models and Approaches. EMBO Mol. Med. 2021, 13, e12606. [Google Scholar] [CrossRef]
- Ito, T.; Yagi, S.; Yamakuchi, M. MicroRNA-34a Regulation of Endothelial Senescence. Biochem. Biophys. Res. Commun. 2010, 398, 735–740. [Google Scholar] [CrossRef]
- Hua, C.-C.; Liu, X.-M.; Liang, L.-R.; Wang, L.-F.; Zhong, J.-C. Targeting the microRNA-34a as a Novel Therapeutic Strategy for Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 8, 784044. [Google Scholar] [CrossRef]
- Fang, Y.; Davies, P.F. Site-Specific MicroRNA-92a Regulation of Krüppel-like Factors 4 and 2 in Atherosusceptible Endothelium. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 979–987. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, M.; Sewani, M.A.; Wang, J. The miR-17-92 Cluster in Cardiac Health and Disease. Birth Defects Res. 2024, 116, e2273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lei, J.; Lei, H.; Ruan, X.; Liu, Q.; Chen, Y.; Huang, W. MicroRNA-101 Overexpression by IL-6 and TNF-α Inhibits Cholesterol Efflux by Suppressing ATP-Binding Cassette Transporter A1 Expression. Exp. Cell Res. 2015, 336, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjärn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M.; et al. Antagonism of microRNA-122 in Mice by Systemically Administered LNA-antimiR Leads to up-Regulation of a Large Set of Predicted Target mRNAs in the Liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef]
- Fitzsimons, S.; Oggero, S.; Bruen, R.; McCarthy, C.; Strowitzki, M.J.; Mahon, N.G.; Ryan, N.; Brennan, E.P.; Barry, M.; Perretti, M.; et al. microRNA-155 Is Decreased During Atherosclerosis Regression and Is Increased in Urinary Extracellular Vesicles During Atherosclerosis Progression. Front. Immunol. 2020, 11, 576516. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Jahantigh, M.; Wei, Y.; Noels, H.; Akhtar, S.; Zhou, Z.; Koenen, R.R.; Heyll, K.; Gremse, F.; Kiessling, F.; Grommes, J.; et al. MicroRNA-155 Promotes Atherosclerosis by Repressing Bcl6 in Macrophages. J. Clin. Investig. 2012, 122, 4190–4202. [Google Scholar] [CrossRef]
- Du, F.; Yu, F.; Wang, Y.; Hui, Y.; Carnevale, K.; Fu, M.; Lu, H.; Fan, D. MicroRNA-155 Deficiency Results in Decreased Macrophage Inflammation and Attenuated Atherogenesis in Apolipoprotein E–Deficient Mice. Arter. Thromb. Vasc. Biol. 2014, 34, 759–767. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef]
- Ke, F.; Wang, H.; Geng, J.; Jing, X.; Fang, F.; Fang, C.; Zhang, B. MiR-155 Promotes Inflammation and Apoptosis via Targeting SIRT1 in Hypoxic-Ischemic Brain Damage. Exp. Neurol. 2023, 362, 114317. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhang, L.; Yang, P. MicroRNA-210 Induces Endothelial Cell Apoptosis by Directly Targeting PDK1 in the Setting of Atherosclerosis. Cell Mol. Biol. Lett. 2017, 22, 3. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Yang, S.; Li, R.; Yang, Y.; Chen, Y.; Zhang, W. Inhibitory Role of Ginsenoside Rb2 in Endothelial Senescence and Inflammation Mediated by microRNA-216a. Mol. Med. Rep. 2021, 23, 415. [Google Scholar] [CrossRef]
- Su, M.; Wang, J.; Wang, C.; Wang, X.; Dong, W.; Qiu, W.; Wang, Y.; Zhao, X.; Zou, Y.; Song, L.; et al. MicroRNA-221 Inhibits Autophagy and Promotes Heart Failure by Modulating the P27/CDK2/mTOR Axis. Cell Death Differ. 2015, 22, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mao, H.; Chen, J.; Wen, S.; Li, D.; Ye, M.; Lv, Z. Increased Expression of microRNA-221 Inhibits PAK1 in Endothelial Progenitor Cells and Impairs Its Function via c-Raf/MEK/ERK Pathway. Biochem. Biophys. Res. Commun. 2013, 431, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Dentelli, P.; Rosso, A.; Orso, F.; Olgasi, C.; Taverna, D.; Brizzi, M.F. microRNA-222 Controls Neovascularization by Regulating Signal Transducer and Activator of Transcription 5A Expression. Arter. Thromb. Vasc. Biol. 2010, 30, 1562–1568. [Google Scholar] [CrossRef]
- Wei, Y.; Nazari-Jahantigh, M.; Chan, L.; Zhu, M.; Heyll, K.; Corbalán-Campos, J.; Hartmann, P.; Thiemann, A.; Weber, C.; Schober, A. The microRNA-342-5p Fosters Inflammatory Macrophage Activation Through an Akt1- and microRNA-155-Dependent Pathway During Atherosclerosis. Circulation 2013, 127, 1609–1619. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, Z.; Feng, Z.; Wang, Y.; Ji, N. MicroRNA-499-5p Promotes Vascular Smooth Muscle Cell Proliferation and Migration via Inhibiting SOX6. Physiol. Genom. 2023, 55, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Kumar, S.; Takabe, W.; Woo Kim, C.; Ni, C.-W.; Alberts-Grill, N.; Jang, I.-H.; Kim, S.; Kim, W.; Won Kang, S.; et al. The Atypical Mechanosensitive microRNA-712 Derived from Pre-Ribosomal RNA Induces Endothelial Inflammation and Atherosclerosis. Nat. Commun. 2013, 4, 3000. [Google Scholar] [CrossRef]
- Fang, Y.; Shi, C.; Manduchi, E.; Civelek, M.; Davies, P.F. MicroRNA-10a Regulation of Proinflammatory Phenotype in Athero-Susceptible Endothelium in Vivo and in Vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 13450–13455. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.; Wang, Y.; Tang, Z.; Cui, Q.; Xi, J.; Li, Y.-S.J.; Chien, S.; Wang, N. MicroRNA-19a Mediates the Suppressive Effect of Laminar Flow on Cyclin D1 Expression in Human Umbilical Vein Endothelial Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3240–3244. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Rotllan, N.; Zhang, X.; Fernández-Fuertes, M.; Ramírez-Hidalgo, C.; Araldi, E.; Daimiel, L.; Busto, R.; Fernández-Hernando, C.; Suárez, Y. Macrophage Deficiency of miR-21 Promotes Apoptosis, Plaque Necrosis, and Vascular Inflammation during Atherogenesis. EMBO Mol. Med. 2017, 9, 1244–1262. [Google Scholar] [CrossRef]
- Wang, K.-C.; Garmire, L.X.; Young, A.; Nguyen, P.; Trinh, A.; Subramaniam, S.; Wang, N.; Shyy, J.Y.; Li, Y.-S.; Chien, S. Role of microRNA-23b in Flow-Regulation of Rb Phosphorylation and Endothelial Cell Growth. Proc. Natl. Acad. Sci. USA 2010, 107, 3234–3239. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Liu, G.; Qi, X.; Cao, X. MicroRNA-24 Inhibits the Proliferation and Migration of Endothelial Cells in Patients with Atherosclerosis by Targeting Importin-α3 and Regulating Inflammatory Responses. Exp. Ther. Med. 2017, 15, 338–344. [Google Scholar] [CrossRef]
- Chen, K.-C.; Wang, Y.-S.; Hu, C.-Y.; Chang, W.-C.; Liao, Y.-C.; Dai, C.-Y.; Juo, S.-H.H. OxLDL Up-regulates microRNA-29b, Leading to Epigenetic Modifications of MMP-2/MMP-9 Genes: A Novel Mechanism for Cardiovascular Diseases. FASEB J. 2011, 25, 1718–1728. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, F.; Wang, J.; Jing, J.; Zhou, S.-S.; Chen, Y.-D. Endothelial Cell Autophagy in Atherosclerosis Is Regulated by miR-30-Mediated Translational Control of ATG6. Cell Physiol. Biochem. 2015, 37, 1369–1378. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Park, N.; Kang, H. BMP-Induced MicroRNA-101 Expression Regulates Vascular Smooth Muscle Cell Migration. Int. J. Mol. Sci. 2020, 21, 4764. [Google Scholar] [CrossRef]
- Chao, C.-T.; Liu, Y.-P.; Su, S.-F.; Yeh, H.-Y.; Chen, H.-Y.; Lee, P.-J.; Chen, W.-J.; Lee, Y.-M.; Huang, J.-W.; Chiang, C.-K.; et al. Circulating MicroRNA-125b Predicts the Presence and Progression of Uremic Vascular Calcification. Arter. Thromb. Vasc. Biol. 2017, 37, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Cao, H.; Fang, L.; Ye, H.; Zhou, Y.; Jiang, L.; Su, W.; Xu, H.; He, W.; Dai, C.; et al. miR-125b/Ets1 Axis Regulates Transdifferentiation and Calcification of Vascular Smooth Muscle Cells in a High-Phosphate Environment. Exp. Cell Res. 2014, 322, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Wang, B.; Yang, J.; Gong, Z.; Zhao, X.; Zhang, C.; Du, K. MiR-126 Inhibits Vascular Endothelial Cell Apoptosis through Targeting PI3K/Akt Signaling. Ann. Hematol. 2016, 95, 365–374. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; Sanagawa, A.; Mori, C.; Ito, S.; Iwaki, S.; Satoh, H.; Fujii, S. Circulating microRNA-126 in Patients with Coronary Artery Disease: Correlation with LDL Cholesterol. Thromb. J. 2012, 10, 16. [Google Scholar] [CrossRef]
- Hao, X.-Z.; Fan, H.-M. Identification of miRNAs as Atherosclerosis Biomarkers and Functional Role of miR-126 in Atherosclerosis Progression through MAPK Signalling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2725–2733. [Google Scholar]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.-H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 Regulate Smooth Muscle Cell Fate and Plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef]
- Hou, J.; Deng, Q.; Deng, X.; Zhong, W.; Liu, S.; Zhong, Z. MicroRNA-146a-5p Alleviates Lipopolysaccharide-Induced NLRP3 Inflammasome Injury and pro-Inflammatory Cytokine Production via the Regulation of TRAF6 and IRAK1 in Human Umbilical Vein Endothelial Cells (HUVECs). Ann. Transl. Med. 2021, 9, 1433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, T.; Yang, L.; Li, Z.; Wong, M.M.; Zheng, X.; Pan, X.; Zhang, L.; Yan, H. Regulation of MicroRNA-155 in Atherosclerotic Inflammatory Responses by Targeting MAP3K10. PLoS ONE 2012, 7, e46551. [Google Scholar] [CrossRef]
- Rachmawati, E.; Sargowo, D.; Rohman, M.S.; Widodo, N.; Kalsum, U. miR-155–5p Predictive Role to Decelerate Foam Cell Atherosclerosis through CD36, VAV3, and SOCS1 Pathway. Non-Coding RNA Res. 2021, 6, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; He, S.; Li, P.; Jiang, S.; Li, D.; Lin, J.; Feinberg, M.W. MicroRNA-181 in Cardiovascular Disease: Emerging Biomarkers and Therapeutic Targets. FASEB J. 2024, 38, e23635. [Google Scholar] [CrossRef] [PubMed]
- Di Gregoli, K.; Mohamad Anuar, N.N.; Bianco, R.; White, S.J.; Newby, A.C.; George, S.J.; Johnson, J.L. MicroRNA-181b Controls Atherosclerosis and Aneurysms Through Regulation of TIMP-3 and Elastin. Circ. Res. 2017, 120, 49–65. [Google Scholar] [CrossRef]
- Yuan, F.; Peng, W.; Yang, Y.; Xu, J.; Liu, Y.; Xie, Y.; Huang, T.; Shi, C.; Ding, Y.; Li, C.; et al. Endothelial Progenitor Cell-Derived Exosomes Promote Anti-Inflammatory Macrophages via SOCS3/JAK2/STAT3 Axis and Improve the Outcome of Spinal Cord Injury. J. Neuroinflamm. 2023, 20, 156. [Google Scholar] [CrossRef]
- Meiler, S.; Baumer, Y.; Toulmin, E.; Seng, K.; Boisvert, W.A. MicroRNA 302a Is a Novel Modulator of Cholesterol Homeostasis and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 323–331. [Google Scholar] [CrossRef]
- Zhou, T.; Li, S.; Yang, L.; Xiang, D. microRNA-363-3p Reduces Endothelial Cell Inflammatory Responses in Coronary Heart Disease via Inactivation of the NOX4-Dependent P38 MAPK Axis. Aging 2021, 13, 11061–11082. [Google Scholar] [CrossRef]
- Yang, B.; Yang, H.; Lu, X.; Wang, L.; Li, H.; Chen, S.; Wang, X.; Shen, C.; Huang, J.; Lu, X.; et al. MiR-520b Inhibits Endothelial Activation by Targeting NF-κB P65-VCAM1 Axis. Biochem. Pharmacol. 2021, 188, 114540. [Google Scholar] [CrossRef]
- Yan, J.; Yang, Y.; Liu, Y.; Shi, X.; Wu, H.; Dai, M. MicroRNA Let-7g Links Foam Cell Formation and Adipogenic Differentiation: A Key Regulator of Paeonol Treating Atherosclerosis-Osteoporosis. Phytomedicine 2024, 126, 155447. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Zhang, Y.; Lou, X.; Cheng, Y.; Zhou, H. Protective Effects of Let-7a and Let-7b on Oxidized Low-Density Lipoprotein Induced Endothelial Cell Injuries. PLoS ONE 2014, 9, e106540. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Q.; Qi, D.; Niu, F.; Li, Q.; Yang, H.; Gao, C. Atherosclerosis-associated Endothelial Cell Apoptosis by miRNA Let7-b-mediated Downregulation of HAS-2. J. Cell Biochem. 2020, 121, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective Effect of Let-7 miRNA Family in Regulating Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 2017, 66, 2266–2277. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; Lorente-Sorolla, C.; Naharro, S.; Rodrigo-Muñoz, J.M.; Del Pozo, V. Advances and Highlights of miRNAs in Asthma: Biomarkers for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1628. [Google Scholar] [CrossRef] [PubMed]
- Feketea, G.; Bocsan, C.I.; Popescu, C.; Gaman, M.; Stanciu, L.A.; Zdrenghea, M.T. A Review of Macrophage MicroRNAs’ Role in Human Asthma. Cells 2019, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, M.; Lecuona, E.; Angulo, M.; Homma, T.; Rodríguez, D.A.; Gonzalez-Gonzalez, F.J.; Welch, L.C.; Amarelle, L.; Kim, S.-J.; Kaminski, N.; et al. Hypercapnia Increases Airway Smooth Muscle Contractility via Caspase-7–Mediated miR-133a–RhoA Signaling. Sci. Transl. Med. 2018, 10, eaat1662. [Google Scholar] [CrossRef]
- Perdomo, C.; Campbell, J.D.; Gerrein, J.; Tellez, C.S.; Garrison, C.B.; Walser, T.C.; Drizik, E.; Si, H.; Gower, A.C.; Vick, J.; et al. MicroRNA 4423 Is a Primate-Specific Regulator of Airway Epithelial Cell Differentiation and Lung Carcinogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 18946–18951. [Google Scholar] [CrossRef] [PubMed]
- Calvén, J.; Ax, E.; Rådinger, M. The Airway Epithelium—A Central Player in Asthma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef]
- Pinkerton, M.; Chinchilli, V.; Banta, E.; Craig, T.; August, A.; Bascom, R.; Cantorna, M.; Harvill, E.; Ishmael, F.T. Differential Expression of microRNAs in Exhaled Breath Condensates of Patients with Asthma, Patients with Chronic Obstructive Pulmonary Disease, and Healthy Adults. J. Allergy Clin. Immunol. 2013, 132, 217–219.e2. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, L.; Zhang, Z.; Hu, G.; Zhang, J.; Li, H. Let-7a Inhibits Proliferation and Promotes Apoptosis of Human Asthmatic Airway Smooth Muscle Cells. Exp. Ther. Med. 2019, 17, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díazcouder, A.; Romero-Nava, R.; Del-Río-Navarro, B.E.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A.; Reyes-Noriega, N.; Rodríguez-Cortés, O.; Leija-Martínez, J.J.; Vélez-Reséndiz, J.M.; Villafaña, S.; et al. The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation. Nutrients 2024, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Allegra, A.; Pioggia, G.; Picardi, G.; Musolino, C.; Gangemi, S. MicroRNA Profiling in Asthma: Potential Biomarkers and Therapeutic Targets. Am. J. Respir. Cell Mol. Biol. 2017, 57, 642–650. [Google Scholar] [CrossRef]
- Bélanger, É.; Madore, A.-M.; Boucher-Lafleur, A.-M.; Simon, M.-M.; Kwan, T.; Pastinen, T.; Laprise, C. Eosinophil microRNAs Play a Regulatory Role in Allergic Diseases Included in the Atopic March. Int. J. Mol. Sci. 2020, 21, 9011. [Google Scholar] [CrossRef]
- Liu, X.; Cui, H.; Bai, Q.; Piao, H.; Song, Y.; Yan, G. miR-128-3p Alleviates Airway Inflammation in Asthma by Targeting SIX1 to Regulate Mitochondrial Fission and Fusion. Int. Immunopharmacol. 2024, 130, 111703. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Y.; Do, D.C.; Ke, X.; Zhang, S.; Lambert, K.; Kumar, S.; Hu, C.; Zhou, Y.; Ishmael, F.T.; et al. miR-155 Modulates Cockroach Allergen- and Oxidative Stress-Induced Cyclooxygenase-2 in Asthma. J. Immunol. 2018, 201, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.; Viggiani, G.; Chen, Y.-W.; Coulis, G.; Castaldi, A. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int. J. Mol. Sci. 2020, 21, 4370. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, W.; Jing, W. Indoor Air Pollution Aggravates Asthma in Chinese Children and Induces the Changes in Serum Level of miR-155. Int. J. Environ. Health Res. 2019, 29, 22–30. [Google Scholar] [CrossRef]
- Li, J.J.; Tay, H.L.; Maltby, S.; Xiang, Y.; Eyers, F.; Hatchwell, L.; Zhou, H.; Toop, H.D.; Morris, J.C.; Nair, P.; et al. MicroRNA-9 Regulates Steroid-Resistant Airway Hyperresponsiveness by Reducing Protein Phosphatase 2A Activity. J. Allergy Clin. Immunol. 2015, 136, 462–473. [Google Scholar] [CrossRef]
- Hou, C.; Chen, Y.; Huang, X.; Huang, Q.; Li, M.; Tan, X. miR-19 Targets PTEN and Mediates High Mobility Group Protein B1(HMGB1)-Induced Proliferation and Migration of Human Airway Smooth Muscle Cells. PLoS ONE 2019, 14, e0219081. [Google Scholar] [CrossRef]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 Is Up-Regulated in Allergic Airway Inflammation and Regulates IL-12p35 Expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef]
- Lu, T.X.; Hartner, J.; Lim, E.-J.; Fabry, V.; Mingler, M.K.; Cole, E.T.; Orkin, S.H.; Aronow, B.J.; Rothenberg, M.E. MicroRNA-21 Limits In Vivo Immune Response-Mediated Activation of the IL-12/IFN-γ Pathway, Th1 Polarization, and the Severity of Delayed-Type Hypersensitivity. J. Immunol. 2011, 187, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Q.; Jiang, S.; Shan, H.; Yu, T. MicroRNA-26a in Respiratory Diseases: Mechanisms and Therapeutic Potential. Mol. Biol. Rep. 2024, 51, 627. [Google Scholar] [CrossRef] [PubMed]
- Mattes, J.; Collison, A.; Plank, M.; Phipps, S.; Foster, P.S. Antagonism of microRNA-126 Suppresses the Effector Function of TH 2 Cells and the Development of Allergic Airways Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 18704–18709. [Google Scholar] [CrossRef]
- Siddiqui, S.; Johansson, K.; Joo, A.; Bonser, L.R.; Koh, K.D.; Le Tonqueze, O.; Bolourchi, S.; Bautista, R.A.; Zlock, L.; Roth, T.L.; et al. Epithelial miR-141 Regulates IL-13–Induced Airway Mucus Production. JCI Insight 2021, 6, e139019. [Google Scholar] [CrossRef]
- Yamada, Y.; Kosaka, K.; Miyazawa, T.; Kurata-Miura, K.; Yoshida, T. miR-142-3p Enhances FcεRI-Mediated Degranulation in Mast Cells. Biochem. Biophys. Res. Commun. 2014, 443, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, X.; Fan, L.; Chen, Q.; Zhang, H.; Pan, H.; Xu, A.; Wang, H.; Yu, Y. MicroRNA-145 Influences the Balance of Th1/Th2 via Regulating RUNX3 in Asthma Patients. Exp. Lung Res. 2016, 42, 417–424. [Google Scholar] [CrossRef]
- Xiong, T.; Du, Y.; Fu, Z.; Geng, G. MicroRNA-145-5p Promotes Asthma Pathogenesis by Inhibiting Kinesin Family Member 3A Expression in Mouse Airway Epithelial Cells. J. Int. Med. Res. 2019, 47, 3307–3319. [Google Scholar] [CrossRef]
- Malmhäll, C.; Alawieh, S.; Lu, Y.; Sjöstrand, M.; Bossios, A.; Eldh, M.; Rådinger, M. MicroRNA-155 Is Essential for TH2-Mediated Allergen-Induced Eosinophilic Inflammation in the Lung. J. Allergy Clin. Immunol. 2014, 133, 1429–1438.e7. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.; Cheng, S.; Xu, Y.; Xuefei, Q.; Cao, Y.; Xie, J.; Wang, C.; Xu, Y.; Xiong, W. Small Interfering RNA Directed against microRNA-155 Delivered by a Lentiviral Vector Attenuates Asthmatic Features in a Mouse Model of Allergic Asthma. Exp. Ther. Med. 2017, 14, 4391–4396. [Google Scholar] [CrossRef]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; Van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of Bic/microRNA-155 for Normal Immune Function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, J.; Gao, P.; Wang, Q.; Zhang, J. miR-155: A Novel Target in Allergic Asthma. Int. J. Mol. Sci. 2016, 17, 1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Liu, J.; Gai, L.; Yan, X.; Guo, Z.; Liu, F. Emerging Advances of Non-Coding RNAs and Competitive Endogenous RNA Regulatory Networks in Asthma. Bioengineered 2021, 12, 7820–7836. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Xu, H.; Zhang, J.; Deng, H.; Gao, H.; Yang, J.; Zhao, D.; Liu, F. miRNA-221-3p Enhances the Secretion of Interleukin-4 in Mast Cells through the Phosphatase and Tensin Homolog/P38/Nuclear Factor-kappaB Pathway. PLoS ONE 2016, 11, e0148821. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y.; Ma, Y.; Yang, J. MiR-223 Plays a Protecting Role in Neutrophilic Asthmatic Mice through the Inhibition of NLRP3 Inflammasome. Respir. Res. 2020, 21, 116. [Google Scholar] [CrossRef]
- Li, P.; Lang, X.; Xia, S. Elevated Expression of microRNA-378 in Children with Asthma Aggravates Airway Remodeling by Promoting the Proliferation and Apoptosis Resistance of Airway Smooth Muscle Cells. Exp. Ther. Med. 2018, 17, 1529–1536. [Google Scholar] [CrossRef]
- Maes, T.; Cobos, F.A.; Schleich, F.; Sorbello, V.; Henket, M.; De Preter, K.; Bracke, K.R.; Conickx, G.; Mesnil, C.; Vandesompele, J.; et al. Asthma Inflammatory Phenotypes Show Differential microRNA Expression in Sputum. J. Allergy Clin. Immunol. 2016, 137, 1433–1446. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, N.; Chen, Z.; Sun, Z.; Wu, C.; Yu, W.; Hu, F.; Huang, M.; Zhang, M. MiR-1165-3p Suppresses Th2 Differentiation via Targeting IL-13 and PPM1A in a Mouse Model of Allergic Airway Inflammation. Allergy Asthma Immunol. Res. 2020, 12, 859. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Zhang, F.; Peng, X.; Mao, X.; Lu, W.; Wu, R.; Huang, B.; Bao, Y.; Ma, L.; et al. Divergent Roles of miR-3162-3p in Pulmonary Inflammation in Normal and Asthmatic Mice as Well as Antagonism of miR-3162-3p in Asthma Treatment. Int. Arch. Allergy Immunol. 2020, 181, 594–605. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, J.; Ye, Q.; Gu, X. Interference of miR-943-3p with Secreted Frizzled-Related Proteins4 (SFRP4) in an Asthma Mouse Model. Cell Tissue Res. 2019, 378, 67–80. [Google Scholar] [CrossRef]
- Tian, M.; Zhou, Y.; Jia, H.; Zhu, X.; Cui, Y. The Clinical Significance of Changes in the Expression Levels of MicroRNA-1 and Inflammatory Factors in the Peripheral Blood of Children with Acute-Stage Asthma. BioMed Res. Int. 2018, 2018, 7632487. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, B.; Huang, M.; Wang, X. miR-30a-3p Participates in the Development of Asthma by Targeting CCR3. Open Med. 2020, 15, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human Airway Epithelial Extracellular Vesicle miRNA Signature Is Altered upon Asthma Development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, M.; Ouyang, L.; Wang, Q.; Guo, Y.; Huang, L.; Jiang, S. miR-142-5p and miR-130a-3p Regulate Pulmonary Macrophage Polarization and Asthma Airway Remodeling. Immunol. Cell Biol. 2020, 98, 715–725. [Google Scholar] [CrossRef]
- Huang, X.; Qin, C.; Gao, Y. miR-135a Inhibits Airway Inflammatory Response in Asthmatic Mice via Regulating JAK/STAT Signaling Pathway. Braz. J. Med. Biol. Res. 2021, 54, e10023. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, S.-G.; Xu, L.; Che, Y.-Y.; Jiang, S.-Y.; Zhu, L.; Zhao, M.; Teng, Y.-C. MiR-135b Alleviates Airway Inflammation in Asthmatic Children and Experimental Mice with Asthma via Regulating CXCL12. Immunol. Investig. 2022, 51, 496–510. [Google Scholar] [CrossRef]
- Huang, H.; Sun, B.; Li, B.; Wei, B. miR-142-3p Regulates Airway Inflammation Through PTEN/AKT in Children and Mice with Asthma. Immunol. Investig. 2024, 54, 297–316. [Google Scholar] [CrossRef]
- Lyu, B.; Wei, Z.; Jiang, L.; Ma, C.; Yang, G.; Han, S. MicroRNA-146a Negatively Regulates IL-33 in Activated Group 2 Innate Lymphoid Cells by Inhibiting IRAK1 and TRAF6. Genes Immun. 2020, 21, 37–44. [Google Scholar] [CrossRef]
- Duan, X.-J.; Zhang, X.; Li, L.-R.; Zhang, J.-Y.; Chen, Y.-P. MiR-200a and miR-200b Restrain Inflammation by Targeting ORMDL3 to Regulate the ERK/MMP-9 Pathway in Asthma. Exp. Lung Res. 2020, 46, 321–331. [Google Scholar] [CrossRef]
- Yang, Z.; Qu, Z.; Yi, M.; Lv, Z.; Wang, Y.; Shan, Y.; Ran, N.; Liu, X. MiR-204-5p Inhibits Transforming Growth Factor-Β1-Induced Proliferation and Extracellular Matrix Production of Airway Smooth Muscle Cells by Regulating Six1 in Asthma. Int. Arch. Allergy Immunol. 2020, 181, 239–248. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, Y.; Wu, W.; Chang, C.; Chen, D.; Chen, S.; Zhen, G. microRNA-218-5p Plays a Protective Role in Eosinophilic Airway Inflammation via Targeting Δ-catenin, a Novel Catenin in Asthma. Clin. Exp. Allergy 2020, 50, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, H.; Liang, L.; Zhi, Y.; Huo, B.; Wu, L.; Xu, L.; Shen, Z. MicroRNA-744 Inhibits Proliferation of Bronchial Epithelial Cells by Regulating Smad3 Pathway via Targeting Transforming Growth Factor-Β1 (TGF-Β1) in Severe Asthma. Med. Sci. Monit. 2019, 25, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular Mechanisms of Oxidative Stress in Asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.W.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; Van Der Vliet, A. Redox-Based Regulation of Signal Transduction: Principles, Pitfalls, and Promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Cheng, L.; Jiang, Z.; Chen, K.; Zhou, C.; Sun, L.; Cao, J.; Qian, W.; Li, J.; Shan, T.; et al. Resveratrol Inhibits ROS-Promoted Activation and Glycolysis of Pancreatic Stellate Cells via Suppression of miR-21. Oxid. Med. Cell Longev. 2018, 2018, 1346958. [Google Scholar] [CrossRef]
- Zhang, X.; Ng, W.-L.; Wang, P.; Tian, L.; Werner, E.; Wang, H.; Doetsch, P.; Wang, Y. MicroRNA-21 Modulates the Levels of Reactive Oxygen Species by Targeting SOD3 and TNFα. Cancer Res. 2012, 72, 4707–4713. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, I.C.; Meng, S.; Xu, J. miR-146a Decreases Inflammation and ROS Production in Aged Dermal Fibroblasts. Int. J. Mol. Sci. 2024, 25, 6821. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Obora, K.; Mori, T.; Fukuda, K. miR-155 Induces ROS Generation through Downregulation of Antioxidation-related Genes in Mesenchymal Stem Cells. Aging Cell 2017, 16, 1369–1380. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Yu, B.; Xu, Y.; Rappold, A.G.; Diaz-Sanchez, D.; Samet, J.M.; Tong, H. Circulating microRNAs as Putative Mediators in the Association between Short-Term Exposure to Ambient Air Pollution and Cardiovascular Biomarkers. Ecotoxicol. Environ. Saf. 2022, 239, 113604. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yuan, W.; Yao, L.; Wang, S.; Jia, Z.; Wu, P.; Li, L.; Wei, P.; Wang, X.; et al. MicroRNA-155-5p Is a Key Regulator of Allergic Inflammation, Modulating the Epithelial Barrier by Targeting PKIα. Cell Death Dis. 2019, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hu, J.; Zhang, A.; Li, F.; Li, X. miR-155 Induces Endothelial Cell Apoptosis and Inflammatory Response in Atherosclerosis by Regulating Bmal1. Exp. Ther. Med. 2020, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- ElKashef, S.M.M.A.E.; Ahmad, S.E.-A.; Soliman, Y.M.A.; Mostafa, M.S. Role of microRNA-21 and microRNA-155 as Biomarkers for Bronchial Asthma. Innate Immun. 2021, 27, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Thavarajah, T.; Gu, W.; Cai, J.; Xu, Q. Impact of miRNA in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2018, 38, E159–E170. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Ferreira, V.V.; Pereira-da-Silva, T.; Ferreira, R.C. The Role of miRNAs in the Diagnosis of Stable Atherosclerosis of Different Arterial Territories: A Critical Review. Front. Cardiovasc. Med. 2022, 9, 1040971. [Google Scholar] [CrossRef]
- Schober, A.; Blay, R.M.; Saboor Maleki, S.; Zahedi, F.; Winklmaier, A.E.; Kakar, M.Y.; Baatsch, I.M.; Zhu, M.; Geißler, C.; Fusco, A.E.; et al. MicroRNA-21 Controls Circadian Regulation of Apoptosis in Atherosclerotic Lesions. Circulation 2021, 144, 1059–1073. [Google Scholar] [CrossRef]
- Tian, M.; Ji, Y.; Wang, T.; Zhang, W.; Zhou, Y.; Cui, Y. Changes in Circulating microRNA-126 Levels Are Associated with Immune Imbalance in Children with Acute Asthma. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418779243. [Google Scholar] [CrossRef]
- Santovito, D.; Egea, V.; Bidzhekov, K.; Natarelli, L.; Mourão, A.; Blanchet, X.; Wichapong, K.; Aslani, M.; Brunßen, C.; Horckmans, M.; et al. Noncanonical Inhibition of Caspase-3 by a Nuclear microRNA Confers Endothelial Protection by Autophagy in Atherosclerosis. Sci. Transl. Med. 2020, 12, eaaz2294. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Vogiatzi, G.; Sagris, M.; Antonopoulos, A.S.; Siasos, G.; Iliopoulos, D.C.; Perrea, D.; Vavouranakis, M.; Tsioufis, K.; et al. The Role of MicroRNA-126 in Atherosclerotic CardiovascularDiseases. Curr. Med. Chem. 2023, 30, 1902–1921. [Google Scholar] [CrossRef]
- Chin, D.D.; Poon, C.; Wang, J.; Joo, J.; Ong, V.; Jiang, Z.; Cheng, K.; Plotkin, A.; Magee, G.A.; Chung, E.J. miR-145 Micelles Mitigate Atherosclerosis by Modulating Vascular Smooth Muscle Cell Phenotype. Biomaterials 2021, 273, 120810. [Google Scholar] [CrossRef]
- Issouf, M.; Vargas, A.; Boivin, R.; Lavoie, J.-P. MicroRNA-221 Is Overexpressed in the Equine Asthmatic Airway Smooth Muscle and Modulates Smooth Muscle Cell Proliferation. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L748–L757. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yang, Q.; Zhou, Y.; Deng, H.; Zhu, Y.; Zhao, D.; Liu, F. MicroRNA-221 Modulates Airway Remodeling via the PI3K/AKT Pathway in OVA-Induced Chronic Murine Asthma. Front. Cell Dev. Biol. 2020, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, Y.; Rong, W.; Fan, L.; Cai, Y.; Qu, Q.; Gao, Y.; Zhao, H. miR-221 Participates in the Airway Epithelial Cells Injury in Asthma via Targeting SIRT1. Exp. Lung Res. 2018, 44, 272–279. [Google Scholar] [CrossRef]
- Guan, Y.; Ma, Y.; Tang, Y.; Liu, X.; Zhao, Y.; An, L. MiRNA-221-5p Suppressed the Th17/Treg Ratio in Asthma via RORγt/Foxp3 by Targeting SOCS1. Allergy Asthma Clin. Immunol. 2021, 17, 123. [Google Scholar] [CrossRef]
- Bazan, H.A.; Hatfield, S.A.; O’Malley, C.B.; Brooks, A.J.; Lightell, D.; Woods, T.C. Acute Loss of miR-221 and miR-222 in the Atherosclerotic Plaque Shoulder Accompanies Plaque Rupture. Stroke 2015, 46, 3285–3287. [Google Scholar] [CrossRef] [PubMed]
- Roffel, M.P.; Bracke, K.R.; Heijink, I.H.; Maes, T. miR-223: A Key Regulator in the Innate Immune Response in Asthma and COPD. Front. Med. 2020, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lu, Q.; Zhao, Y.; Ding, Z.; Yu, S.; Li, J.; Ji, M.; Fan, H.; Hou, S. miR-223: A Key Regulator of Pulmonary Inflammation. Front. Med. 2023, 10, 1187557. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Qiao, Q.; Ono, K.; Wei, M.; Tan, W.; Wang, C.; Liu, Y.; Liu, G.; Zheng, M. miR-223-3p Inhibits the Progression of Atherosclerosis via down-Regulating the Activation of MEK1/ERK1/2 in Macrophages. Aging 2022, 14, 1865–1878. [Google Scholar] [CrossRef]
- Datta, N.; Johnson, C.; Kao, D.; Gurnani, P.; Alexander, C.; Polytarchou, C.; Monaghan, T.M. MicroRNA-Based Therapeutics for Inflammatory Disorders of the Microbiota-Gut-Brain Axis. Pharmacol. Res. 2023, 194, 106870. [Google Scholar] [CrossRef]
- Greene, M.A.; Worley, G.A.; Udoka, A.N.S.; Powell, R.R.; Bruce, T.; Klotz, J.L.; Bridges, W.C.; Duckett, S.K. Use of AgomiR and AntagomiR Technologies to Alter Satellite Cell Proliferation in Vitro, miRNA Expression, and Muscle Fiber Hypertrophy in Intrauterine Growth-Restricted Lambs. Front. Mol. Biosci. 2023, 10, 1286890. [Google Scholar] [CrossRef]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as Therapeutic Targets in Cardiovascular Disease. J. Clin. Investig. 2022, 132, e159179. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hur, J.; Kang, J.Y.; Rhee, C.K.; Lee, S.Y. MicroRNA-21 Inhibition Suppresses Alveolar M2 Macrophages in an Ovalbumin-Induced Allergic Asthma Mice Model. Allergy Asthma Immunol. Res. 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-L.; Zhang, L.; Bo, X.-H. MiR-126 on Mice with Coronary Artery Disease by Targeting S1PR2. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 893–904. [Google Scholar] [CrossRef]
- Li, P.; Wei, J.; Li, X.; Cheng, Y.; Chen, W.; Cui, Y.; Simoncini, T.; Gu, Z.; Yang, J.; Fu, X. 17β-Estradiol Enhances Vascular Endothelial Ets-1/miR-126-3p Expression: The Possible Mechanism for Attenuation of Atherosclerosis. J. Clin. Endocrinol. Metab. 2017, 102, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liang, T.; Zhou, Y.; Ju, H.; Song, D.; Fang, H. Telocytes Reduce Oxidative Stress by Downregulating DUOX2 Expression in Inflamed Lungs of Mice. Acta Biochim. Biophys. Sin. 2022, 54, 574–582. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Yuan, Y.; Nie, C.; Wei, K.; Zhang, T.; Chen, T.; Chu, X. Neutrophil-Membrane-Coated Biomineralized Metal–Organic Framework Nanoparticles for Atherosclerosis Treatment by Targeting Gene Silencing. ACS Nano 2023, 17, 7721–7732. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Wang, C.; Hu, W.; Zou, S.; Ren, H.; Zuo, Y.; Qu, L. MiR-127-3p Enhances Macrophagic Proliferation via Disturbing Fatty Acid Profiles and Oxidative Phosphorylation in Atherosclerosis. J. Mol. Cell Cardiol. 2024, 193, 36–52. [Google Scholar] [CrossRef]
- Qin, B.; Shu, Y.; Long, L.; Li, H.; Men, X.; Feng, L.; Yang, H.; Lu, Z. MicroRNA-142-3p Induces Atherosclerosis-Associated Endothelial Cell Apoptosis by Directly Targeting Rictor. Cell Physiol. Biochem. 2018, 47, 1589–1603. [Google Scholar] [CrossRef]
- Nalbant, E.; Akkaya-Ulum, Y.Z. Exploring Regulatory Mechanisms on miRNAs and Their Implications in Inflammation-Related Diseases. Clin. Exp. Med. 2024, 24, 142. [Google Scholar] [CrossRef]
- Johnson, J.L. Elucidating the Contributory Role of microRNA to Cardiovascular Diseases (a Review). Vascul. Pharmacol. 2019, 114, 31–48. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef] [PubMed]
- Kp, A.; Kaliaperumal, K.; Sekar, D. microRNAs and Their Therapeutic Strategy in Phase I and Phase II Clinical Trials. Epigenomics 2024, 16, 259–271. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, F.-Y.; Lin, Y.-W.; Cheng, S.-M.; Chang, C.-C.; Lin, R.-H.; Chuang, C.-L.; Sheu, J.-S.; Chen, S.-M.; Tsai, C.-S. Platelet MicroRNA 365-3p Expression Correlates with High On-Treatment Platelet Reactivity in Coronary Artery Disease Patients. Cardiovasc. Drugs Ther. 2019, 33, 129–137. [Google Scholar] [CrossRef]
- Suzuki, M.; Tomoike, H.; Dai, Z.; Hosoda, T.; Sumiyoshi, T.; Hosoda, S.; Isobe, M. Polyvascular Disease and the Incidence of Cancer in Patients with Coronary Artery Disease. JMA J. 2022, 5, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, J.; Mahendra, L.; Fageeh, H.N.; Fageeh, H.I.; Ibraheem, W.; Abdulkarim, H.H.; Kanakamedala, A.; Prakash, P.; Srinivasan, S.; Balaji, T.M.; et al. miRNA-146a and miRNA-126 as Potential Biomarkers in Patients with Coronary Artery Disease and Generalized Periodontitis. Materials 2021, 14, 4692. [Google Scholar] [CrossRef] [PubMed]
- Howlett, P.; Cleal, J.K.; Wu, H.; Shah, N.; Horton, A.; Curzen, N.; Mahmoudi, M. MicroRNA 8059 as a Marker for the Presence and Extent of Coronary Artery Calcification. Open Heart 2018, 5, e000678. [Google Scholar] [CrossRef]
- Pasca, S.; Jurj, A.; Petrushev, B.; Tomuleasa, C.; Matei, D. MicroRNA-155 Implication in M1 Polarization and the Impact in Inflammatory Diseases. Front. Immunol. 2020, 11, 625. [Google Scholar] [CrossRef]

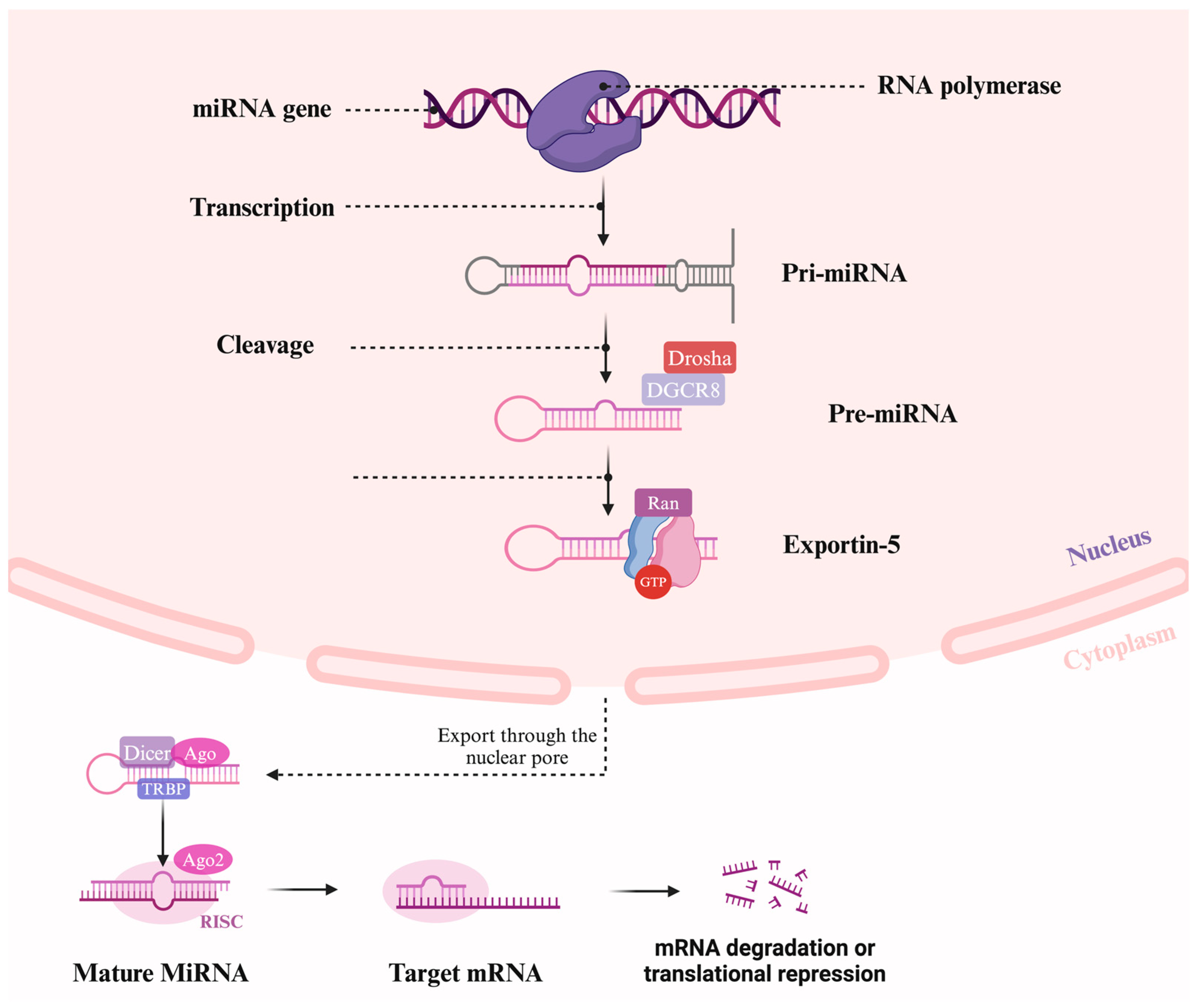
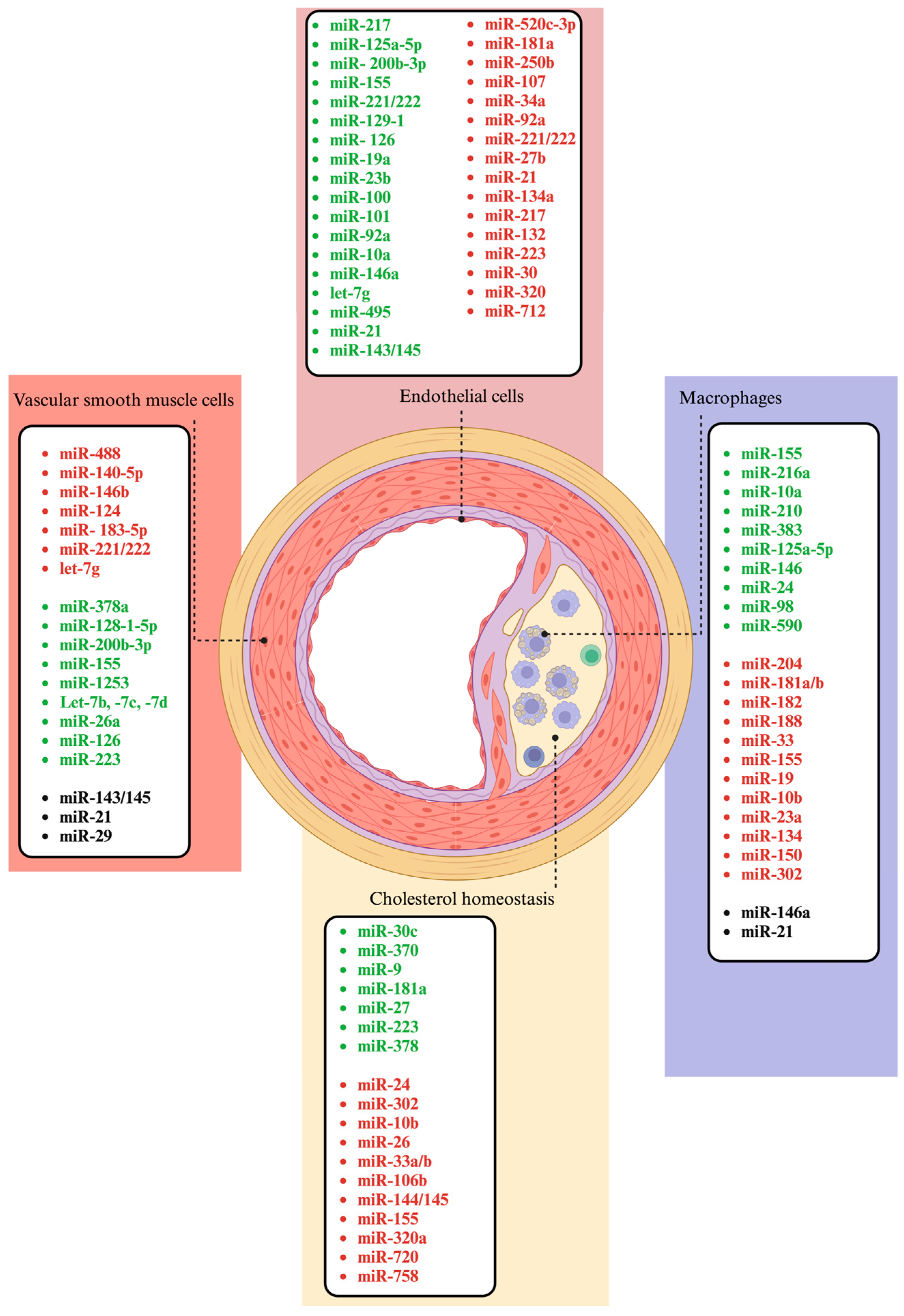
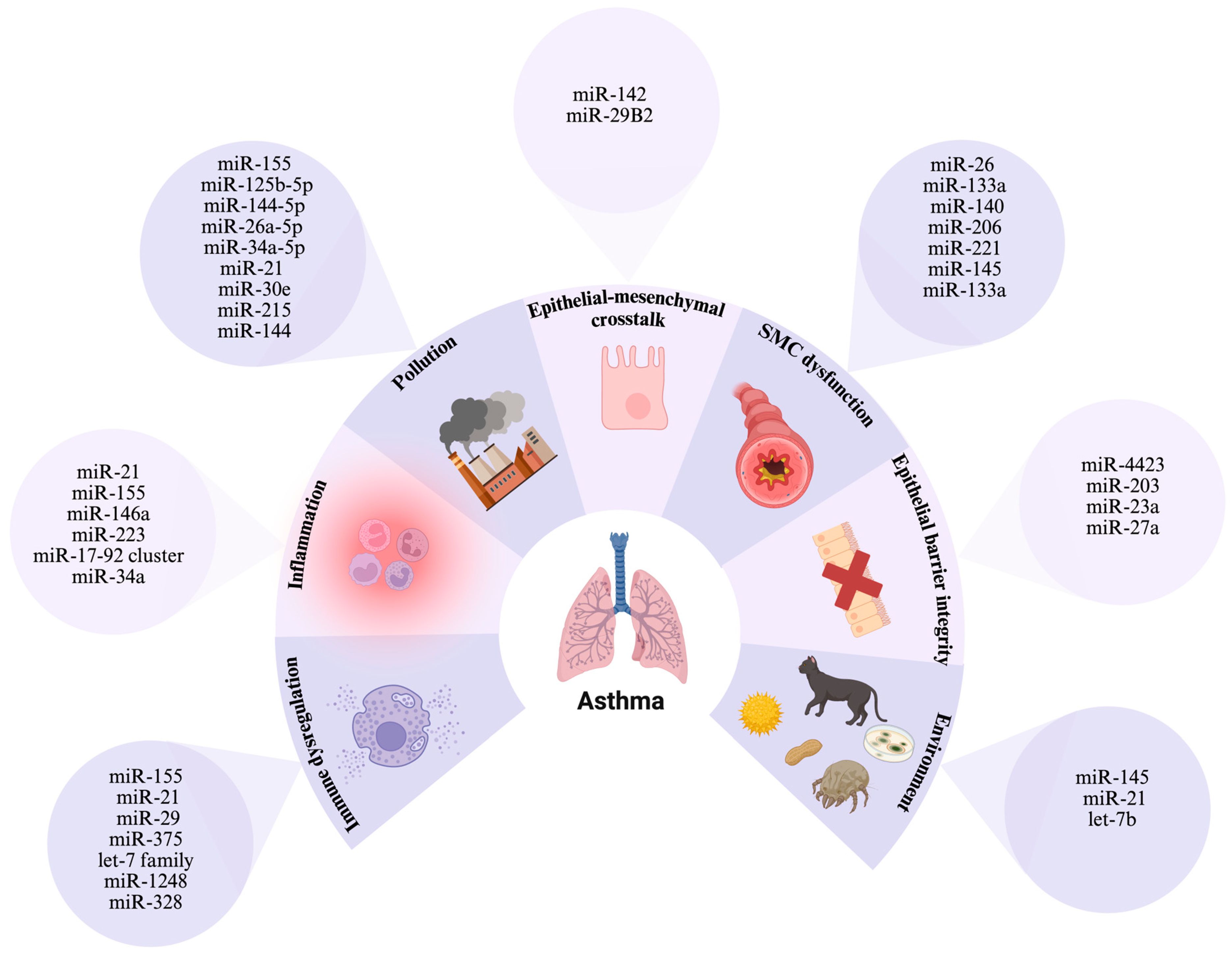
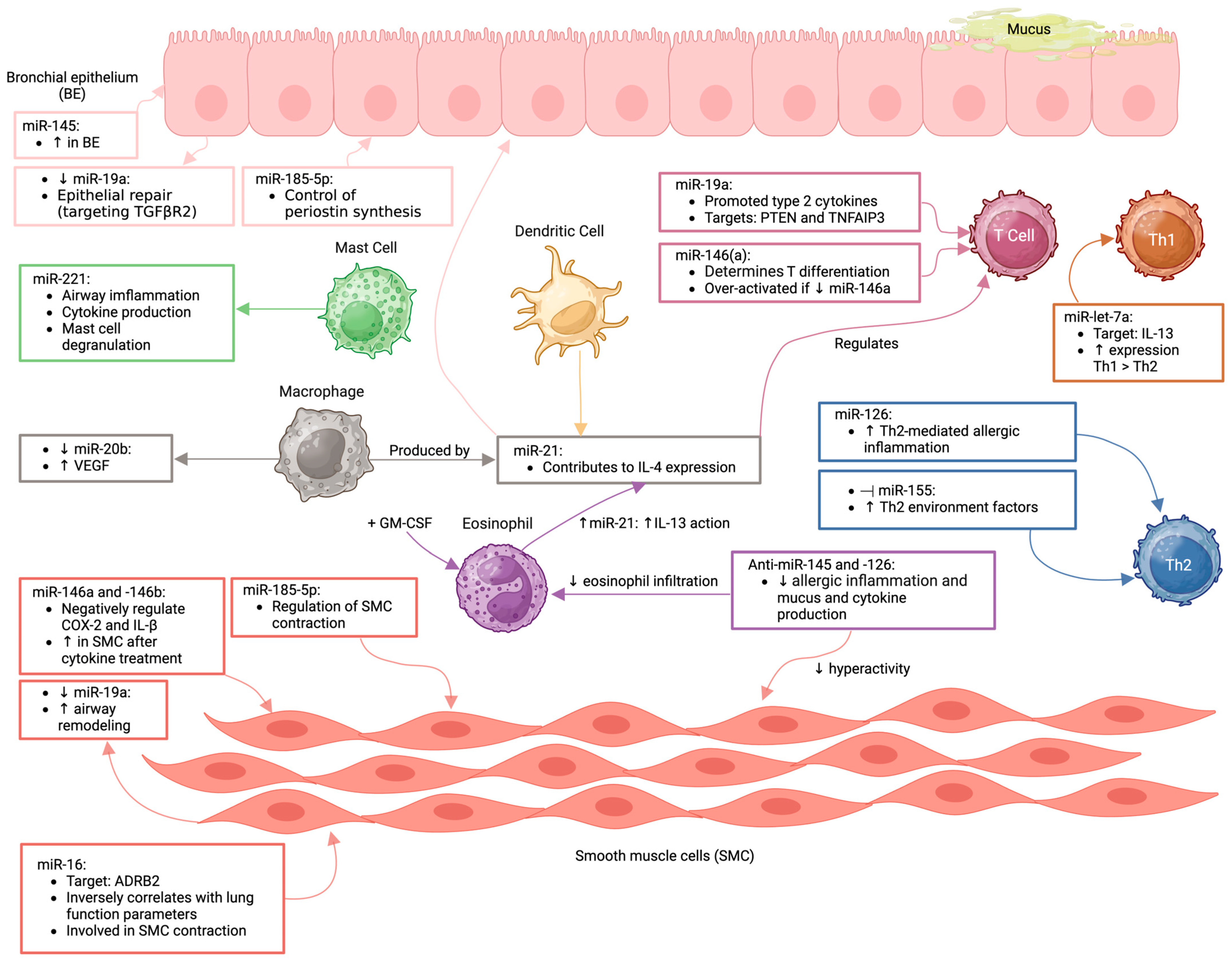
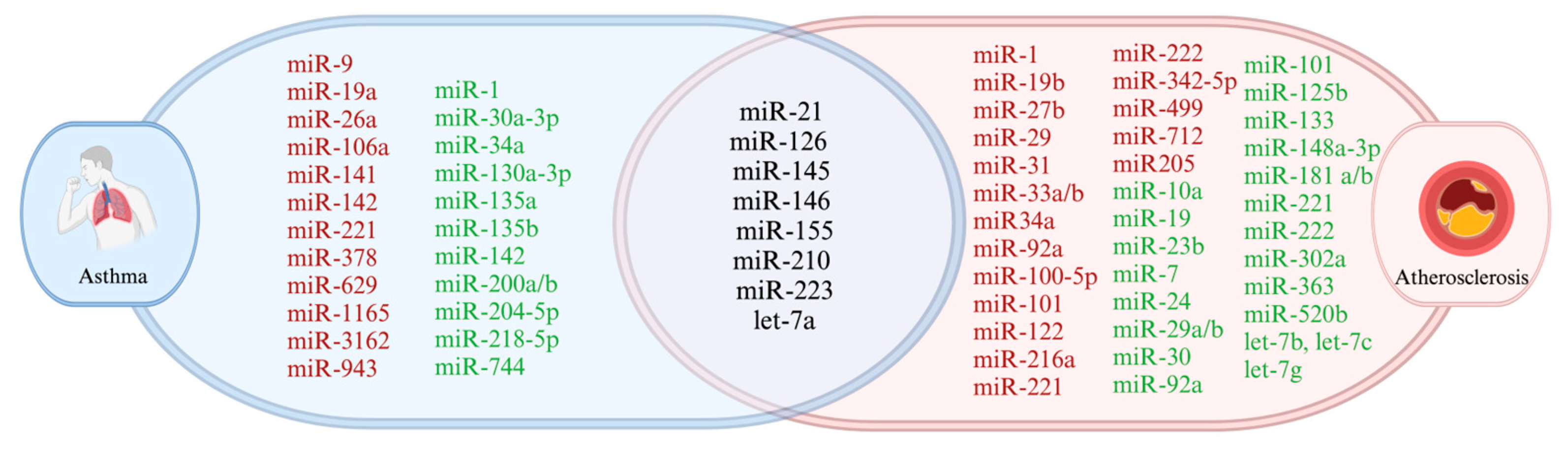
| MiRNA | Effect | Target/Pathway | References |
|---|---|---|---|
| miR-1 | Induction of vSMC differentiation, promotion of inflammation | KLF4, NF-κB | [151] |
| miR-19b | Promotion of macrophage foam cell formation | ABCA1, PPARγ | [152,153] |
| miR-21 | Promotion of EC inflammatory phenotype | NF-κB | [154] |
| Stimulation of vSMC proliferation | PTEN, SPRY1, RASA1 | [104] | |
| miR-27b | Plaque progression and development | Pparg, Angptl3, Gpam | [155,156] |
| miR-29 | Mediation of vSMC calcification | ADAMTS-7 | [157] |
| miR-31 | Regulation of macrophage cell proliferation and apoptosis | NOX4 | [67] |
| miR-33a/b | Regulation of cholesterol and fatty acid homeostasis | ABCA1, ABCG1 | [142,158] |
| miR34a | Promotion of EC senescence | SIRT1 | [159,160] |
| miR-92a | Induction of endothelial dysfunction and inflammation, inhibits angiogenesis | KLF2, KLF4, SOCS5 | [161,162,163] |
| miR-100-5p | Inhibition of cell progression and inflammatory response in eosinophils | FZD5/Wnt/β-catenin pathway | [79] |
| miR-101 | Promotion of foam cell formation | ABCA1 | [164] |
| miR-122 | Regulation of cholesterol and fatty acid homeostasis | HMGCR, SREBP2, fatty acid synthase | [165] |
| miR-145 | Regulation of vSMC phenotypic modulation | KLF4/KLF5, ELK1, ABCA1/SCARB1 | [121] |
| miR-155 | Promotion of inflammation | Bcl6 | [166,167,168,169,170] |
| Enhancement of inflammation, impairment of cholesterol efflux | SOCS1 | ||
| Suppression of anti-inflammatory signals | BCL-6, pSTAT3 | ||
| miR-210 | Induction of EC apoptosis | PDK1 | [171] |
| miR-216a | Promotion of senescence of EC | SMAD3/IκBα | [172] |
| miR-221 | Induction of EC dysfunction | AMPK | [173,174] |
| miR-222 | Promotion of vSMC proliferation and migration | eNOS | [175] |
| miR-223 | Promotion of EC apoptosis Thrombus formation | HMG-CoA synthase 1, methyl sterol, monooxygenase 1 | [132] |
| miR-342-5p | Enhancement of the inflammatory stimulation of macrophages | AKT1 | [176] |
| miR-499 | Promotion of vSMC proliferation and migration | SOX6 | [177] |
| miR-712-miR205 | Stimulation of pro-inflammatory EC responses | TIMP3 | [178] |
| MiRNA | Effect | Target/Pathway | References |
|---|---|---|---|
| miR-10a | Anti-inflammatory effect | MAP3K7, βTRC | [179] |
| Antiapoptotic effect | Bim1 | ||
| miR-19 | Inhibition of shear stress-induced cellular proliferation | cyclin D1 | [180] |
| miR-21 | Reduces plaque necrosis and inflammation | MAP2K3 | [181] |
| Enhances EC survival by decreasing apoptosis and activating the NO Pathway | EC | [154] | |
| miR-23b | Inhibits EC proliferation | E2F1, Rb | [182] |
| miR-7 | Inhibits the final steps of cholesterol synthesis | DHCR24, SC5D, SREBP2 | [150] |
| miR-24 | Inhibits the proliferation and migration of ECs | importin-α3, MMP14 | [183] |
| miR-29a/b | Inhibition of calcification of vSMCs | ADAMTS-7 | [157] |
| Regulates cell migration | MMP-2/MMP-9 | [184] | |
| miR-30 | Regulation of endothelial cell autophagy | ATG6 | [185] |
| miR-92a | Blocks angiogenesis and reduces migration of endothelial cells | Integrin-alpha5 | [186] |
| miR-101 | Regulates Vascular Smooth Muscle Cell Migration | DOCK4 | [187] |
| miR-125b | Inhibition of calcification of vSMCs | Ets-1, SP-7 | [188,189] |
| miR-126 | Inhibits apoptosis of EC | SIRT1, SOD2 | [190] |
| Reduction of lipid accumulation | Mac | [191] | |
| Reduction of inflammatory response | MAP3K10 | [192] | |
| miR-133 | Reduction of oxidative stress-induced apoptosis | caspase-9/caspase-3 | [11] |
| Suppression of EC proliferation rate, viability and migration activity | VEGFR2 and FGFR1 | [95] | |
| miR-143/145 cluster | Inhibition of vSMC proliferation | KLF4, PDGFR-β, TPM4 | [120,193] |
| Promotion of the contractile phenotype of vSMCs | ELK1 | ||
| Reduction of inflammatory response | ADAM17 | ||
| miR-145 | Regulates vSMC fate and plasticity | KLF4 | [193] |
| miR-146a | Delays endothelial cell senescence Delays both inflammatory response and ox-LDL accumulation | NOX4 TRAF6/IRAK1 TLR4 | [85,88,194] |
| miR-148a-3p | Inhibition of ox-LDL-induced cell apoptosis and inflammation | CNTN4 | [111] |
| miR-155 | Reduction of inflammation | MAP3K10 | [195,196] |
| Enhancement of cholesterol efflux from macrophages | SOCS1 | ||
| Internalization and endocytosis of oxidized LDL in macrophages | CD36, Vav3 | ||
| miR-181a/b | Modulates endothelial integrity and reduces inflammation | TAB2, NEMO, MAP3K3, importin-α3, TIMP3/ELN | [197,198] |
| miR-210 | Attenuation of lipid accumulation and inflammation | IGF2 | [146] |
| miR-221 | Inhibition of endothelial progenitor cell proliferation | PAK1 | [174] |
| miR-222 | Enhances anti-inflammatory response | SOCS3/JAK2/STAT3 | [199] |
| miR-223 | Regulation of cholesterol metabolism and inflammation | ABCA1, IKKα, NLRP3 | [132] |
| miR-302a | Modulation of cholesterol efflux | ABCA1 | [200] |
| miR-363 | Reduction of EC inflammatory responses | NOX4 | [201] |
| miR-520b | Inhibition of endothelial activation | NF-κB/p65/ICAM1/VCAM1 | [202] |
| let-7g | Inhibition of foam cell formation and adipocyte differentiation | HMGA2/CEBPβ | [203] |
| let-7a | Inhibition of ox-LDL-induced endothelial cell apoptosis, NO deficiency, ROS overproduction, LOX-1 upregulation and eNOS downregulation | LOX-1 | [204] |
| let-7b, let-7c, let-7c | Inhibition of EC apoptosis and dysfunction, vSMC migration/proliferation Attenuation of pro-inflammatory cytokine release from human plaques Reduction of plaque size in vivo | LOX-1, HAS-2 | [204,205,206] |
| MiRNA | Effect | Target/Pathway | References |
|---|---|---|---|
| miR-9 | Promotion of inflammatory responses | Protein phosphatase 2A regulatory subunit B, SLC26A2 | [221] |
| miR-19a | Inhibition of proliferation and migration of airway smooth muscle cells (aSMCs) | PTEN | [222] |
| miR-21 | Enhancement of Th2 polarization by suppressing Th1-polarizing cytokines and allergic airway inflammation | PDCD4 PTEN | [223,224] |
| miR-26a | Promotion of aSMC hypertrophy | GSK-3β | [225] |
| miR-106a | Regulation of immune pathways | IL-10 | [164] |
| miR-126 | Modulation of gene translation to bridge TLR4/ MyD88 signaling and Th2 response in a BALB/c mouse model of HDM-induced allergic asthma | OBF.1→ ↓ GATA3 | [226] |
| miR-141 | Stimulation of IL-13-induced goblet cell metaplasia, promotes airway remodeling and pathological airway mucus production | CLCA1 | [227] |
| miR-142 | Enhances FcεRI-mediated degranulation and rescues the reduction of degranulation Modulation of M2 polarization and profibrotic activities | LPP SOCS1 | [228] |
| miR-145-5p | Enhancement of eosinophilic inflammation, mucus hypersecretion, AHR and Th2 cytokine production Stimulation of epithelial barrier dysfunction and suppression of epithelial repair | KLF 4/5 RUNX3 ↓ KIF3A | [229,230] |
| miR-155 | Enhancement of airway remodeling Modulation of Th2 responses through the transcription factor PU.1 Enhancement of IL-4, -5, -13 and -17a secretion; enhancement of mucus secretion; stimulation of T cell activation and proliferative response | ↓ SOCS1, Pu.1, c-Maf ↓ COX-2 MAF bZIP transcription factor CTLA-4 | [218,231,232,233,234] |
| miR-221 | Reduces the permeability of inflammatory cells, regulates the cell cycle of mast cells and promotes their proliferation; increases IL-4 secretion and promotes the differentiation of Th cells into Th2 cells | P27KIP1; PTEN and p38/NF-κB pathway | [235,236] |
| miR-223 | Inhibition of airway inflammation, NLRP3 levels and IL-1β release | NLRP3 | [237] |
| miR-378 | Promotion of aSMC proliferation and apoptosis resistance | ErbB, RAS, MAPK and calcium signaling pathway | [238] |
| miR-629 | Enhancement of IL-1β and IL-8 protein, which show a positive correlation with increased neutrophil presence in sputum | IL-8 mRNA | [239] |
| miR-1165 | Inhibition of bronchial hyper-responsiveness, airway inflammation and differentiation of T cells toward Th2 | IL-13, PPM1A | [240] |
| miR-3162 | Enhancement of bronchial hyper-responsiveness and airway inflammation; regulation of Th1/Th2 balance | β-catenin | [241] |
| miR-943 | Increases the number of macrophages, eosinophils, lymphocytes and neutrophils; enhances the expression of collagen, β-catenin and c-Myc in lung tissue; promotes subepithelial fibrosis and aSMC proliferation | SFRP4 | [242] |
| MiRNA | Effect | Target/Pathway | References |
|---|---|---|---|
| Let-7a | Inhibits proliferation and promotes apoptosis of human aSMCs | STAT3 | [213] |
| miR-1 | Inhibits secretion of IL-4, -5, -8 and TNF-α; regulates Th1/Th2 balance | - | [243] |
| miR-30a-3p | Inhibition of the secretion of specific IgE, eotaxin-1, IL-5 and IL-4 | CCR3 | [244] |
| miR-34a | Enhancement of the polarization of Th2 cells | Wnt signaling pathway | [245] |
| miR-130a-3p | Modulation of M2 polarization and profibrotic activities | Proliferator-activated receptor γ | [246] |
| miR-135a | Inhibition of bronchial hyper-responsiveness and lung pathological changes; reduction of the secretion of TNF-α, IL-6, IL-5 and Eotaxin | STAT family | [247] |
| miR-135b | Inhibition of the immune response of Th17 cells, goblet cell proliferation and bronchial hyper-responsiveness; reduction of the number of eosinophils and lymphocytes | CXCL12 | [248] |
| miR-142 | Regulation of airway inflammation | PTEN/AKT | [249] |
| miR-146a/b | Regulation of inflammatory responses | IRAK1, TRAF6 | [250] |
| miR-200a/b | Inhibition of the secretion of TNF-α, IL-4, -5, -13 and -1β | ORMDL3 | [251] |
| miR-204-5p | Inhibition of aSMC proliferation and extracellular matrix production | SIX1 | [252] |
| miR-210 | Enhancement of the polarization of Th2 cells | FOXP3 | [245] |
| miR-218-5p | Inhibition of bronchial hyper-responsiveness, eosinophilic airway inflammation and the expression of CCL26 | CTNND2 | [253] |
| miR-744 | Inhibition of bronchial epithelial cell proliferation | TGF-β1 | [254] |
| MiRNA | Title | Status | Identifier | Sponsor | Enrollment | Study Type | References |
|---|---|---|---|---|---|---|---|
| miR-365-3p | The Relationship of Platelet Micro-RNA Expression and Platelet Reactivity in Patients Under Clopidogrel or Ticagrelor Treatment | Completed (2017) | NCT02101437 | Taipei City Hospital | 175 | Interventional | [295] * |
| panel of miRNAs | Circulating microRNAs and Adverse Cardiovascular Outcomes in Patients With Coronary Artery Disease | Recruiting | NCT03635255 | National Taiwan University Hospital | 460 (estimated) | Observational | * |
| miRNA 92a miRNA 210 | Extracellular microRNA: Biomarkers of Endothelial Dysfunction in Obese Adolescents & Adults With Obstructive Sleep Apnea | Unknown | NCT03546751 | University of California, San Diego | 100 (estimated) | Interventional | * |
| panel of miRNAs | Study of microRNAs in the Evolution of Carotid Plaque. Physiopathological and Therapeutic Interest | Unknown | NCT03149406 | Centre Hospitalier Universitaire, Amiens | 245 (estimated) | Observational | * |
| miR-100, miR-125a, miR-127, miR-133a, miR-145, miR-221 | Physiopathological and Therapeutic Value of microRNA in the Progression of Carotid Artery Plaques: Carotid Protocol and microRNA (CAMIA2) | Unknown | NCT02804659 | Centre Hospitalier Universitaire, Amiens | 120 (estimated) | Observational | * |
| panel of miRNAs | microRNAs in the Diagnosis of Atherosclerotic Plaque Instability | Unknown | NCT05680935 | I.M. Sechenov First Moscow State Medical University | 60 (estimated) | Interventional | * |
| panel of miRNAs | Pathogenic Mechanisms of Cancer and Cardiovascular Diseases | Completed | NCT03051191 | Sakakibara Heart Institute | 66 | Observational | [296] * |
| miR-146a, miR-126 | Interplay of miRNA-146a and miRNA-126 in Chronic Periodontitis Patients With Coronary Artery Disease | Completed | NCT04583085 | Meenakshi Ammal Dental College and Hospital | 75 | Observational | [297] * |
| panel of miRNAs | miRNAs in Critical Limb Ischemia (miRNACLI) (miRNACLI) | Recruiting | NCT06066268 | IRCCS Policlinico S. Donato | 80 (estimated) | Observational | * |
| panel of miRNAs | The Effect of Structured Lifestyle Modification and Yoga Practice on Metabolic Processes Associated With Cardiovascular Disease (SLYM II) | Recruiting | NCT05250310 | Icahn School of Medicine at Mount Sinai | 120 (estimated) | Interventional | * |
| panel of miRNAs | RNA Sequencing in the Framingham Heart Study Third Generation Cohort Exam 2 | Completed | NCT03225183 | National Heart, Lung, and Blood Institute (NHLBI) | 1700 | Observational | * |
| panel of miRNAs | Extracellular RNAs in Relation to Cardiometabolic Risk | Completed | NCT03225196 | National Heart, Lung, and Blood Institute (NHLBI) | 4495 | Observational | * |
| panel of miRNAs | Circulating microRNAs and Degenerative Abdominal Aorta Aneurysm (ACTA-miRNA) | Completed | NCT03974958 | Assistance Publique Hopitaux De Marseille | 106 | Interventional | * |
| panel of miRNAs | Assessment of the TGF-beta Pathway and Micro-RNA in Pediatric Pulmonary Arterial Hypertension | Unknown | NCT04489251 | Medical College of Wisconsin | 40 (estimated) | Observational | * |
| miR-8059 | MAP-Calcification: MicroRNAs as Potential Biomarkers for Coronary Artery Calcification | Completed | NCT01992848 | University of Surrey | 45 | Interventional | [298] * |
| panel of miRNAs | Potential Diagnostic and Prognostic Value of microRNAs for the Patients of Acute Coronary Syndrome | Unknown | NCT02755207 | Xinhua Hospital, Shanghai Jiao Tong University School of Medicine | 100 (estimated) | Observational | * |
| miR-32 | Coronary Artery Calcification in Type 2 Diabetes Mellitus (USCAC Study) | Recruiting | NCT04889053 | University of South China | 1400 (estimated) | Observational | * |
| miR-210 | The Role of microRNA-210 in Regulating Oxidative Stress in Patients With PAD | Active, not recruiting | NCT04089943 | University of West Florida | 230 | Interventional | * |
| panel of ~200 miRNAs | Role of microRNAs in T Cell-Driven Inflammation in Asthma (RITA) | Completed | NCT01484691 | University of California, San Francisco | 55 | Interventional | * |
| miR-141 | Study of the Inflammation and Airway Changes That Occur After Exposure to Allergen in Asthmatics (ACE) | Completed | NCT02230189 | University of California, San Francisco | 28 | Interventional | [227] * |
| miR-7, miR-20a, miR-21, miR-22, miR-145 and miR-155 | Circulating Micro RNAs Expression in Egyptian Bronchial Asthma and COPD Patients | Completed | NCT02719145 | Tanta University | 30 | Observational | [299] * |
| panel of miRNAs | Interaction Between Benralizumab and Basophils in Eosinophilic Asthma (BASEAS) | Completed | NCT04742504 | Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz | 20 | Observational | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimbru, R.-I.; Zimbru, E.-L.; Bojin, F.-M.; Haidar, L.; Andor, M.; Harich, O.O.; Tănasie, G.; Tatu, C.; Mailat, D.-E.; Zbîrcea, I.-M.; et al. Connecting the Dots: How MicroRNAs Link Asthma and Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 3570. https://doi.org/10.3390/ijms26083570
Zimbru R-I, Zimbru E-L, Bojin F-M, Haidar L, Andor M, Harich OO, Tănasie G, Tatu C, Mailat D-E, Zbîrcea I-M, et al. Connecting the Dots: How MicroRNAs Link Asthma and Atherosclerosis. International Journal of Molecular Sciences. 2025; 26(8):3570. https://doi.org/10.3390/ijms26083570
Chicago/Turabian StyleZimbru, Răzvan-Ionuț, Elena-Larisa Zimbru, Florina-Maria Bojin, Laura Haidar, Minodora Andor, Octavia Oana Harich, Gabriela Tănasie, Carmen Tatu, Diana-Evelyne Mailat, Iulia-Maria Zbîrcea, and et al. 2025. "Connecting the Dots: How MicroRNAs Link Asthma and Atherosclerosis" International Journal of Molecular Sciences 26, no. 8: 3570. https://doi.org/10.3390/ijms26083570
APA StyleZimbru, R.-I., Zimbru, E.-L., Bojin, F.-M., Haidar, L., Andor, M., Harich, O. O., Tănasie, G., Tatu, C., Mailat, D.-E., Zbîrcea, I.-M., Hirtie, B., Uța, C., Bănărescu, C.-F., & Panaitescu, C. (2025). Connecting the Dots: How MicroRNAs Link Asthma and Atherosclerosis. International Journal of Molecular Sciences, 26(8), 3570. https://doi.org/10.3390/ijms26083570







