How Does a Porcine Herpesvirus, PCMV/PRV, Induce a Xenozoonosis
Abstract
1. PCMV/PRV—Molecular Biology
2. Pathogenesis in the Pig
3. Pathogenesis in Non-Human Primate Xenotransplant Recipients
4. Transmission to a Human Pig Heart Recipient
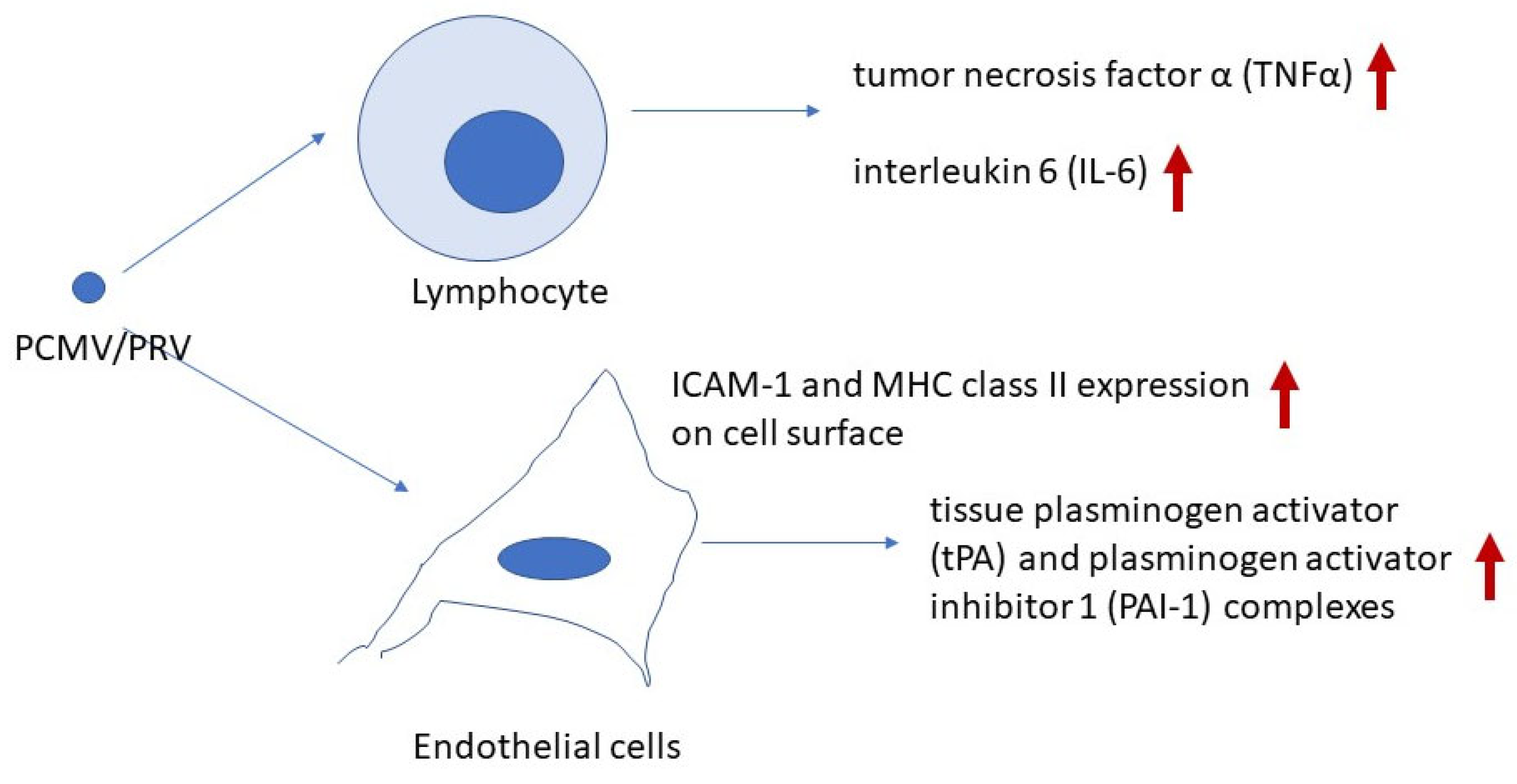
5. Molecular Insights into PCMV/PRV-Induced Xenozoonosis
6. PCMV/PRV and HHV-6
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BaCMV | baboon cytomegalovirus |
| CXCL-9 | chemokine (C-X-C motif) ligand 9 |
| HCMV | human cytomegalovirus |
| HHV6, 7 | human herpesvirus 6, 7 |
| HUVEC | human umbilical vein endothelial cells |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFNγ | interferon γ |
| IL-1α, -1β, -4, -5, -6, -7, -8, -12B, -15 | interleukin-1α, -1β, -6, -7, -8, -12B, 15 |
| ITCV | International Committee on Taxonomy of Viruses |
| IVIG | intravenous immunoglobulin |
| MCP-1, -2 | monocyte chemoattractant protein-1, -2 |
| MDMs | monocyte-derived macrophages |
| MIP-1α, -3 | macrophage inflammatory protein-1α, -3 |
| MHC | major histocompatibility complex |
| miRNA | micro RNA |
| MIG | monokine induced by gamma interferon |
| PAEC | porcine aortic endothelial cells |
| PAI-1 | plasminogen activator inhibitor 1 |
| PCMV/PRV | porcine cytomegalovirus/porcine roseolovirus |
| PTEC | proximal tubular epithelial cells |
| SuBHV2 | suid betaherpesvirus 2 |
| TCR | T cell receptor |
| TGF-β1 | transforming growth factor β1 |
| TF | porcine tissue factor |
| TNFα | tumor necrosis factor α |
| tPA | tissue plasminogen activator |
References
- Denner, J.; Bigley, T.M.; Phan, T.L.; Zimmermann, C.; Zhou, X.; Kaufer, B.B. Comparative Analysis of Roseoloviruses in Humans, Pigs, Mice, and Other Species. Viruses 2019, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zeng, N.; Zhou, L.; Ge, X.; Guo, X.; Yang, H. Genomic organization and molecular characterization of porcine cytomegalovirus. Virology 2014, 460–461, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Genus: Roseolovirus. Available online: https://ictv.global/report/chapter/orthoherpesviridae/orthoherpesviridae/roseolovirus (accessed on 6 February 2025).
- Edington, N.; Plowright, W.; Watt, R.G. Generalized porcine cytomegalic inclusion disease: Distribution of cytomegalic cells and virus. J. Comp. Pathol. 1976, 86, 191–202. [Google Scholar] [CrossRef]
- Jhelum, H.; Kaufer, B.; Denner, J. Application of Methods Detecting Xenotransplantation-Relevant Viruses for Screening German Slaughterhouse Pigs. Viruses 2024, 16, 1119. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Reduction of the survival time of pig xenotransplants by porcine cytomegalovirus. Virol. J. 2018, 15, 171. [Google Scholar] [CrossRef]
- Michaels, M.G.; Alcendor, D.J.; St George, K.; Rinaldo, C.R., Jr.; Ehrlich, G.D.; Becich, M.J.; Hayward, G.S. Distinguishing baboon cytomegalovirus from human cytomegalovirus: Importance for xenotransplantation. J. Infect. Dis. 1997, 176, 1476–1483. [Google Scholar] [CrossRef]
- Michaels, M.G.; Jenkins, F.J.; St George, K.; Nalesnik, M.A.; Starzl, T.E.; Rinaldo, C.R., Jr. Detection of infectious baboon cytomegalovirus after baboon-to-human liver xenotransplantation. J. Virol. 2001, 75, 2825–2828. [Google Scholar] [CrossRef]
- Degré, M.; Ranneberg-Nilsen, T.; Beck, S.; Rollag, H.; Fiane, A.E. Human cytomegalovirus productively infects porcine endothelial cells in vitro. Transplantation 2001, 72, 1334–1337. [Google Scholar] [CrossRef]
- Whitteker, J.L.; Dudani, A.K.; Tackaberry, E.S. Human fibroblasts are permissive for porcine cytomegalovirus in vitro. Transplantation 2008, 86, 155–162. [Google Scholar] [CrossRef]
- Tucker, A.W.; Galbraith, D.; McEwan, P.; Onions, D. Evaluation of porcine cytomegalovirus as a potential zoonotic agent in xenotransplantation. Transplant. Proc. 1999, 31, 915. [Google Scholar] [CrossRef]
- Plowright, W.; Edington, N.; Watt, R.G. The behaviour of porcine cytomegalovirus in commercial pig herds. J. Hyg. 1976, 76, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liao, S.; Zhu, L.; Xu, Z.; Zhou, Y. Molecular epidemiology of porcine Cytomegalovirus (PCMV) in Sichuan Province, China: 2010–2012. PLoS ONE 2013, 8, e64648. [Google Scholar] [CrossRef]
- Edington, N.; Wrathall, A.; Done, J. Porcine cytomegalovirus (PCMV) in early gestation. Vet. Microbiol. 1988, 17, 117–128. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Zhu, L.; Liao, S.; Guo, W. Transcriptome analysis of porcine thymus following porcine cytomegalovirus infection. PLoS ONE 2014, 9, e113921. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, Y.; Peng, L.; Ma, J.; Tang, Z.; Gao, W.; Zhu, Z.; Yao, Z. The role of the CD95, CD38 and TGF-b 1 during active human cytomegalovirus infection in liver transplantation. Cytokine 2006, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liao, S.; Xu, Z.; Zhu, L.; Yang, F.; Guo, W. Identification and Analysis of the Porcine MicroRNA in Porcine Cytomegalovirus-Infected Macrophages Using Deep Sequencing. PLoS ONE 2016, 11, e0150971. [Google Scholar] [CrossRef]
- Liu, X.; Wei, H.; Liao, S.; Ye, J.; Zhu, L.; Xu, Z. MicroRNA transcriptome analysis of porcine vital organ responses to immunosuppressive porcine cytomegalovirus infection. Virol. J. 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Kavanová, L.; Moutelíková, R.; Prodělalová, J.; Faldyna, M.; Toman, M.; Salát, J. Monocyte derived macrophages as an appropriate model for porcine cytomegalovirus immunobiology studies. Vet. Immunol. Immunopathol. 2018, 197, 58–62. [Google Scholar] [CrossRef]
- Gollackner, B.; Mueller, N.J.; Houser, S.; Qawi, I.; Soizic, D.; Knosalla, C.; Buhler, L.; Dor, F.J.; Awwad, M.; Sachs, D.H.; et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation 2003, 75, 1841–1847. [Google Scholar] [CrossRef]
- Mueller, N.J.; Barth, R.N.; Yamamoto, S.; Kitamura, H.; Patience, C.; Yamada, K.; Cooper, D.K.; Sachs, D.H.; Kaur, A.; Fishman, J.A. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J. Virol. 2002, 76, 4734–4740. [Google Scholar] [CrossRef]
- Levi, M.; Sivapalaratnam, S. Disseminated intravascular coagulation: An update on pathogenesis and diagnosis. Expert Rev. Hematol. 2018, 11, 663–672. [Google Scholar] [CrossRef]
- Kim, Y.-J. A new pathological perspective on thrombotic microangiopathy. Kidney Res. Clin. Pract. 2022, 41, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Längin, M.; Mayr, T.; Reichart, B.; Michel, S.; Buchholz, S.; Guethoff, S.; Dashkevich, A.; Baehr, A.; Egerer, S.; Bauer, A.; et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018, 564, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Denner, J.; Längin, M.; Reichart, B.; Krüger, L.; Fiebig, U.; Mokelke, M.; Radan, J.; Mayr, T.; Milusev, A.; Luther, F.; et al. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci. Rep. 2020, 10, 17531. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.A.; Abicht, J.M.; Reichart, B.; Mayr, T.; Guethoff, S.; Denner, J. Active replication of porcine cytomegalovirus (PCMV) following transplantation of a pig heart into a baboon despite undetected virus in the donor pig. Ann. Virol. Res. 2016, 2, 1018. [Google Scholar]
- Fiebig, U.; Abicht, J.M.; Mayr, T.; Längin, M.; Bähr, A.; Guethoff, S.; Falkenau, A.; Wolf, E.; Reichart, B.; Shibahara, T.; et al. Distribution of Porcine Cytomegalovirus in Infected Donor Pigs and in Baboon Recipients of Pig Heart Transplantation. Viruses 2018, 10, 66. [Google Scholar] [CrossRef]
- Jhelum, H.; Bender, M.; Reichart, B.; Mokelke, M.; Radan, J.; Neumann, E.; Krabben, L.; Abicht, J.M.; Kaufer, B.; Längin, M.; et al. Evidence for Microchimerism in Baboon Recipients of Pig Hearts. Viruses 2023, 15, 1618. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Cabezas, L.; Jouve, T.; Malvezzi, P.; Janbon, B.; Giovannini, D.; Rostaing, L.; Noble, J. Tocilizumab and Active Antibody-Mediated Rejection in Kidney Transplantation: A Literature Review. Front. Immunol. 2022, 13, 839380. [Google Scholar] [CrossRef]
- Goetz, F.W.; Planas, J.V.; MacKenzie, S.I. Tumor necrosis factors. Dev. Comp. Immunol. 2004, 28, 487–497. [Google Scholar] [CrossRef]
- Li, W.; Chen, P.; Zhao, Y.; Cao, M.; Hu, W.; Pan, L.; Sun, H.; Huang, D.; Wu, H.; Song, Z.; et al. Human IL-17 and TNF-alpha Additively or Synergistically Regulate the Expression of Proinflammatory Genes, Coagulation-Related Genes, and Tight Junction Genes in Porcine Aortic Endothelial Cells. Front. Immunol. 2022, 13, 857311. [Google Scholar]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Scobie, L.; Goerlich, C.E.; Grazioli, A.; Saharia, K.; Crossan, C.; Burke, A.; Drachenberg, C.; Oguz, C.; et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: A case report. Lancet 2023, 402, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. First transplantation of a pig heart from a multiple gene-modified donor porcine cytomegalovirus/roseolovirus, and antiviral drugs. Xenotransplantation 2023, 30, e12800. [Google Scholar] [CrossRef]
- Plotzki, E.; Keller, M.; Ivanusic, D.; Denner, J. A new Western blot assay for the detection of porcine cytomegalovirus (PCMV). J. Immunol. Methods 2016, 437, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Halecker, S.; Hansen, S.; Krabben, L.; Ebner, F.; Kaufer, B.; Denner, J. How, where and when to screen for porcine cytomegalovirus (PCMV) in donor pigs for xenotransplantation. Sci. Rep. 2022, 12, 21545. [Google Scholar] [CrossRef]
- Yamada, K.; Tasaki, M.; Sekijima, M.; Wilkinson, R.A.; Villani, V.; Moran, S.G.; Cormack, T.A.; Hanekamp, I.M.; Hawley, R.J.; Arn, J.S.; et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation 2014, 98, 411–418. [Google Scholar] [CrossRef]
- Sedmak, D.D.; Knight, D.A.; Vook, N.C.; Waldman, J.W. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation 1994, 58, 1379. [Google Scholar]
- van Dorp, W.T.; van Wieringen, P.A.; Marselis-Jonges, E.; Bruggeman, C.A.; Daha, M.R.; Es, L.V.; van Der Woude, F.J. Cytomegalovirus directly enhances MHC class I and intercellular adhesion molecule-1 expression on cultured proximal tubular epithelial cells. Transplantation 1993, 55, 1367. [Google Scholar] [CrossRef]
- Pugliese, A.; Vidotto, V.; Beltramo, T.; Petrini, S.; Torre, D. A review of HIV-1 Tat protein biological effects. Cell Biochem. Funct. 2005, 23, 223–227. [Google Scholar] [CrossRef]
- Romani, B.; Engelbrecht, S.; Glashoff, R.H. Functions of Tat: The versatile protein of human immunodeficiency virus type 1. J. Gen. Virol. 2010, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. The transmembrane proteins contribute to immunodeficiencies induced by HIV-1 and other retroviruses. AIDS 2014, 28, 1081–1090. [Google Scholar] [CrossRef]
- Denner, J.; Eschricht, M.; Lauck, M.; Semaan, M.; Schlaermann, P.; Ryu, H.; Akyüz, L. Modulation of cytokine release and gene expression by the immunosuppressive domain of gp41 of HIV-1. PLoS ONE 2013, 8, e55199. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Zoonosis and xenozoonosis in xenotransplantation: A proposal for a new classification. Zoonoses Public Health 2023, 70, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, U.; Holzer, A.; Ivanusic, D.; Plotzki, E.; Hengel, H.; Neipel, F.; Denner, J. Antibody Cross-Reactivity between Porcine Cytomegalovirus (PCMV) and Human Herpesvirus-6 (HHV-6). Viruses 2017, 9, 317. [Google Scholar] [CrossRef]
- Looney, R.J.; Huggins, J. Use of intravenous immunoglobulin G (IVIG). Best Pract. Res. Clin. Haematol. 2006, 19, 3–25. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Human Herpesviruses 6A, 6B, and 7. Microbiol. Spectr. 2016, 4, 157–176. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denner, J. How Does a Porcine Herpesvirus, PCMV/PRV, Induce a Xenozoonosis. Int. J. Mol. Sci. 2025, 26, 3542. https://doi.org/10.3390/ijms26083542
Denner J. How Does a Porcine Herpesvirus, PCMV/PRV, Induce a Xenozoonosis. International Journal of Molecular Sciences. 2025; 26(8):3542. https://doi.org/10.3390/ijms26083542
Chicago/Turabian StyleDenner, Joachim. 2025. "How Does a Porcine Herpesvirus, PCMV/PRV, Induce a Xenozoonosis" International Journal of Molecular Sciences 26, no. 8: 3542. https://doi.org/10.3390/ijms26083542
APA StyleDenner, J. (2025). How Does a Porcine Herpesvirus, PCMV/PRV, Induce a Xenozoonosis. International Journal of Molecular Sciences, 26(8), 3542. https://doi.org/10.3390/ijms26083542





