The Role of Tetrahydrocurcumin in Tumor and Neurodegenerative Diseases Through Anti-Inflammatory Effects
Abstract
1. Introduction
2. Chemical and Biological Properties of THC
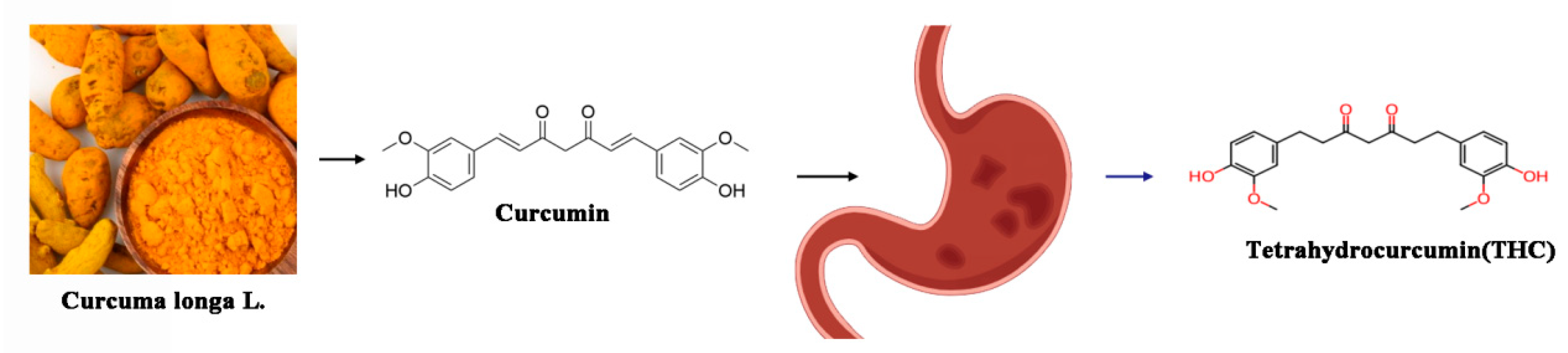
2.1. Chemical Structure of THC Compared with Curcumin
2.2. Solubility, Stability, and Bioavailability of THC
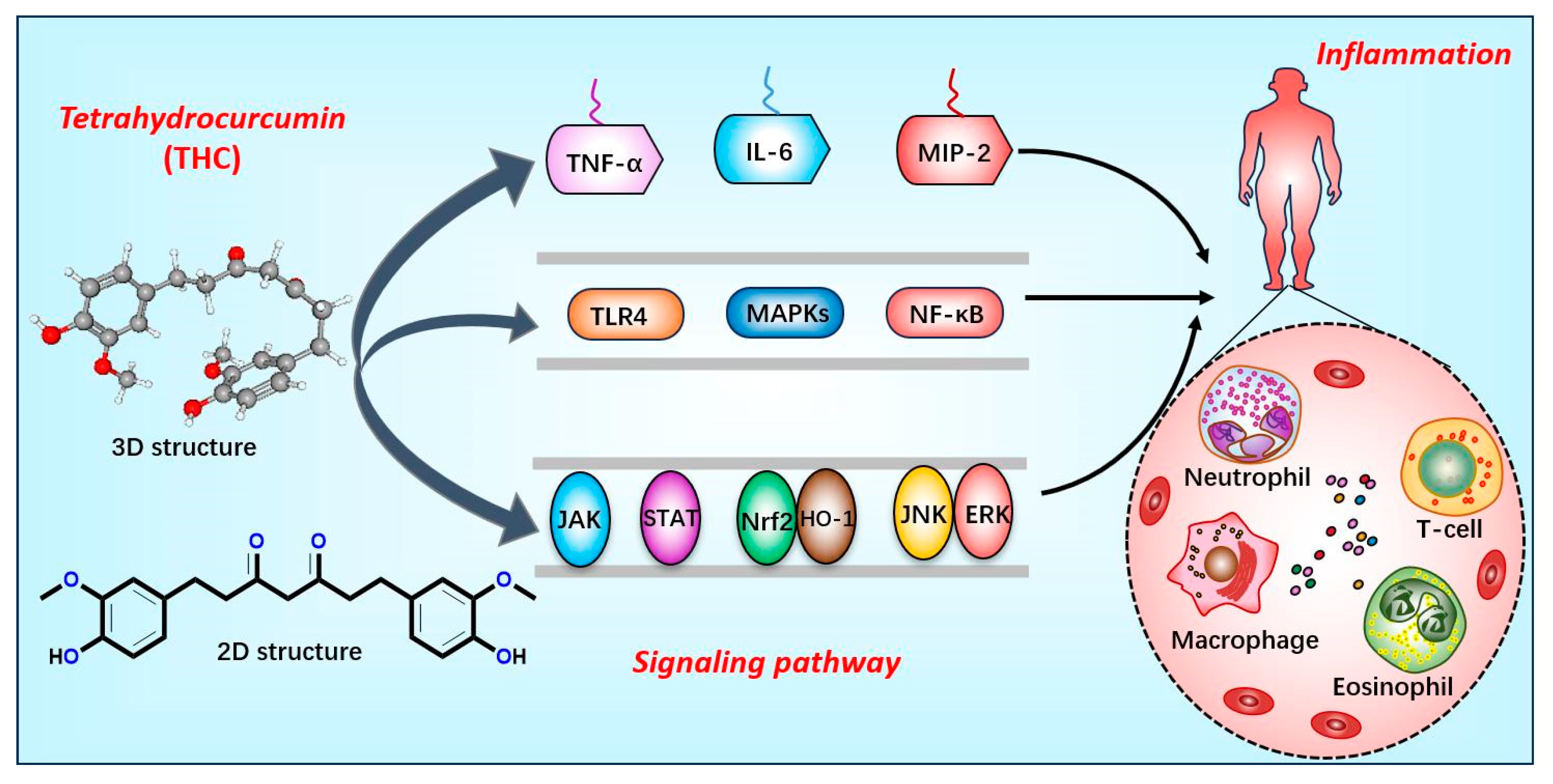
2.3. Anti-Inflammatory Mechanisms of THC
2.3.1. Inhibiting Inflammatory Factors and Signaling Pathways
2.3.2. Improved Vascular Function and Structure
2.3.3. Antioxidant Effect
2.3.4. Modulating Immune Response
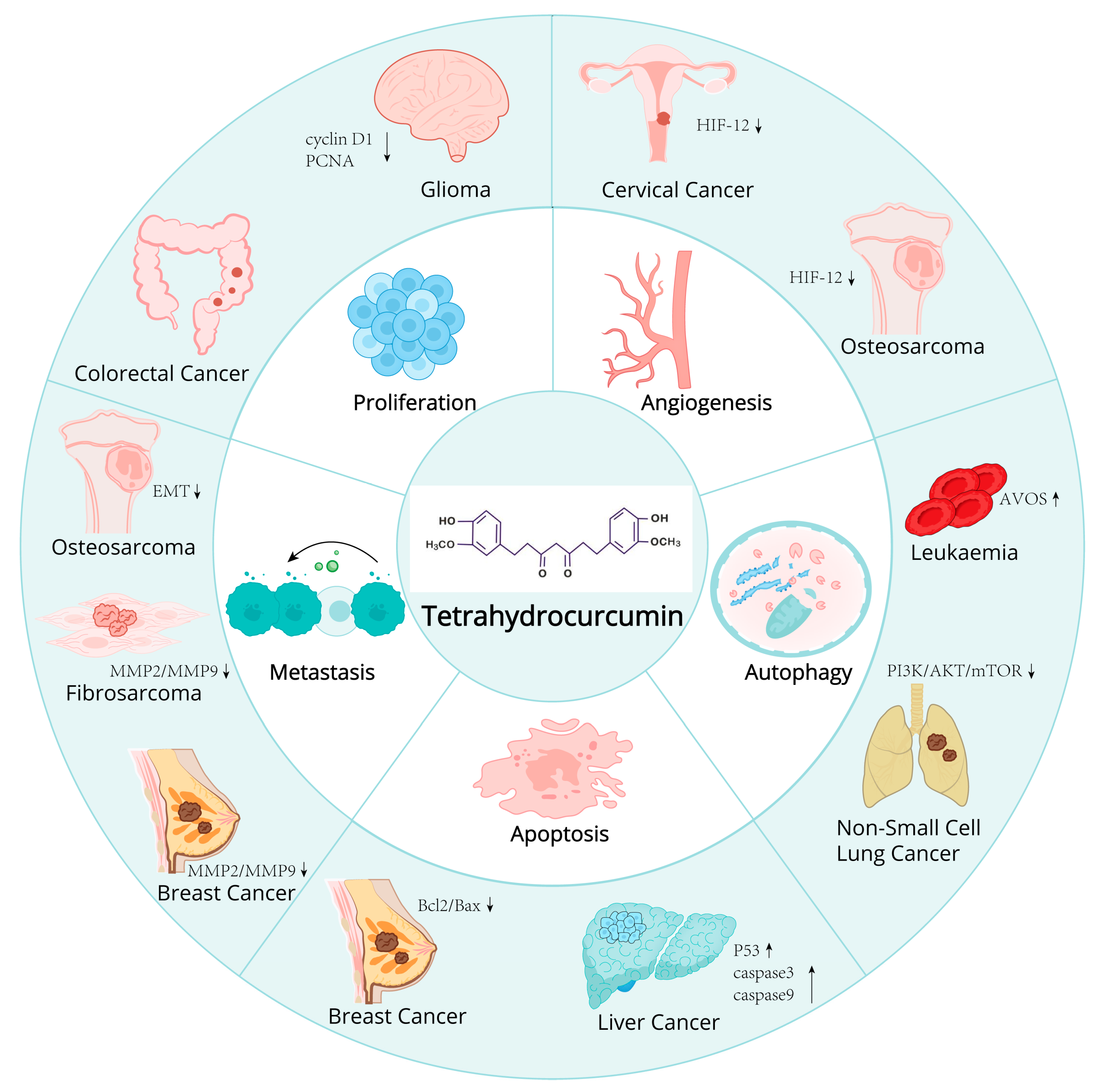
2.4. Potential of THC in Cancer Prevention and Therapy
2.4.1. Mechanism of Action of THC on Cancer Cells
Anti-Proliferative Effect
Proapoptotic Effect
Anti-Angiogenic Effect
2.4.2. Research Progress of THC in Different Cancer Models
Cervical Cancer
Breast Cancer
Liver Cancer
Acute Myeloid Leukemia
Fibrosarcoma
Colon Cancer
Osteosarcoma
Non-Small Cell Lung Cancer
Glioma
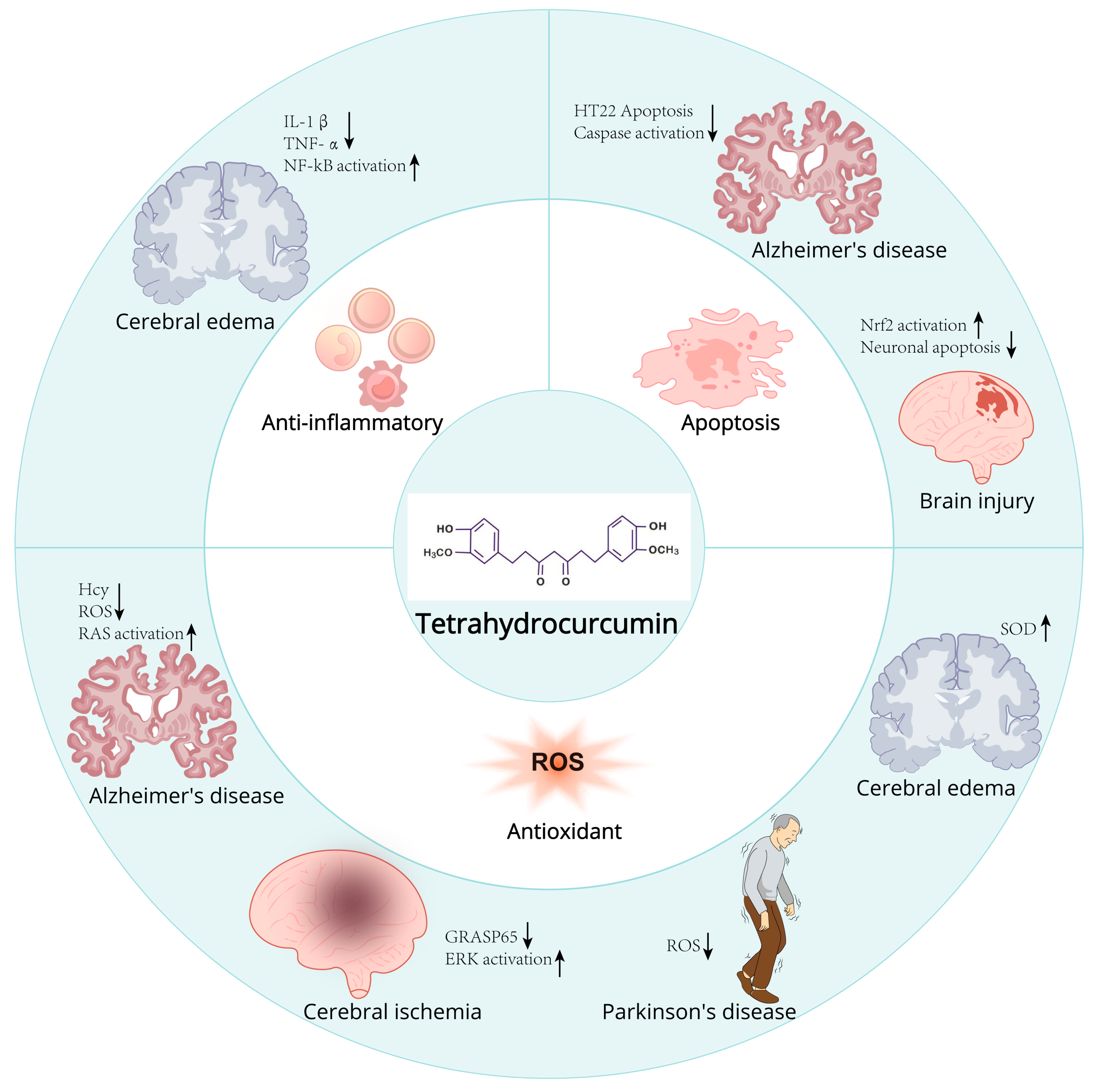
2.5. Protective Effects of THC in Neurodegenerative Diseases
2.5.1. Potential Mechanisms of THC on Neuroprotection and Repair
Antioxidation
Anti-Inflammatory Effect
Inhibition of Apoptosis
Other Potential Mechanisms
2.5.2. Potential Use of THC in Neurodegenerative Diseases
Brain Injury
Cerebral Edema
Cerebral Ischemia
Parkinson’s Disease
Alzheimer’s Disease
- Improve the Neurotoxicity Caused by Aβ
- Neuroprotective Effect
2.6. Clinical Applications and Safety Considerations
Dose, Mode of Administration, and Potential Side Effects of THC
2.7. Drug Delivery Systems and Pharmacokinetics
Nanoparticle
2.8. Future Research Directions and Challenges
2.8.1. Development of New Dosage Forms
2.8.2. Applications of Synthetic Biology
2.8.3. Challenge
Low Bioavailability
Long-Term Toxicity
Individual Difference
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kothaplly, S.; Alukapally, S.; Nagula, N.; Maddela, R. Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans Under Fasting Conditions. Adv. Ther. 2022, 39, 2128–2138. [Google Scholar] [CrossRef]
- Yuan, T.; Yin, Z.; Yan, Z.; Hao, Q.; Zeng, J.; Li, L.; Zhao, J. Tetrahydrocurcumin ameliorates diabetes profiles of db/db mice by altering the composition of gut microbiota and up-regulating the expression of GLP-1 in the pancreas. Fitoterapia 2020, 146, 104665. [Google Scholar] [CrossRef]
- Ramirez, B.G.; Blazquez, C.; del Pulgar, T.G.; Guzman, M.; de Ceballos, M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Zimmer, A.M.; Hohmann, A.G.; Herkenham, M.; Bonner, T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 5780–5785. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamamoto, I.; Oguri, K.; Yoshimura, H. Metabolic disposition of delta 8-tetrahydrocannabinol and its active metabolites, 11-hydroxy-delta 8-tetrahydrocannabinol and 11-oxo-delta 8-tetrahydrocannabinol, in mice. Drug Metab. Dispos 1981, 9, 261–264. [Google Scholar] [CrossRef]
- Johansson, E.; Agurell, S.; Hollister, L.E.; Halldin, M.M. Prolonged apparent half-life of delta 1-tetrahydrocannabinol in plasma of chronic marijuana users. J. Pharm. Pharmacol 1988, 40, 374–375. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Lin, G.; Luo, D.; Chen, H.; Yang, H.; Liang, J.; Liu, Y.; Xie, J.; Su, Z.; et al. Tetrahydrocurcumin is more effective than curcumin in inducing the apoptosis of H22 cells via regulation of a mitochondrial apoptosis pathway in ascites tumor-bearing mice. Food Funct. 2017, 8, 3120–3129. [Google Scholar] [CrossRef]
- Chen, B.L.; Chen, Y.Q.; Ma, B.H.; Yu, S.F.; Li, L.Y.; Zeng, Q.X.; Zhou, Y.T.; Wu, Y.F.; Liu, W.L.; Wan, J.B.; et al. Tetrahydrocurcumin, a major metabolite of curcumin, ameliorates allergic airway inflammation by attenuating Th2 response and suppressing the IL-4Ralpha-Jak1-STAT6 and Jagged1/Jagged2 -Notch1/Notch2 pathways in asthmatic mice. Clin. Exp. Allergy 2018, 48, 1494–1508. [Google Scholar] [CrossRef]
- Lai, C.S.; Ho, C.T.; Pan, M.H. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Peferoen, L.A.; Vogel, D.Y.; Breur, M.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Whitney, N.P.; Eidem, T.M.; Peng, H.; Huang, Y.; Zheng, J.C. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J. Neurochem. 2009, 108, 1343–1359. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Beebe-Dimmer, J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. CA Cancer J. Clin. 2006, 56, 69–83. [Google Scholar] [CrossRef]
- Trinchieri, G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012, 30, 677–706. [Google Scholar] [CrossRef]
- Sgambato, A.; Cittadini, A. Inflammation and cancer: A multifaceted link. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 263–268. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.D.; Templeton, D.J.; Cross, J.V. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J. Immunol. 2012, 189, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes. Dev 1999, 13, 1382–1397. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.O.N. Hernadez de la Cruz and J. S. Lopez-Gonzalez, Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef]
- Ono, M. Molecular links between tumor angiogenesis and inflammation: Inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008, 99, 1501–1506. [Google Scholar] [CrossRef]
- Kimura, Y.N.; Watari, K.; Fotovati, A.; Hosoi, F.; Yasumoto, K.; Izumi, H.; Kohno, K.; Umezawa, K.; Iguchi, H.; Shirouzu, K.; et al. Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci. 2007, 98, 2009–2018. [Google Scholar] [CrossRef]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef]
- Li, T.; Lu, L.; Pember, E.; Li, X.; Zhang, B.; Zhu, Z. New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention. Cells 2022, 11, 1925. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Srivastava, S.Y.; Brickey, W.J.; Iocca, H.; Toews, A.; Morrison, J.P.; Chen, V.S.; Gris, D.; Matsushima, G.K.; Ting, J.P. The inflammasome sensor; NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J. Neurosci. 2010, 30, 15811–15820. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Deleidi, M.; Isacson, O. Viral and inflammatory triggers of neurodegenerative diseases. Sci. Transl. Med. 2012, 4, 121ps3. [Google Scholar] [CrossRef]
- Patrick, K.L.; Bell, S.L.; Weindel, C.G.; Watson, R.O. Exploring the “Multiple-Hit Hypothesis” of Neurodegenerative Disease: Bacterial Infection Comes Up to Bat. Front. Cell Infect. Microbiol. 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuroinflammation 2023, 20, 165. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Pari, L.; Amali, D.R. Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J. Pharm. Pharm. Sci. 2005, 8, 115–123. [Google Scholar]
- Qiu, Y.; Wang, Y.; Lu, J.; Zhu, Q.; Jia, L.; Lei, F.; Shen, L.; Jiang, L.; Wu, A. Synthesis, spectroscopic analysis, DFT, docking, MD and antioxidant activity of tetrahydrocurcumin. J. Biomol. Struct. Dyn. 2023, 42, 13447–13459. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin Ameliorates Diabetic Cardiomyopathy by Attenuating High Glucose-Induced Oxidative Stress and Fibrosis via Activating the SIRT1 Pathway. Oxid. Med. Cell Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- He, W.; Yuan, K.; Ji, B.; Han, Y.; Li, J. Protective effects of curcumin against neuroin fl ammation induced by Abeta25-35 in primary rat microglia: Modulation of high-mobility group box 1, toll-like receptor 4 and receptor for advanced glycation end products expression. Ann. Transl. Med. 2020, 8, 88. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Chen, T.C.; Yeh, J.H.; Tsou, S.C.; Wang, I.; Shen, T.J.; Chuang, C.J.; Chang, Y.Y. Suppressive Effect of Tetrahydrocurcumin on Pseudomonas aeruginosa Lipopolysaccharide-Induced Inflammation by Suppressing JAK/STAT and Nrf2/HO-1 Pathways in Microglial Cells. Oxid. Med. Cell Longev. 2022, 2022, 4978556. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Mojica-Flores, R.; Moreno-Labrador, D.; Pecchio, M.; Rao, K.S.J.; Ahumedo-Monterrosa, M.; Fernandez, P.L.; Larionov, O.V.; Lakey-Beitia, J. Tetrahydrocurcumin Derivatives Enhanced the Anti-Inflammatory Activity of Curcumin: Synthesis, Biological Evaluation, and Structure-Activity Relationship Analysis. Molecules 2023, 28, 7787. [Google Scholar] [CrossRef]
- Wu, J.C.; Tsai, M.L.; Lai, C.S.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014, 5, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Jia, H.; Xu, L.; Cao, Y.; Zhai, M.; Li, K.; Xia, L.; Jiang, L.; Li, X.; et al. Tetrahydrocurcumin improves lipopolysaccharide-induced myocardial dysfunction by inhibiting oxidative stress and inflammation via JNK/ERK signaling pathway regulation. Phytomedicine 2022, 104, 154283. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Yuan, J.; Ma, X.; Zhao, Y.; Li, Y.; Li, F.; Gong, X.; Zhao, J.; Tang, H.; et al. Tetrahydrocurcumin mitigates acute hypobaric hypoxia-induced cerebral oedema and inflammation through the NF-kappaB/VEGF/MMP-9 pathway. Phytother. Res. 2020, 34, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Sangartit, W.; Kukongviriyapan, U.; Donpunha, W.; Pakdeechote, P.; Kukongviriyapan, V.; Surawattanawan, P.; Greenwald, S.E. Tetrahydrocurcumin protects against cadmium-induced hypertension, raised arterial stiffness and vascular remodeling in mice. PLoS ONE 2014, 9, e114908. [Google Scholar] [CrossRef]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef]
- Xu, P.; Huang, Z.; Xu, Y.; Liu, H.; Liu, Y.; Wang, L. Editorial: Antioxidants and inflammatory immune-related diseases. Front. Immunol. 2024, 15, 1476887. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Luo, X.; Wang, Y.; Li, J.; Guo, L.; Wu, G.; Li, Q. Tetrahydrocurcumin protects against spinal cord injury and inhibits the oxidative stress response by regulating FOXO4 in model rats. Exp. Ther. Med. 2019, 18, 3681–3687. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Li, Q.; Ye, X.; Guo, X.; Sun, L.; Zou, J.; Shen, Y.; Mao, Y.; Li, C.; et al. Tetrahydrocurcumin alleviates allergic airway inflammation in asthmatic mice by modulating the gut microbiota. Food Funct. 2021, 12, 6830–6840. [Google Scholar] [CrossRef]
- Zeng, A.; Yu, X.; Chen, B.; Hao, L.; Chen, P.; Chen, X.; Tian, Y.; Zeng, J.; Hua, H.; Dai, Y.; et al. Tetrahydrocurcumin regulates the tumor immune microenvironment to inhibit breast cancer proliferation and metastasis via the CYP1A1/NF-kappaB signaling pathway. Cancer Cell Int. 2023, 23, 12. [Google Scholar] [CrossRef]
- Lai, C.S.; Wu, J.C.; Yu, S.F.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol. Nutr. Food Res. 2011, 55, 1819–1828. [Google Scholar] [CrossRef]
- Yoysungnoen, B.; Bhattarakosol, O.; Changtam, C.; Patumraj, S. Combinational Treatment Effect of Tetrahydrocurcumin and Celecoxib on Cervical Cancer Cell-Induced Tumor Growth and Tumor Angiogenesis in Nude Mice. J. Med. Assoc. Thai 2016, 99 (Suppl. S4), S23–S31. [Google Scholar]
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamrarn, A.; Patumraj, S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008, 14, 2003–2009. [Google Scholar] [CrossRef]
- Yoysungnoen, B.; Bhattarakosol, P.; Changtam, C.; Patumraj, S. Effects of Tetrahydrocurcumin on Tumor Growth and Cellular Signaling in Cervical Cancer Xenografts in Nude Mice. Biomed. Res. Int. 2016, 2016, 1781208. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, B.; Bhattarakosol, P.; Patumraj, S.; Changtam, C. Effects of tetrahydrocurcumin on hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in cervical cancer cell-induced angiogenesis in nude mice. Biomed. Res. Int. 2015, 2015, 391748. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-kappaB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Deng, S.; Wang, N.; Liu, Y.; Yang, X. Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells. Food Nutr. Res. 2016, 60, 30616. [Google Scholar] [CrossRef]
- Kang, N.; Wang, M.M.; Wang, Y.H.; Zhang, Z.N.; Cao, H.R.; Lv, Y.H.; Yang, Y.; Fan, P.H.; Qiu, F.; Gao, X.M. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Qian, K.; Zhang, F.; Allison, S.K.; Zheng, C.; Yang, X. Image-guided locoregional non-intravascular interventional treatments for hepatocellular carcinoma: Current status. J. Interv. Med. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chiou, S.S.; Weng, J.P.; Lin, P.C. Curcumin and tetrahydrocurcumin induce cell death in Ara-C-resistant acute myeloid leukemia. Phytother. Res. 2019, 33, 1199–1207. [Google Scholar] [CrossRef]
- Wu, J.C.; Lai, C.S.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol. Nutr. Food Res. 2011, 55, 1646–1654. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Garbisa, S.; Limtrakul, P. Tetrahydrocurcumin inhibits HT1080 cell migration and invasion via downregulation of MMPs and Upa. Acta Pharmacol. Sin. 2008, 29, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Plyduang, T.; Lomlim, L.; Yuenyongsawad, S.; Wiwattanapatapee, R. Carboxymethylcellulose-tetrahydrocurcumin conjugates for colon-specific delivery of a novel anti-cancer agent, 4-amino tetrahydrocurcumin. Eur. J. Pharm. Biopharm. 2014, 88, 351–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zou, J.; Yan, L.; Du, W.; Zhang, Y.; Sun, H.; Lu, P.; Geng, S.; Gu, R.; et al. Tetrahydrocurcumin induces mesenchymal-epithelial transition and suppresses angiogenesis by targeting HIF-1alpha and autophagy in human osteosarcoma. Oncotarget 2017, 8, 91134–91149. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lu, H.; Chen, F.; Wang, Y.; Fan, W.; Shao, W.; Lu, H.; Lin, B. Tetrahydrocurcumin-induced autophagy via suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma cells. Mol. Med. Rep. 2018, 17, 5964–5969. [Google Scholar]
- Zhang, X.; Peng, L.; Liu, A.; Ji, J.; Zhao, L.; Zhai, G. The enhanced effect of tetrahydrocurcumin on radiosensitivity of glioma cells. J. Pharm. Pharmacol. 2018, 70, 749–759. [Google Scholar] [CrossRef]
- Perez, A.; Huse, J.T. The Evolving Classification of Diffuse Gliomas: World Health Organization Updates for 2021. Curr. Neurol. Neurosci. Rep. 2021, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef]
- Park, C.H.; Song, J.H.; Kim, S.N.; Lee, J.H.; Lee, H.J.; Kang, K.S.; Lim, H.H. Neuroprotective Effects of Tetrahydrocurcumin against Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2019, 25, 144. [Google Scholar] [CrossRef]
- Gao, Y.; Zhuang, Z.; Gao, S.; Li, X.; Zhang, Z.; Ye, Z.; Li, L.; Tang, C.; Zhou, M.; Han, X.; et al. Tetrahydrocurcumin reduces oxidative stress-induced apoptosis via the mitochondrial apoptotic pathway by modulating autophagy in rats after traumatic brain injury. Am. J. Transl. Res. 2017, 9, 887–899. [Google Scholar]
- Bateman, E.A.; VanderEnde, J.; Sequeira, K.; MacKenzie, H.M. Postural neurologic deficits after decompressive craniectomy: A case series of sinking skin flap syndrome in traumatic brain injury. NeuroRehabilitation 2021, 49, 663–672. [Google Scholar] [CrossRef]
- Wei, G.; Chen, B.; Lin, Q.; Li, Y.; Luo, L.; He, H.; Fu, H. Tetrahydrocurcumin Provides Neuroprotection in Experimental Traumatic Brain Injury and the Nrf2 Signaling Pathway as a Potential Mechanism. Neuroimmunomodulation 2017, 24, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, L.; He, B.; Wang, G.; Bianbazhuoma; Kong, D. Characteristics of High Altitude Pulmonary Edema in Naqu at the Altitude of 4500 m. Am. J. Med. Sci. 2021, 362, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Nathal, E.; Serrano-Rubio, A.; Maciel, E.; Arauz, A. Moyamoya disease in Mexico: Our experience. Neurologia (Engl. Ed.) 2021, 36, 603–610. [Google Scholar] [CrossRef]
- Mondal, N.K.; Behera, J.; Kelly, K.E.; George, A.K.; Tyagi, P.K.; Tyagi, N. Tetrahydrocurcumin epigenetically mitigates mitochondrial dysfunction in brain vasculature during ischemic stroke. Neurochem. Int. 2019, 122, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Qipshidze, N.; Munjal, C.; Vacek, J.C.; Metreveli, N.; Givvimani, S.; Tyagi, S.C. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. J. Mol. Neurosci. 2012, 47, 128–138. [Google Scholar] [CrossRef]
- Lin, B.; Yu, H.; Lin, Y.; Cai, C.; Lu, H.; Zhu, X. Suppression of GRASP65 phosphorylation by tetrahydrocurcumin protects against cerebral ischemia/reperfusion injury via ERK signaling. Mol. Med. Rep. 2016, 14, 4775–4780. [Google Scholar] [CrossRef]
- Islam, A.; Alcock, L.; Nazarpour, K.; Rochester, L.; Pantall, A. Effect of Parkinson’s disease and two therapeutic interventions on muscle activity during walking: A systematic review. NPJ Parkinsons Dis. 2020, 6, 22. [Google Scholar] [CrossRef]
- Rajeswari, A.; Sabesan, M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology 2008, 16, 96–99. [Google Scholar] [CrossRef]
- Altuna-Azkargorta, M.; Mendioroz-Iriarte, M. Blood biomarkers in Alzheimer’s disease. Neurologia (Engl. Ed.) 2021, 36, 704–710. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, M.; Seth, P.; Sharma, S.K. Tetrahydrocurcumin confers protection against amyloid beta-induced toxicity. Neuroreport 2011, 22, 23–27. [Google Scholar] [CrossRef]
- Xiao, Y.; Dai, Y.; Li, L.; Geng, F.; Xu, Y.; Wang, J.; Wang, S.; Zhao, J. Tetrahydrocurcumin ameliorates Alzheimer’s pathological phenotypes by inhibition of microglial cell cycle arrest and apoptosis via Ras/ERK signaling. Biomed. Pharmacother. 2021, 139, 111651. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Samra, Y.A.; Elsherbiny, N.M.; Al-Shabrawey, M. Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules 2020, 10, 1119. [Google Scholar] [CrossRef]
- De la Fuente-Munoz, C.E.; Arias, C. The therapeutic potential of mitochondrial transplantation for the treatment of neurodegenerative disorders. Rev. Neurosci. 2021, 32, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Alaghehbandan, R.; Montiel, D.P.; Luis, A.S.; Hes, O. Molecular Genetics of Renal Cell Tumors: A Practical Diagnostic Approach. Cancers 2019, 12, 85. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Cas, M.D.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: In vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef]
- Stielow, M.; Witczynska, A.; Kubryn, N.; Fijalkowski, L.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs-The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Kao, Y.W.; Hsu, S.K.; Chen, J.Y.; Lin, I.L.; Chen, K.J.; Lee, P.Y.; Ng, H.S.; Chiu, C.C.; Cheng, K.C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2020, 22, 212. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Thammathong, J.; Evans, B.; Dubey, K.D.; Banerjee, S.; Dunbar, G.L. Tetrahydrocurcumin Has Similar Anti-Amyloid Properties as Curcumin: In Vitro Comparative Structure-Activity Studies. Antioxidants 2021, 10, 1592. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Khazaeli, M.; Savoj, J.; Manekia, K.; Bangash, M.; Thakurta, R.G.; Dang, A.; Vaziri, N.D.; Singh, B. Dietary tetrahydrocurcumin reduces renal fibrosis and cardiac hypertrophy in 5/6 nephrectomized rats. Pharmacol. Res. Perspect. 2018, 6, e00385. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef]
- Majeed, M.; Natarajan, S.; Pandey, A.; Bani, S.; Mundkur, L. Subchronic and Reproductive/Developmental Toxicity Studies of Tetrahydrocurcumin in Rats. Toxicol. Res. 2019, 35, 65–74. [Google Scholar] [CrossRef]
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D. Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to Drug-Drug Interactions. J. Clin. Med. 2020, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin--from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y.; Liu, H.; Bai, G.; Mayl, J.; Lin, X.; Sutherland, K.; Nabar, N.; Cai, J. The potential therapeutic effects of THC on Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 973–984. [Google Scholar] [CrossRef]
| Disease Name | Targets and Signaling Pathways | Effect | References |
|---|---|---|---|
| Cervical Cancer | Tumor angigenesis HIF-1-α | Suppressed tumor angiogenesis, volume, and growth rate | [63,64,65,66,67,68,69,70,71,72,73,74] |
| Breast Cancer | Bcl-2/Bax protein MMP-2 MMP-9 Apoptosis G2/M cell cycle arrest | Inhibited proliferation, facilitated apoptosis Diminished metastatic potential | [65,66,67] |
| Liver Cancer (HCC) | Caspase-3 Caspase-9 p53 gene Anti-angiogenic properties | Enhanced survival rate Suppressed proliferation Diminished ascites volume and abdominal girth | [63,68] |
| Acute Myeloid Leukemia | Apoptosis Autophagy AVOs | Elicited cell death in drug-resistant cells | [69,70] |
| Fibrosarcoma | MMP-2 MMP-9 Upa MT1-MMP TIMP-2 | Reduced invasive and migratory capabilities Diminished cellular adhesion | [71] |
| Colon Cancer | BCL-2/BAX Caspase3 MMP-2 MMP-9 E-Cadherin N-Cadherin Vimentin | Inhibitted cell proliferation and transfer Promote apoptosis Inhibition of EMT transformation | [59,72] |
| Osteosarcoma | HIF-1α Akt/mTOR p38 MAPK signaling Autophagy MET process | Diminished proliferation, migration, and invasion Fostered MET Curbed angiogenesis | [73] |
| Non-Small Cell Lung Cancer (NSCLC) | Autophagy Beclin-1 LC3-II/LC3-I PI3K/Akt/mTOR signaling | Induced autophagy Suppressed growth and proliferation | [74] |
| Glioma | GSH levels Cyclin D1 PCNA expression G0/G1 cell cycle arrest | Augmented radiosensitivity Reduced tumor cell viability Increased apoptosis rate | [7,75] |
| Disease Name | Targets and Signaling Pathways | Effect | References |
|---|---|---|---|
| Traumatic Brain Injury | Oxidative stress Mitochondrial dysfunction Nrf2 signaling pathway | Mitigates cerebral edema Reduces neuronal apoptosis | [80,81] |
| High-Altitude Cerebral Edema | IL-1β TNF-α SOD VEGF MMP-9 NF-κB | Reduces cerebral water content Diminishes IL-1β and TNF-α | [53,82] |
| Cerebral Ischemia | THcy levels Mitochondrial oxidative stress MMP-9 Tight junction proteins Autophagy markers ERK pathway | Enhances cerebral function Reduces infarct volume | [83,84,85,86] |
| Parkinson’s Disease | Dopaminergic neurons DA and DOPAC levels | Counteracts MPTP-induced depletion of DA and DOPAC | [87,88] |
| Alzheimer’s Disease | Aβ plaques Tau protein neurofibrillary tangles Ras signaling pathway Mitochondrial remodeling Oxidative stress Calcium ion influx Glutamate-induced injury | Mitigates Aβ-induced neurotoxicity Enhances learning/memory Enhances resilience to oxidative stress Reduces cell death | [72,89,90,91,92,93,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, A.; Quan, Y.; Tao, H.; Dai, Y.; Song, L.; Zhao, J. The Role of Tetrahydrocurcumin in Tumor and Neurodegenerative Diseases Through Anti-Inflammatory Effects. Int. J. Mol. Sci. 2025, 26, 3561. https://doi.org/10.3390/ijms26083561
Zeng A, Quan Y, Tao H, Dai Y, Song L, Zhao J. The Role of Tetrahydrocurcumin in Tumor and Neurodegenerative Diseases Through Anti-Inflammatory Effects. International Journal of Molecular Sciences. 2025; 26(8):3561. https://doi.org/10.3390/ijms26083561
Chicago/Turabian StyleZeng, Anqi, Yunyun Quan, Hongxia Tao, Ying Dai, Linjiang Song, and Junning Zhao. 2025. "The Role of Tetrahydrocurcumin in Tumor and Neurodegenerative Diseases Through Anti-Inflammatory Effects" International Journal of Molecular Sciences 26, no. 8: 3561. https://doi.org/10.3390/ijms26083561
APA StyleZeng, A., Quan, Y., Tao, H., Dai, Y., Song, L., & Zhao, J. (2025). The Role of Tetrahydrocurcumin in Tumor and Neurodegenerative Diseases Through Anti-Inflammatory Effects. International Journal of Molecular Sciences, 26(8), 3561. https://doi.org/10.3390/ijms26083561





